Back to Journals » OncoTargets and Therapy » Volume 11
Lipoprotein (a): a promising prognostic biomarker in patients with hepatocellular carcinoma after curative resection
Authors Gao XH, Zhang SS, Chen H, Wang K, Xie W, Wang F
Received 1 February 2018
Accepted for publication 2 July 2018
Published 17 September 2018 Volume 2018:11 Pages 5917—5924
DOI https://doi.org/10.2147/OTT.S164273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Xing-hui Gao,1,* Shuang-shuang Zhang,2,* Hao Chen,3 Kun Wang,4 Wen Xie,1 Fu-Bing Wang1
1Department of Laboratory Medicine, Zhongnan Hospital of Wuhan University, Wuhan 430071, People’s Republic of China; 2Department of Dermatology, Shanghai Skin Disease Hospital, Shanghai 200443, People’s Republic of China; 3Department of Pathology, Zhongnan Hospital of Wuhan University, Wuhan 430071, People’s Republic of China; 4Department of Laboratory Medicine, Hubei Cancer Hospital, Wuhan 430079, People’s Republic of China
*These authors contributed equally to this work
Purpose: This study aimed to explore serum lipoprotein (a) (Lp(a)) levels and investigate their prognostic value in hepatocellular carcinoma (HCC) patients after curative resection.
Materials and methods: One cohort of 102 healthy individuals, one cohort of 172 HCC patients, and one cohort of 171 HCC patients undergoing curative resection were studied to evaluate serum Lp(a) levels and their prognostic significance, using Kaplan–Meier curves and log-rank tests.
Results: The Lp(a) levels in HCC patients were significantly lower than those in healthy individuals. Furthermore, the levels in HCC patients were significantly associated with recurrence. HCC patients were stratified into high Lp(a) (>20 mg/L) and low Lp(a) (≤20 mg/L) groups, using an optimal cutoff point for the Lp(a) of 20 mg/L. Low Lp(a) levels significantly correlated with tumor recurrence and survival time; HCC patients with low Lp(a) levels had higher recurrence rates and shorter survival time than those with high Lp(a) levels; Lp(a) was an independent prognostic factor for relapse-free survival and overall survival, and retained its prognostic value for α-fetoprotein ≤400 ng/mL and tumor size ≤5 cm subgroups in the training and validation cohorts.
Conclusion: Lp(a) was a promising and useful marker for assessing and monitoring recurrence and prognosis of patients with HCC, and improving Lp(a) levels may be a promising therapeutic strategy in HCC patients.
Keywords: lipoprotein (a), hepatocellular carcinoma, recurrence, prognosis, survival, LP(a), HCC, recurrence, biomarker
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant cancers, which is the second leading cause of tumor-related mortality worldwide.1 Although there are still various therapeutic methods, such as surgical resection, radiotherapy, chemotherapy, and immunotherapy for HCC patients at present, the prognosis of HCC patients after surgical treatments remains poor because of high metastasis and recurrence in tumor progression.2–5 Thus, a sufficient and convenient biomarker will play an important role in monitoring tumor metastasis and recurrence in the prognosis of post-operative HCC patients, which may help clinicians to adjust and choose rational treatments in time so as to improve the survival time for post-operative HCC patients. It is essential to find a novel and potential biomarker for predicting tumor metastasis and recurrence in HCC patients after surgery.
The mechanism underlying high metastasis and recurrence in the progression of HCC is multifactorial and complex.6 Liver damage, as a major factor in HCC progression, reflects the ratio of normal hepatic cells and tumor cells indicating metastasis and invasion. As the liver plays an important role in lipid and lipoprotein metabolism, the significant impairment of the hepatic function occurring during liver diseases, such as HCC, can influence plasma lipoprotein profiles. Since the liver is the organ that synthesizes lipoprotein (a) (Lp(a)), reduced levels of Lp(a) during liver diseases may be attributed to the relative decrease in the synthesis by a damaged liver. Serum Lp(a) levels in patients with liver diseases, induced by hepatitis viral infections, are significantly decreased when compared to healthy controls. Recent studies have shown that serum Lp(a) levels were significantly lower in HCC patients than healthy individuals, revealing that Lp(a) may contribute to a complete assessment and monitoring of the liver function in HCC patients.7,8 Lp(a) levels in chronic hepatitis C patients with 6 months of intramuscular interferon treatment significantly increased from baseline serum lipoprotein concentrations, representing improved liver functions of patients. Serum Lp(a) levels in HCC patients might be caused by HBV relating to long-term liver dysfunction, indicating the potential role of Lp(a) in predicting operative outcomes in HCC patients.7–9 However, Lp(a) levels in tumor recurrence and metastasis of HCC patients have not yet been studied completely.
