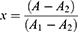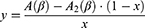Back to Journals » Eye and Brain » Volume 13
Lipocalin-type Prostaglandin D Synthase Concentration Gradients in the Cerebrospinal Fluid in Normal-tension Glaucoma Patients with Optic Nerve Sheath Compartmentation
Authors Pircher A , Neutzner A, Montali M, Huber A, Scholl HP, Berberat J, Remonda L, Killer HE
Received 17 December 2020
Accepted for publication 19 February 2021
Published 14 April 2021 Volume 2021:13 Pages 89—97
DOI https://doi.org/10.2147/EB.S297274
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Achmed Pircher, 1, 2 Albert Neutzner, 3 Margherita Montali, 4 Andreas Huber, 5 Hendrik PN Scholl, 2, 6 Jatta Berberat, 7 Luca Remonda, 7 Hanspeter E Killer 3
1Department of Neuroscience/Ophthalmology, Uppsala University, Uppsala, Sweden; 2Department of Ophthalmology, University Hospital Basel & University Basel, Basel, Switzerland; 3Department of Biomedicine, University Hospital Basel & University Basel, Basel, Switzerland; 4Department of Ophthalmology, San Bassiano Hospital, Bassano del Grappa, Italy; 5Department of Medicine, Private University of the Principality of Lie Triesen, Triesen, Liechtenstein; 6Institute of Molecular and Clinical Ophthalmology Basel, Basel, Switzerland; 7Department of Neuroradiology, Cantonal Hospital Aarau, Aarau, Switzerland
Correspondence: Achmed Pircher
Department of Neuroscience/Ophthalmology, Uppsala University, Uppsala, Sweden
Tel +46 76 496 37 51
Email [email protected]
Objective: To report on the lipocalin-type prostaglandin D synthase (L-PGDS) concentrations in the cerebrospinal fluid (CSF) of the perioptic and lumbar subarachnoid space (SAS) in patients with radiologically proven optic nerve (ON) sheath compartmentation presenting as normal-tension glaucoma (NTG).
Methods: Retrospective biochemical analysis of CSF in thirteen patients with ON sheath compartmentation presenting as NTG (four females, mean age 70± 8 years). CSF was sampled from the SAS of the ON during ON sheath fenestration for ON sheath compartmentation and from the lumbar SAS at the time of lumbar puncture. Nephelometry was used for the quantification of L-PGDS and albumin concentration. Albumin was measured in order to assess the amount of contamination with serum in the CSF samples taken from the ON SAS. Main outcome measures were L-PGDS concentrations in the CSF of the perioptic and lumbar SAS.
Results: Mean L-PGDS concentration was 24± 8 mg/L in the lumbar SAS compared to 33± 27 mg/L without correction of serum contamination and 45± 39 mg/L after correction of serum contamination in the perioptic SAS. The difference between the lumbar and the perioptic SAS was statistically significant (P=0.0047 without correction of serum contamination, P=0.0002 with correction of serum contamination; Mann–Witney U-test).
Conclusion: This study demonstrates a concentration gradient of L-PGDS levels within the CSF with a statistically significant higher concentration in the compartmentalized perioptic SAS compared to that in the lumbar SAS. Biochemical changes in the perioptic SAS might be involved in the pathophysiology in NTG patients with ON sheath compartmentation.
Keywords: lipocalin-type prostaglandin D synthase, cerebrospinal fluid, optic nerve sheath compartment syndrome, normal-tension glaucoma, optic nerve
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Normal-tension glaucoma (NTG) is a multifactorial, yet still poorly understood disease of the optic nerve (ON). In contrast to primary open-angle glaucoma, intraocular pressure (IOP) appears to play a minor role in NTG.1,2 Vascular dysregulation,3 oxidative stress,4 genetic factors5 and autoinflammatory processes6 are considered to be involved in the pathogenesis of NTG.
In recent years, the role of cerebrospinal fluid (CSF) in the pathogenesis of NTG was recognized7 with CSF circulatory dysfunction8,9 and abnormal translaminar pressure gradients10 as contributing factors. Studies8,9 with computed tomography (CT) assisted cisternography demonstrated sequestration of CSF in the subarachnoid space (SAS) of the ON in a cohort of NTG patients, thus suggesting a disturbance of CSF exchange in this location. These patients also presented with enlarged retrobulbar ON sheath diameters, as seen typical in patients with papilledema due to elevated intracranial pressure. As intracranial pressure was normal in all of the patients described, distension of the ON sheath is likely to be caused by a higher local CSF pressure or a change in the compliance of the dural sheath. We hypothesize that the compartmentation of the perioptic SAS might contribute to the pathophysiology of NTG in these patients with ON sheath compartmentation and considered ON sheath decompression as a therapeutic option to lower the pressure in the ON SAS and therefore to facilitate newly formed CSF to flow into this location.
ON sheath compartmentation may have been reported before, but under different names. In 1968 Bane described a patient with a “cyst” of the dural sheath of the ON.11 Garrity et al reported on thirteen patients with “optic nerve sheath meningoceles”.12 The common finding in all these cases was the enlargement of the ON sheath. In some of these patients, ON sheath decompression was performed and a “gush” of “crystal clear” fluid was observed exiting the fistula that was surgically created, thus suggesting an elevated “opening pressure”. Probably “cysts of the dural sheath”, “optic nerve sheath meningoceles” and the “optic nerve sheath compartment syndrome” are all features of the same underlying process and NTG with ON sheath compartmentation might be in the spectrum of this process.
Lipocalin-type prostaglandin D synthase (L-PGDS), also known as beta-trace protein, is a brain-specific glycoprotein that consists of 168 amino acids and belongs to the lipocalin superfamily. It is one of the most abundant proteins in the CSF (15–17 mg/L)13,14 and represents about 3% of the total CSF protein.15 In the brain, L-PGDS is produced by the cells of the leptomeninges, the choroid plexus and the arachnoid membrane.16 In the blood, L-PGDS is present in a much lower concentration (0.6 mg/L)14 and it is therefore used as a clinical marker for CSF leakage into the nasal cavity. It has been hypothesized that L-PGDS enters the serum by diffusion and is eliminated by the liver. The role of L-PGDS is manifold. L-PGDS is considered to be a major chaperone in the human CSF.17,18 Moreover, L-PGDS is involved in diverse cellular processes like apoptosis, inflammation, cell differentiation and cell cycle progression. L-PGDS has been shown to have a critical role in protection against cerebral ischemia and to be involved in multiple sclerosis,13 spinal canal stenosis, bacterial meningitis,19 normal-pressure hydrocephalus,20 and dementia.19,21
The current study compares the L-PGDS levels in the perioptic SAS with the concentration in the lumbar SAS in thirteen NTG patients with radiologically proven ON sheath compartmentation for a better understanding of the biochemical environment of the ON following compartmentalization of the perioptic SAS.
Methods
The study was approved by the local ethical commission (Ethikkommission Nordwest- und Zentralschweiz) and followed the tenets of the Declaration of Helsinki. The informed consent from the subjects was obtained before inclusion in the study. This study retrospectively analyses and compares CSF L-PGDS concentration in the perioptic SAS with the concentration in the lumbar SAS in Caucasian NTG patients with ON sheath compartmentation and progressive visual field defects despite normal or low IOP.
Optic nerve sheath fenestration (ONSF) was performed as clinical care following the diagnostic procedure (CT-assisted cisternography) that proved compartmentation of the SAS. All patients were informed about the rationale for CT-assisted cisternography and ONSF.
Thirteen patients with NTG underwent lumbar puncture and CT-assisted cisternography after MRI demonstrated dilatation of the ON sheath (measurement of the ON sheath diameter at 3 mm distance from the posterior globe). The indication for this work-up was progressive loss of visual fields despite normal and low IOP. ONSF was only performed in eyes with fast (>2.5dB over the last year) and severe progression of visual field defects (mean deviation (MD) >15 dB) demonstrated by using standard automated perimetry (Program G2, Octopus Haag-Streit, Switzerland) in whom impaired CSF flow in the ON SAS of the affected eye was measured. CT cisternography demonstrated diminished contrast loaded CSF along the intraorbital ON with a minimum of contrast in the bulbar region of the ON behind the lamina cribrosa. The rationale for ONSF was to lower the pressure and the resistance at the location of the most pronounced compartmentation and therefore to increase velocity and outflow of stagnant CSF, thus allowing new CSF inflow into the SAS. No complications such as retrobulbar hematoma, immediate postoperative vision loss and infections that occurred during and after the surgical procedure for ONSF. One patient suffered from transient double vision postoperatively. The most likely reason was the procedure performed on the medial rectus muscle in combination with fusion problems due to markedly compromised visual fields.