The purpose of this study was to investigate the prognostic significance of Lp(a) on the recurrence and overall survival (OS) time in HCC patients undergoing resection in two independent cohorts. We found that Lp(a) was a potential biomarker for predicting the prognosis of HCC patients undergoing resection.
Materials and methods
Study design
We recruited 172 HCC patients as a training cohort at Hubei Cancer Hospital, People’s Republic of China, from June 2012 to July 2015, and 171 HCC patients as a validation cohort at Zhongnan Hospital, People’s Republic of China, from January 2013 to December 2015 (Table 1). Serum samples of HCC patients and healthy controls were collected from Hubei Cancer Hospital under approval of the Institutional Review Board and Zhongnan Hospital of Wuhan University under the approval of Institutional Review Board. Written informed consent was obtained from all participants. HCC patients were defined using the data of biochemical assays and imaging scans, and their diagnosis was confirmed by histopathology according to the criteria of the American Association for the Study of Liver Diseases guidelines. Tumor size was defined using the diameter of the single largest lesion, and tumor stage was defined according to the TNM classification system established by the 2010 International Union Against Cancer.10
  | Table 1 The clinicopathologic characteristics of patients in the training and validation cohorts |
Follow-up and tumor recurrence
HCC patients were followed up after surgery until January 2016. Time to recurrence (TTR) was determined as the interval between the date of surgery and the date of diagnosed intrahepatic or extrahepatic recurrence. OS was determined as the interval between surgery and death, or the interval between surgery and the last observation.11
Lp(a)
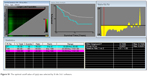
Serum samples of HCC patients were collected 2 days before surgery and stored at −80°C before analysis. The serum Lp(a) concentrations were determined using the Olympus AU5831 automated biochemistry analyzer (Olympus Corporation, Tokyo, Japan). The cutoff value of Lp(a) for predicting HCC recurrence in the training cohort was determined by X-tile 3.6.1 software (Yale University, New Haven, CT, USA).12 Results from X-tile analysis showed an optimal cutoff point for the Lp(a) of 20 mg/L in the training cohort (Figure S1). Therefore, HCC patients were stratified into high (>20 mg/L) or low (≤20 mg/L) Lp(a) groups in the study.
Statistical analysis
Statistical analyses in the study were performed using SPSS 19.0 software (IBM Corporation, Armonk, NY, USA). Experimental values for continuous variables were expressed as the mean ± standard error of the mean. The chi-squared test, Fisher’s exact probability tests, and the Student’s t-tests were used to evaluate the significance of differences in data between groups. If variances within groups were not homogeneous, the nonparametric Mann–Whitney U test or the Wilcoxon signed-rank test was used. Kaplan–Meier curves and log-rank tests were used to analyze the relationships between serum Lp(a) levels and TTR and OS. P<0.05 was considered statistically significant.
Results
Patient characteristics
There was no significant difference in clinicopathologic characteristics, including tumor size and vascular invasion (either micro or major) between the training cohort and the validation cohort. The training cohort included 172 HCC patients (150 males and 22 females), and the average age was 55.4±9.9 years. There were 84 HCC patients with recurrence and 45 HCC patients who died before the last follow-up. In the validation cohort, there were 141 male and 30 female HCC patients, with the average age of 56.6±10.5 years; 56 of the 171 HCC patients suffered recurrence after resection, and 23 of the 171 patients died before the last follow-up (Table 1).