Each patient underwent full ophthalmologic examination including slit lamp assisted biomicroscopy, applanation tonometry, gonioscopy, visual acuity, color vision, measurement of central corneal thickness (ultrasound pachymetry), standard automated perimetry and neuroretinal rim assessment by optic coherence tomography (Heidelberg Engineering, California, USA). In each patient, IOP was measured at least four times at different times during 24 h (between 8:00 a.m. and 8:00 p.m.) in a seated position, using Goldmann applanation tonometry and twice at night (between 9:00 p.m. and 6:00 a.m.) in a recumbent position, using Perkins tonometry. All IOP measurements were examined for their dependence on central corneal thickness in order to exclude false negative values.22 IOP lowering treatment consisted of topical applied prostaglandin analogs, β-blockers, carbonic anhydrase inhibitors, α-agonists and combinations of these medications. The IOP remained unchanged after ONSF was performed. Systemic medication influencing the production or resorption of CSF was a study exclusion criterion.
CSF was sampled from the lumbar SAS during lumbar puncture (L3-L4) and from the ON during ONSF. ON sheath decompression was performed via a medial transconjunctival orbitotomy under general anesthesia. Special care was taken not to use fluids to moisten the cornea after the orbitotomy in order not to dilute the CSF. Multiple incisions were performed in the dura of the ON with a 19-gauge blade and the trabeculae in the SAS were loosened with a tenotomy hook. After the first incision, a gush of CSF was observed, followed by further slow outflow. CSF then was sampled with a syringe using a 37-gauge needle and immediately deep frozen (Figure 1, Figure 2, Figure 3, Figure 4).
CSF from both sampling sites was examined for the content of L-PGDS and albumin. L-PGDS was chosen due to its abundance in the CSF and its very low serum concentration to avoid the possibility of false positive results that often occur as a consequence of the presence of serum during CSF sampling. Albumin was measured in order to assess the amount of contamination with serum during the ON sheath fenestration in the CSF sample taken from the ON SAS. The contamination with serum in the perioptic SAS was calculated by setting up two equations where in the first equation the percentage of blood volume that contaminates the perioptic CSF and in the second equation the amount (mg/L) of L-PGDS without serum contamination was calculated. The calculations were based on the L-PGDS levels measured in the serum and the albumin levels measured in the serum and in the lumbar SAS. The following calculations were performed:
where x=hundredths of a liter of perioptic CSF w/o serum (eg 30/100=0.3 L), (100–x)=complement to hundredths of a liter of the serum (eg 70/100=0.7 L), A (mg/L)=albumin in the perioptic CSF with serum, 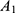 (mg/L)=albumin in the perioptic CSF without serum*,
(mg/L)=albumin in the perioptic CSF without serum*, 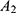 (mg/L)=albumin in serum.
(mg/L)=albumin in serum.
where y (mg/L)=L-PGDS in the perioptic CSF without serum contamination, A(β) (mg/L)=L-PGDS in the perioptic CSF with serum contamination, A2(β) (mg/L)=L-PGDS in serum. *For the albumin levels in the perioptic CSF without serum contamination the albumin levels measured in the lumbar SAS was used in order to deal with the same quantity of contamination in the lumbar SAS and in the perioptic SAS.
Concentration measurements were routinely performed by nephelometry on BNII (Dade Behring, Marburg, Germany) according to the manufacturer`s instructions. If necessary, samples were diluted so that the results would fall in the linear range. At least three measurements were performed from each sample and the mean value was calculated. In addition, L-PGDS and albumin serum levels were determined using above described analytical method.
Statistical analysis was performed using SIGMAPLOT 10.0 (Systat Software, San Jose, CA, USA), Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA), and the SPSS 21.0 (IBM Corporation, Armonk NY, USA) for Windows statistical package. Data were analyzed using the Mann–Whitney U-test when normal distribution was not given and the unpaired Student’s t-test to compare normally distributed variables. The nonparametric Spearman rank order correlation coefficient was used for analysis of correlations between L-PGDS concentrations and age and between L-PGDS concentrations and IOP. All data are expressed as mean and standard deviation or median and interquartile ranges, respectively. Differences were considered significant when the error probability of P<0.05.
Results
Thirteen NTG patients (mean age 70±8 years), four women (66±9 years) and nine men (72±7 years) underwent lumbar puncture, cisternography and ONSF. ONSF was performed in 16 of 26 ONs due to progressive loss of visual field in the presence of low IOP and compartmentation of the SAS with lack of contrast medium flow to the lamina cribrosa. Figures 2–4 illustrates fundus photography of the ON head, visual fields and the CT cisternography of one of the included patients before ONSF was performed (Figures 2–4). Intracranial pressure measured during lumbar puncture was below 20 cm H2O in all patients. Only one randomly selected ON of each patient was included in the statistical analysis. The mean glaucomatous visual field defect (MD) was 23±4 dB and the mean IOP was 14±2 mmHg (Table 1).
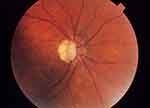 |
Figure 2 Fundus photography of right optic nerve head. Note the glaucomatous optic disc cupping and peripapillary atrophy. |
 |
Figure 3 Right visual field, measured by standard automated perimetry. |
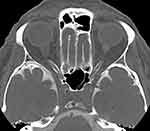 |
Figure 4 Computer tomography assisted cisternography. Note the lack of contrast medium flow into the intraorbital optic nerve subarachnoid space and enlarged optic nerve sheath diameters. |
In the CSF, the mean L-PGDS concentration (n=13) measured 24±8 mg/L in the lumbar SAS and in the perioptic SAS 33±27 mg/L without correction of serum contamination and 45±39 mg/L after correction of serum contamination. In the lumbar SAS, L-PGDS levels ranged from 4 to 34 mg/L and in the perioptic SAS from 11 to 104 mg/L without correction of serum contamination and from 21 to 144 with correction of serum contamination (Table 1). There was a statistically significant difference in L-PGDS concentration between lumbar and perioptic SAS (P=0.0047 without correction of serum contamination and P=0.0002 with correction of serum contamination; Mann–Whitney U-test) (Figure 5). L-PGDS showed no significant difference between females (n=4) and males (n=9) neither in the lumbar SAS (P=0.44; Mann–Whitney U-test) nor in the perioptic SAS (P=0.27 without correction of serum contamination, P=25 with correction of serum contamination; Mann–Whitney U-test).
The mean albumin concentration measured 290±130 mg/L in the lumbar SAS (n=13) and in the perioptic SAS (n=13) 10,800±5455 mg/L. The difference was statistically significant (P<0.001, unpaired t-test) between the lumbar and perioptic SAS.
In the serum the mean L-PGDS concentration (n=13) measured 0.8±0.3 mg/L. The mean albumin concentration (n=13) measured 3725±5469 mg/L.
Bivariate analysis comparing L-PGDS with age showed no statistically significant correlation between age and L-PGDS neither in the lumbar spine (Spearman's correlation; ρ=−0.23, =0.46) nor in the ON SAS (Spearman`s correlation; ρ=−0.03, P=0.93 without correction of serum contamination; ρ=0.17, P=0.59 with correction of serum contamination). No significant correlation was found between IOP and L-PGDS in the ON SAS (Spearman's correlation; ρ=−0.04, P=0.89 without correction of serum contamination; ρ=−0.06, P=0.84 with correction of serum contamination).
Discussion
This study demonstrates a concentration gradient of L-PGDS between the lumbar CSF and the CSF in the perioptic SAS in thirteen patients with clinical features of NTG and radiologically proven ON sheath compartmentation (dilated ON sheath in spite of normal intracranial pressure and reduced contrast agent in the SAS of the ON on cisternography). The concentration of L-PGDS was significantly higher in the perioptic SAS compared to the concentration in the lumbar SAS. This difference indicates that the biochemical environment differs around the ON from that in the lumbar spine.
There are mainly three explanations for a higher concentration of L-PGDS in the perioptic SAS: (a) impaired perioptic recycling or outflow, (b) higher perioptic production of L-PGDS, or (c) a combination of both.