Serum Lp(a) levels were significantly lower in HCC patients and correlate with tumor recurrence
Serum Lp(a) levels in HCC patients were significantly lower than healthy individuals (122.91±11.09 mg/L vs 203.34±19.79 mg/L, P<0.01, Figure 1A). Furthermore, serum Lp(a) levels were significantly lower in HCC patients with recurrence compared with HCC patients without recurrence (97.22±11.25 mg/L vs 145.62±18.23 mg/L, P<0.05, Figure 1B) in the training cohort and (105.28±15.85 mg/L vs 168.50±19.52 mg/L, P<0.05, Figure 1C) in the validation cohort.
Prognostic value of Lp(a) in the training cohort
The optimal cutoff value (20 mg/L) of Lp(a) was set so as to stratify HCC patients into low Lp(a) (≤20 mg/L) and high Lp(a) (>20 mg/L) groups by using the X-tile 3.6.1 software (Figure S1).12 Our results showed that the low Lp(a) group of HCC patients had shorter TTR (P=0.009, Figure 2A) and higher recurrence rates (70.37% vs 43.47%) compared with the high Lp(a) group. Furthermore, the low Lp(a) group of HCC patients had shorter OS (P=0.007, Figure 2B) and higher death rates (48.14% vs 23.60%) compared with the high Lp(a) group. Cox regression analyses demonstrated that the Lp(a) was an independent indicator for TTR (hazard ratio [HR], 0.49; 95% CI: 0.29–0.82; P=0.006; Table 2) and OS (HR, 0.43; 95% CI: 0.23–0.83; P=0.012; Table 2). There was no significant difference in clinicopathologic characteristics, including tumor size and vascular invasion (either micro or major) between the high and low Lp(a) groups. In the training cohort, HCC patients with low Lp(a) levels were more likely to have higher recurrence rates and death rates (P=0.025 and P=0.017, Table 3).
  | Table 3 Correlation between the LP(a) and clinicopathologic characteristics |
Prognostic value of Lp(a) in the validation cohort
The prognostic value of serum Lp(a) levels were further explored using an independent cohort of 171 HCC patients. The results between the training cohort and validation cohort were similar (Table 1). The HCC patients in the low Lp(a) group had shorter TTR (P=0.005, Figure 2A) and shorter OS (P=0.008, Figure 2B) than those in the high Lp(a) group. Multivariate analysis demonstrated that the serum Lp(a) levels were also an independent indicator of TTR (HR, 0.43; 95% CI, 0.22–0.84; P=0.013; Table 2) and OS (HR, 0.35; 95% CI, 0.14–0.91; P=0.031; Table 2). There was also no significant difference in clinical characteristics, including tumor size and vascular invasion (either micro or major) between the high and low Lp(a) groups. In the validation cohort, HCC patients with low Lp(a) levels were more likely to have higher recurrence rates and death rates (P=0.041 and P=0.042, Table 3).
The prognostic significance of Lp(a) for HCC patients with AFP ≤400 ng/mL and tumor size ≤5 cm subgroups
The prognostic significance of Lp(a) for AFP ≤400 ng/mL or tumor size ≤5 cm subgroups was further explored. We found that low Lp(a) levels significantly correlated with TTR and OS of HCC patients for the variables as follows: AFP ≤400 ng/mL for TTR (P=0.019, Figure 3A); tumor size ≤5 cm for TTR (P=0.011, Figure 3A); AFP ≤400 ng/mL for OS (P=0.034, Figure 3B); and tumor size ≤5 cm for OS (P=0.021, Figure 3B) in the training cohort. The prognostic significance for TTR and OS of HCC patients were maintained in AFP ≤400 ng/mL group (P=0.030 and P=0.022, respectively, Figure 3) and tumor size ≤5 cm group (P=0.025 and P=0.018, respectively, Figure 3) in the validation cohort.