All included patients underwent CT-assisted cisternography one month before ONSF. The cisternography in all patients demonstrated impaired contrast loaded CSF dynamics in the SAS of the ON, most pronounced in the retrobulbar ON segment as described in a recent study in 56 patients with NTG.9 In these NTG patients, a gradual reduction of contrast-loaded CSF toward the retrobulbar segment was measured in Hounsfield units. This indicates that CSF in these patients does not behave as a continuum within the ON SAS between the pituitary cistern and the retrobulbar ON segment, a condition that has previously been described as ON sheath compartmentalization.23,24 One possible explanation for this phenomenon is a remodeling of the SAS following a change in the architecture of meningothelial cells via mechanical and biochemical induced effects.25,26 In such a compartment, the CSF inflow is disturbed and most likely also the CSF outflow and thus recycling of various CSF proteins, eg L-PGDS. Interestingly, Tumani et al19 found increased L-PGDS concentrations in the lumbar SAS in patients with spinal canal stenosis. It seems likely that the SAS of the lumbar spine also becomes compartmented in patients with spinal canal stenosis, thus impairing outflow and recycling of CSF proteins and therefore effecting L-PGDS concentrations in a similar fashion. As a consequence of a stagnant CSF, the biochemical environment within the perioptic SAS might undergo changes that result in an alteration of protein and molecule concentrations including upregulation of L-PGDS and other CSF proteins.
Previous studies of L-PGDS indicate a possible relationship between the concentration of L-PGDS and pressure. In patients with spontaneous intracranial hypotension a significant increase of L-PGDS was measured in the lumbar CSF.27 The authors hypothesized that the secretion of L-PGDS and other CSF proteins may depend on CSF pressure and volume. Further, in a cohort of primary open angle glaucoma patients, Bauer et al28 found a positive correlation between IOP and L-PGDS in the aqueous humor. The local pressure in the SAS of the ON is unknown and in fact due to its small size difficult to measure. However, pressure in a CSF compartment is likely to differ from other CSF spaces and might therefore influence the secretion of L-PGDS.
Increased concentration of L-PGDS in the CSF was observed also following stroke during the repair of the damaged brain.13 The authors suggested that L-PGDS overexpression may be a component of a physiological response to the homeostatic imbalance of the brain following damage. Glaucoma is characterized by progressive ON fiber loss. All included NTG patients in this study had marked ON fiber loss as assessed by OCT as well as progressive severe visual field loss. Similar to the upregulation during stroke, L-PGDS overexpression in the perioptic SAS could also represent a physiological response to damaged ON fibers in NTG patients. A recently published study demonstrated a neuroprotective role of L-PGDS.18 This in vitro study18 demonstrated the ability of L-PGDS to act as a chaperone on the pre-formed fibrils of amyloid β, a protein strongly suspected to play a key role in the pathogenesis of Alzheimer’s disease. As NTG is a neurodegenerative disease it is conceivable that increased production of L-PGDS within the ON occurs as a neuroprotective reaction on the ON fiber damage. Alternatively, it has been reported that L-PGDS itself has also apoptosis-inducing properties in neuronal tissue, depending on the receptor pathway it signals to target cells.29,30 The role that L-PGDS plays around the ON during ON sheath compartmentation needs to be studied in more detail.
Suk31 showed in vitro and in vivo that L-PGDS induces glial cell migration. This indicates that L-PGDS might also induce morphological changes and thus play a crucial role in the development of a compartment itself.
Compared to other studies13,14,19 on L-PGDS, the L-PGDS concentration in the lumbar SAS in the NTG patients in this study was significantly higher while the serum L-PGDS concentration was in the same range as reported by other authors.19,32 In our cohort, L-PGDS concentration in the lumbar SAS was 24±8 mg/L while in other studies in healthy subjects L-PGDS measured between 15 and 17 mg/L.13,14 As the patients in our study are slightly older, this might partially explain these results.15 However, a higher overall L-PGDS concentration in the CSF might indicate a possible generalized neurodegenerative pathophysiology in NTG. There is evidence for a correlation between NTG and Alzheimer's disease even though this is still controversial.33,34 The findings of elevated lumbar L-PGDS concentrations points toward generalized reduced CSF dynamics in NTG patients with impaired clearing of CSF L-PGDS and probably other proteins as well.
The albumin concentration in the NTG patients in this study was in a normal range both in serum and in the lumbar CSF.35,36 The significant difference in albumin concentration between the lumbar spine SAS and the perioptic SAS is most likely due to contamination with blood during ONSF. As the albumin concentration is much higher in serum than in the CSF this is the most likely explanation. The concentration of L-PGDS is, however, extremely low in serum and false positive results caused by serum contamination are therefore impossible.
In the data set of L-PGDS measurements in the perioptic SAS two (patients 2 and 13 without correction of serum contamination), respectively three (patients 1, 2 and 13 after correction of serum contamination) measurements present highly elevated L-PGDS levels. Duration and activity of the disease might have contributed on this elevation, this is however pure speculation. A similar effect has been found on the L-PGDS concentration in the subretinal space of detached retinas. The levels there were higher in freshly detached retinas.37
This study has several limitations. First, the study population is rather small. The patients included in this study may not represent the typical NTG patient as in them also ON sheath compartmentation was diagnosed. Larger studies will have to estimate the amount of patients with NTG that also present with ON sheath compartmentation. Second, there should be no misunderstanding concerning ONSF for NTG. We do not suggest that this is the treatment of choice for this disease. The reason for ONSF in our patients was to allow CSF outflow from the compartmented perioptic SAS, in analogy to ON sheath hydrops12 and for compartmentation due to meningioma.24 Third, the lack of a control group is an obvious problem. Recruiting a control group for an invasive diagnostic and treatment, however, is not ethical and therefore out of the scope. Instead of a control group we suggest consideration of the following: according to the second law of thermodynamics, a homogeneous distribution of molecules and proteins can be expected in a closed system with free fluid circulation. The difference between CSF composition in the ON SAS and the lumbar SAS, however, is already evident in this small cohort of NTG patients. Fourth, all included NTG patients are in an advanced stage of glaucoma (MD >15 dB). It is therefore possible that the stage of the disease had an impact on the L-PGDS concentration in the perioptic SAS.
This study demonstrates a significant concentration gradient of L-PGDS within the CSF of the trapped perioptic CSF compared to that in the lumbar spine SAS in a group of NTG patients with optic nerve sheath compartment syndrome. It is to be expected that other CSF proteins might differ in quantity and quality in a comparted perioptic CSF. We hypothesize that alteration in protein concentrations and content can influence the ON function, possibly contributing to NTG in some patients. Larger studies are necessary to support this hypothesis. We would like to encourage MRI studies in NTG patients that will focus on the morphology of the ON sheath and features of an ON sheath compartment syndrome.
Disclosure
Dr Hendrik Scholl is supported by the Swiss National Science Foundation, National Center of Competence in Research Molecular Systems Engineering “Molecular Systems Engineering”, the Wellcome Trust, and the Foundation Fighting Blindness Clinical Research Institute. Dr Scholl is member of the Scientific Advisory Board of: Astellas Institute for Regenerative Medicine; Gensight Biologics; Ionis Pharmaceuticals, Inc.; Gyroscope Therapeutics Ltd; Janssen Research & Development, LLC (Johnson & Johnson); and Pharma Research & Early Development (pRED) of F. Hoffmann-La Roche Ltd; Novartis Pharma AG (CORE). Dr Scholl is a paid consultant of: Boehringer Ingelheim Pharma GmbH & Co; Gerson Lehrman Group; and Guidepoint. Dr Scholl is member of the Data Monitoring and Safety Board/Committee of Belite Bio and ReNeuron Group Plc/Ora Inc. and a member of the Steering Committee of Novo Nordisk (FOCUS trial). Dr Scholl is co-director of the Institute of Molecular and Clinical Ophthalmology Basel (IOB) which is constituted as a nonprofit foundation and receives funding from the University of Basel, the University Hospital Basel, Novartis, and the government of Basel-Stadt. These arrangements have been reviewed and approved by the University of Basel (Universitätsspital Basel, USB) in accordance with its conflict of interest policies. Dr Hendrik Scholl is principal investigator of grants at the USB sponsored by the following entities: IVERIC bio (Ophthotech Corporation); Kinarus AG; and Novartis Pharma AG. Grants at USB are negotiated and administered by the institution (USB) which receives them on its proper accounts. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive support from the institution for their project(s). The authors report no other conflicts of interest in this work.