Discussion
Metastasis and recurrence of tumor is a multiple-step procedure, which involves a large number of factors. Several studies have shown that the Lp(a) correlates with breast cancer and liver cancer, and low serum Lp(a) levels are involved in tumorigenesis and tumor progression. Serum Lp(a) levels were found to be inversely proportional to tumor mass.9,13 Furthermore, Lp(a) levels were significantly lower in HCC patients than in the controls.7,14–16 Lp(a) may play a crucial role in controlling tumor growth and expansion as a competitive inhibitor of plasmin-induced proteolysis and through its adhesive properties to extracellular matrix components because of its unique structure. High Lp(a) levels may protect against tumors, which participate in mitigating extracellular matrix damage during cancer progression, in particular by inhibiting proteolytic processes characteristic of all types of cancer cells.13,17–23 All of these factors might suggest that Lp(a), as one of the major moleculars, affects tumor progression through modulating proteolytic processes during extracellular matrix damage. However, the value of serum Lp(a) in monitoring the recurrence and survival of HCC patients has not been investigated.
In our study, serum Lp(a) levels in HCC patients significantly decreased in comparison to those in healthy individuals. When analyzing the Lp(a) levels of HCC patients, we found that HCC patients with low serum Lp(a) levels had a higher recurrence rate and a higher death risk than HCC patients with high serum Lp(a) levels. Moreover, low serum Lp(a) levels were significantly associated with the TTR and OS of HCC patients and, in two independent cohorts, were an independent factor for predicting possible recurrence and survival time of HCC patients after surgery. These results suggest that Lp(a) plays an important role in the prognosis of HCC patients after surgery. Moreover, the measurement of Lp(a) is based on standard laboratory measurements, which are routinely performed in the clinical setting. Thus, there is a potential for Lp(a) to be used as a marker of tumor recurrence and treatment responsiveness surveillance, which might provide a powerful test, enabling accurate and early decision making to tailor the most effective therapy according to characteristics of individual HCC patient.
To date, it is still challenging to predict tumor recurrence in low recurrence risk HCC subgroups in clinical practice. Our study showed that preoperative Lp(a) levels retain their prognostic significance in low recurrence risk HCC patients whose conventional clinicopathological variables offer little information on predicting their prognosis. Up to now, AFP and tumor size have been the most extensively used biomarkers for assessing recurrence and surveillance of HCC patients.24–26 Monitoring tumor recurrence in 30%–40% of HCC patients with low AFP levels or tumor size ≤5 cm is still difficult.2,26 Our study has shown that the serum Lp(a) level is a convenient and good tool for predicting recurrence and survival in HCC patients with low AFP levels or tumor size ≤5 cm. Detecting Lp(a) levels would help clinicians identify risk of recurrence in HCC patients with low AFP levels or tumor size ≤5 cm.
Conclusion
In our study, we collected an independent cohort of HCC patients to validate the clinical utility of serum Lp(a) levels and found that the prognostic value in the training and validation cohorts was similar, indicating the reliability and universality of our results. However, there are still several limitations in the study. Some recognized factors, such as tumor number, tumor size, vascular invasion, and AFP, are not independent risk factors for TTR and OS for patients with HCC. It might be owing to our short follow-up time and small-cohort of HCC patients with a hepatitis B virus-positive background. Therefore, a large-cohort, multicenter, long-term study including HCC patients with different medical histories is needed to collect and validate the prognostic value of Lp(a) after resection, and this is currently being conducted in our laboratory. In addition, a comprehensive study of the mechanisms of the role of Lp(a) on HCC cells also needs to be investigated.
Acknowledgments
The authors thank the participating patients for the source of clinical blood samples. This work was supported by Applied Basic Research Program of Science and Technology Bureau Foundation of Wuhan (No 2016060101010054) and Science and Technology Innovation Fostering Foundation of Zhongnan Hospital of Wuhan University (cxpy20160025). This work was also funded by “351 talent project (Luojia Young Scholars)” of Wuhan University and National Natural Science Foundation of China (grant No 81672114).