References
1. Mi XS, Yuan TF, So KF. The current research status of normal tension glaucoma. Clin Interv Aging. 2014;9:1563–1571. doi:10.2147/CIA.S67263
2. Killer HE, Pircher A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (Lond). 2018;32:924–930. doi:10.1038/s41433-018-0042-2
3. Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393.
4. Harada T, Harada C, Nakamura K, et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest. 2007;117(7):1763–1770. doi:10.1172/JCI30178
5. Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009;88(4):837–844. doi:10.1016/j.exer.2008.11.003
6. Kremmer S, Kreuzfelder E, Klein R, et al. Antiphosphatidylserine antibodies are elevated in normal tension glaucoma. Clin Exp Immunol. 2001;125(2):211–215. doi:10.1046/j.1365-2249.2001.01578.x
7. Wostyn P, De Groot V, Van Dam D, et al. Senescent changes in cerebrospinal fluid circulatory physiology and their role in the pathogenesis of normal-tension glaucoma. Am J Ophthalmol. 2013;156(1):5–14. doi:10.1016/j.ajo.2013.03.003
8. Killer HE, Miller NR, Flammer J, et al. Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br J Ophthalmol. 2012;96(4):544–548. doi:10.1136/bjophthalmol-2011-300663
9. Pircher A, Montali M, Wostyn P, et al. Impaired cerebrospinal fluid dynamics along the entire optic nerve in normal-tension glaucoma. Acta Ophthalmol. 2018;96(5):e562–e569. doi:10.1111/aos.13647
10. Jonas JB, Wang N, Yang D. Translamina cribrosa pressure difference as potential element in the pathogenesis of glaucomatous optic neuropathy. Asia Pac J Ophthalmol. 2016;5:5–10. doi:10.1097/APO.0000000000000170
11. Bane WV. Cyst of dural sheath of optic nerve. Am J Ophthalmol. 1968;1:17–20. doi:10.1016/S0002-9394(18)91162-1
12. Garrity JA, Trautmann JC, Bartley GB, et al. Optic nerve sheath meningoceles. Clinical and radiographic features in 13 cases with a review of the literature. Ophthalmology. 1990;97(11):1519–1531. doi:10.1016/S0161-6420(90)32382-5
13. Link H, Olsson JE. Beta-trace protein concentration in CSF in neurological disorders. Acta Neurol Scand. 1972;48:57–68. doi:10.1111/j.1600-0404.1972.tb07527.x
14. Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310:173–186. doi:10.1016/S0009-8981(01)00573-3
15. Melegos DN, Freedman MS, Diamandis EP. Prostaglandin D synthase concentration in cerebrospinal fluid and serum of patients with neurological disorders. Prostaglandins. 1997;54(1):463–474. doi:10.1016/S0090-6980(97)00062-2
16. Beuckmann CT, Lazarus M, Gerashchenko D, et al. Cellular localization of lipocalin-type prostaglandin D synthase in the central nervous system of the adult rat. J Comp Neurol. 2000;428:62. doi:10.1002/1096-9861(20001204)428:1<62::AID-CNE6>3.0.CO;2-E
17. Kanekiyo T, Ban T, Aritake K, et al. Lipocalin-type prostaglandin D synthase/beta-trace is a major amyloid beta-chaperone in human cerebrospinal fluid. Proc Natl Acad Sci USA. 2007;104:6412–6417. doi:10.1073/pnas.0701585104
18. Kannaian B, Sharma B, Phillips M, et al. Abundant neuroprotective chaperone lipocalin-type prostaglandin D synthase (L-PGDS) disassembles the amyloid-β fibrils. Sci Rep. 2019;9(1):12579. doi:10.1038/s41598-019-48819-5
19. Tumani H, Nau R, Felgenhauer K. Beta-trace protein in cerebrospinal fluid: a blood-CSF barrier-related evaluation in neurological diseases. Ann Neurol. 1998;44:882–889. doi:10.1002/ana.410440606