Author contributions
Xing-hui Gao and Fubing Wang conceived and designed the experiments. Xing-hui Gao and Shuang-shuang Zhang performed the research, conducted the data analyses, and wrote the manuscript. Hao Chen and Kun Wang contributed to the clinical data collection. Wen Xie and Fubing Wang revised the manuscript and coordinated the research team. All authors have read and approved the final manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. | ||
Hong TS, Wo JY, Yeap BY, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34(5):460–468. | ||
Wang G, Liu J, Cai Y, et al. Loss of Barx1 promotes hepatocellular carcinoma metastasis through up-regulating MGAT5 and MMP9 expression and indicates poor prognosis. Oncotarget. 2017;8(42):71867–71880. | ||
Jariwala N, Rajasekaran D, Mendoza RG, et al. Oncogenic Role of SND1 in Development and Progression of Hepatocellular Carcinoma. Cancer Res. 2017;77(12):3306–3316. | ||
Mao X, Wong SY, Tse EY, et al. Mechanisms through Which Hypoxia-Induced Caveolin-1 Drives Tumorigenesis and Metastasis in Hepatocellular Carcinoma. Cancer Res. 2016;76(24):7242–7253. | ||
Motta M, Giugno I, Ruello P, Pistone G, di Fazio I, Malaguarnera M. Lipoprotein (a) behaviour in patients with hepatocellular carcinoma. Minerva Med. 2001;92(5):301–305. | ||
Malaguarnera M, Giugno I, Trovato BA, Panebianco MP, Siciliano R, Ruello P. Lipoprotein(a) concentration in patients with chronic active hepatitis C before and after interferon treatment. Clin Ther. 1995;17(4):721–728. | ||
Ooi K, Shiraki K, Sakurai Y, Morishita Y, Nobori T. Clinical significance of abnormal lipoprotein patterns in liver diseases. Int J Mol Med. 2005;15(4):655–660. | ||
Wittekind C. Pitfalls in the classification of liver tumors. Pathologe. 2006;27(4):289–293. | ||
Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150(7):1646.e17–1658.e17. | ||
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. | ||
Cha J, Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Lipoprotein(a) and vitamin C impair development of breast cancer tumors in Lp(a)+; Gulo−/− mice. Int J Oncol. 2016;49(3):895–902. | ||
Irshad M. Serum lipoprotein (a) levels in liver diseases caused by hepatitis. Indian J Med Res. 2004;120(6):542–545. | ||
Basili S, Andreozzi P, Vieri M, et al. Lipoprotein (a) serum levels in patients with hepatocarcinoma. Clin Chim Acta. 1997;262(1–2):53–60. | ||
Malaguarnera G, Catania VE, Francaviglia A, et al. Lipoprotein(a) in patients with hepatocellular carcinoma and portal vein thrombosis. Aging Clin Exp Res. 2017;29(Suppl 1):185–190. | ||
Lippi G, Franchini M, Salvagno GL, Guidi GC. Lipoprotein[a] and cancer: anti-neoplastic effect besides its cardiovascular potency. Cancer Treat Rev. 2007;33(5):427–436. | ||
Vere CC, Streba CT, Streba L, Rogoveanu I. Lipid serum profile in patients with viral liver cirrhosis. Med Princ Pract. 2012;21(6):566–568. | ||
Wang Q, Lau WY, Zhang B, et al. Preoperative total cholesterol predicts postoperative outcomes after partial hepatectomy in patients with chronic hepatitis B- or C-related hepatocellular carcinoma. Surgery. 2014;155(2):263–270. | ||
Sawabe M, Tanaka N, Mieno MN, et al. Low lipoprotein(a) concentration is associated with cancer and all-cause deaths: a population-based cohort study (the JMS cohort study). PLoS One. 2012;7(4):e31954. | ||
Rath M, Pauling L. Hypothesis: lipoprotein(a) is a surrogate for ascorbate. Proc Natl Acad Sci U S A. 1990;87(16):6204–6207. | ||
Jiang J, Nilsson-Ehle P, Xu N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006;5:4. | ||
Uccello M, Malaguarnera G, Pelligra EM, Biondi A, Basile F, Motta M. Lipoprotein(a) as a potential marker of residual liver function in hepatocellular carcinoma. Indian J Med Paediatr Oncol. 2011;32(2):71–75. | ||
Sato Y, Nakata K, Kato Y, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328(25):1802–1806. | ||
Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62(4):848–854. | ||
Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5(1):145–159. |
Supplementary materials
  | Figure S1 The optimal cutoff value of Lp(a) was selected by X-tile 3.6.1 software. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.