20. Mase M, Yamada K, Shimazu N, et al. Lipocalin-type prostaglandin D synthase (beta-trace) in cerebrospinal fluid: a useful marker for the diagnosis of normal pressure hydrocephalus. Neurosci Res. 2003;47(4):455–459. doi:10.1016/j.neures.2003.08.009
21. Maesaka JK, Sodam B, Palaia T, et al. Prostaglandin D2 synthase: apoptotic factor in alzheimer plasma, inducer of reactive oxygen species, inflammatory cytokines and dialysis dementia. J Nephropathol. 2013;2:166–180. doi:10.12860/JNP.2013.28
22. Shah S. Accurate intraocular pressure measurement-the myth of modern ophthalmology? Ophthalmology. 2000;107:1805–1807. doi:10.1016/S0161-6420(00)00383-3
23. Killer HE, Jaggi GP, Flammer J, et al. The optic nerve: a new window into cerebrospinal fluid composition? Brain. 2006;129(4):1027–1030. doi:10.1093/brain/awl045
24. Jaggi GP, Mironov A, Huber AR, Killer HE. Optic nerve compartment syndrome in a patient with optic nerve sheath meningioma. Eur J Ophthalmol. 2007;7(3):454. doi:10.1177/112067210701700334
25. Xin X, Fan B, Flammer J, et al. Meningothelial cells react to elevated pressure and oxidative stress. PLoS One. 2011;6(5):e20142. doi:10.1371/journal.pone.0020142
26. Pircher A, Montali M, Berberat J, et al. The optic canal: a bottleneck for cerebrospinal fluid dynamics in normal-tension glaucoma? Front Neurol. 2017;8:47. doi:10.3389/fneur.2017.00047
27. Murakami Y, Takahashi K, Hoshi K, et al. Spontaneous intracranial hypotension is diagnosed by a combination of lipocalin-type prostaglandin D synthase and brain-type transferrin in cerebrospinal fluid. Biochim Biophys Acta. 2018;1862(8):1835–1842. doi:10.1016/j.bbagen.2018.03.027
28. Bauer G, Killer HE, Forrer A, et al. Lipocalinlike Prostaglandin D synthase (L-PGDS) concentration in aqueous humor in patients with open-angle glaucoma. J Glaucoma. 2014;23:164–168. doi:10.1097/IJG.0b013e31826a7dea
29. Taniike M, Mohri I, Eguchi N, et al. Perineuronal oligodendrocytes protect against neuronal apoptosis through the production of lipocalin-type prostaglandin D synthase in a genetic demyelinating model. J Neurosci. 2002;22(12):4885–4896. doi:10.1523/JNEUROSCI.22-12-04885.2002
30. Liang X, Wu L, Hand T, et al. ProstaglandinD2mediatesneuronal protection via the DP1 receptor. J Neurochem. 2005;92:477–486. doi:10.1111/j.1471-4159.2004.02870.x
31. Suk K. Unexpected role of lipocalin-type prostaglandin D synthase in brain: regulation of glial cell migration and morphology. Cell Adh Migr. 2012;6:160–163. doi:10.4161/cam.20251
32. Petereit HF, Bachmann G, Nekic MA, et al. A new nephelometric assay for b-trace protein (prostaglandin D synthase) as an indicator of liquorrhea. J Neurol Neurosurg Psychiatry. 2001;71:347–351. doi:10.1136/jnnp.71.3.347
33. Lai SW, Lin CL, Liao KF. Glaucoma may be a non-memory manifestation of alzheimer’s disease in older people. Int Psychogeriatr. 2017;1–7. doi:10.1017/S1041610217000801
34. Wostyn P, De Groot V, Van Dam D, et al. Glaucoma considered as an imbalance between production and clearance of neurotoxins. Invest Ophthalmol Vis Sci. 2014;55(8):5351–5352. doi:10.1167/iovs.14-15041
35. Burtis CA, Ashwood MD. Tietz Textbook of Clinical Chemistry.
36. Roos K. Principles of Neurologic Infectious Diseases. New York: McGraw-Hill; 2005.
37. Jaggi GP, Flammer J, Huber AR, Killer HE. Lipocalin-like prostaglandin D synthase in subretinal fluid of detached retinas in humans. Retina. 2008;28(6):858–863. doi:10.1097/IAE.0b013e3181631975
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.