Back to Journals » International Journal of Nanomedicine » Volume 18
Lipid Nanoparticles-Based Therapy in Liver Metastasis Management: From Tumor Cell-Directed Strategy to Liver Microenvironment-Directed Strategy
Authors Wang Y , Yin Z, Gao L, Ma B, Shi J, Chen H
Received 14 January 2023
Accepted for publication 15 May 2023
Published 2 June 2023 Volume 2023:18 Pages 2939—2954
DOI https://doi.org/10.2147/IJN.S402821
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Yuhan Wang,1 Zhenyu Yin,1 Lei Gao,1 Bin Ma,1 Jianming Shi,1 Hao Chen2
1Lanzhou University Second Hospital, Lanzhou, 730030, People’s Republic of China; 2Department of Surgical Oncology, Key Laboratory of the Digestive System Tumors of Gansu Province, Lanzhou University Second Hospital, Lanzhou, 730030, Gansu Province, People’s Republic of China
Correspondence: Hao Chen, Tel +8615009467790, Fax +8609315109551, Email [email protected]
Abstract: Metastasis to the liver, as one of the most frequent metastatic patterns, was associated with poor prognosis. Major drawbacks of conventional therapies in liver metastasis were the lack of metastatic-targeting ability, predominant systemic toxicities and incapability of tumor microenvironment modulations. Lipid nanoparticles-based strategies like galactosylated, lyso-thermosensitive or active-targeting chemotherapeutics liposomes have been explored in liver metastasis management. This review aimed to summarize the state-of-art lipid nanoparticles-based therapies in liver metastasis management. Clinical and translational studies on the lipid nanoparticles in treating liver metastasis were searched up to April, 2023 from online databases. This review focused not only on the updates in drug-encapsulated lipid nanoparticles directly targeting metastatic cancer cells in treating liver metastasis, but more importantly on research frontiers in drug-loading lipid nanoparticles targeting nonparenchymal liver tumor microenvironment components in treating liver metastasis, which showed promise for future clinical oncological practice.
Keywords: liver neoplasms, neoplasm metastasis, liposomes, tumor microenvironment
Key Points
- Liver metastasis, which is highly prevalent in advanced malignancies, was associated with poor prognosis.
- Contemporary therapies in liver metastasis were associated with the lack of metastatic-targeting ability, significant systemic toxicities and incapability of tumor microenvironment (TME) modulations.
- Lipid nanoparticles (LNP)-based strategies would overcome the deficiencies of conventional approaches in liver metastasis management.
- The switch from directly targeting metastatic tumor cells to nonparenchymal cells in liver TME has been explored using LNP-based strategies in treating liver metastasis.
- Current challenges included the optimization of LNP properties and identifications of liver-specific ligands.
Overview of Liver Metastasis and Its Current Treatment Landscapes
Metastasis, which is the process of tumor cells evading their primary sites and spreading to other distant sites, was reportedly to cause over 90% of cancer-related death.1 Metastasis to the liver was one of the most common metastatic patterns which would be frequently seen in all cancer types,2 such as colorectal cancer,3 melanoma and neuroendocrine tumors.4,5 Also, liver metastasis was a negative factor for the treatment response and survival in various cancer types.3,6–13 As much of tumor-related morbidity and mortality resulted from liver metastasis progression, it would be critical to generate effective treatment modalities for liver metastasis. However, at present only limited treatment approaches were used in patients with liver metastasis. Although surgery has long been regarded as the only curative modality to treat resectable hepatic metastasis, the eligibility, efficacy and safety of surgical procedures in patients with liver metastasis remained unsatisfactory.14–17 For systemic approaches, many patients were ineligible for chemotherapy as their intolerance for chemotherapy-caused severe adverse effects or being unresponsive to systemic chemotherapy.18 Strategies combining systemic therapy with locoregional therapy were thought to be effective in many cancers with distant metastasis.19 However, its efficacy in liver metastasis was far from satisfactory.20 Large randomized clinical trials have revealed that peri-operative chemotherapy or targeted therapeutics would not improve overall survival otherwise increasing drug-related side effects in patients with resectable colorectal liver metastasis.16,20,21 Accordingly, conventional therapeutics were associated with considerable systemic toxicities, treatment resistance to single-target therapies and limited local drug concentrations in liver metastasis treatment. Novel chemotherapeutic formulations were urgently needed to overcome the deficiencies of conventional therapeutics. Recently, with the development of novel liposomal drug delivery systems, the efficacy, tolerability and targeting ability of contemporary systemic therapy modalities in cancer treatment have been largely improved.22 However, the role of LNP-based therapeutic strategies in liver metastasis remained equivocal. As the liver tumor microenvironment (TME) has been regarded as a significant obstacle for pronounced treatment response,23 the liver-TME-modulating strategy would further synergize the anti-tumor effect of conventional therapeutics in liver metastasis treatment.
Overview of Liposomal Delivery Systems
Liposomal nanoparticles (LNPs) were unilamellar or multilamellar lipid bilayer vesicles encapsulating drugs.24 Liposomal delivery systems were composed of LNPs which embedded chemotherapy or gene therapy agents. During the COVID-19 pandemic, the applications of various LNP-based, mRNA-loading SARS-CoV-2 vaccines such as the BNT162b2 have demonstrated tremendous efficacy in preventing the infection of coronavirus.25 In vivo studies have also demonstrated that lipid nanoparticles would improve tumor-targeting ability while decreasing the accumulation of drugs in the non-tumor area, thus reducing drug-induced systemic adverse events. The high density and permeability of tumor vessels enabled drug-encapsulated lipid nanoparticles to accumulate in neoplasm lesions, which led to high drug concentrations and selective anti-cancer activity.22,26 The first FDA-approved liposomal chemotherapy nanoparticle, namely Doxil, has achieved influential anti-tumor effects in clinical practice.27 Doxil, which was made of polyethylene glycol-modified doxorubicin-encapsulated liposomes, has been observed to specifically accumulate in breast cancer lesions.28 Compared with conventional intravenous doxorubicin formulations, Doxil showed better pharmacokinetics as a lower half-life, 250-fold reduced clearance rate and nearly 300-fold greater Area Under Curve (AUC) after a dose of 50 mg/m2.29 Correspondingly, doxorubicin-induced cardiotoxicity has been drastically reduced in patients treated with Doxil.29 A Phase I clinical study30 demonstrated the benefit of Doxil + vinorelbine in four breast cancer patients with liver metastasis. Besides drug-loading liposomes, recent advances have been achieved in LNP-based gene therapy. Liposome-coated small interfering RNAs (siRNA) would effectively silence the expression of the target gene ex vivo. Tabernero et al31 reported results from a phase I clinical trial which evaluated the anti-tumor efficacy and safety profile of ALN-VSP, an LNP formulation of siRNAs targeting VEGF and kinesin spindle protein in treating the liver metastasis of various cancer types. Results from both pre-clinical and clinical experiments showed that the ALN-VSP was well-tolerated and exhibited complete response in an endometrial cancer patient with multiple liver metastasis. However, the lack of liver-targeting limited its application in contemporary liver metastasis management. Recently, Onpattro, a clinically approved non-viral LNP-based siRNA delivery system with liver-targeting ability, has been successfully used intravenously to improve neuropathy in patients with hereditary transthyretin amyloidosis.32 As low-density lipoprotein receptor (LDL-r) was heavily expressed on the surface of hepatocytes, researchers coated Onpattro with LDL-r ligand apolipoprotein E (Apo-E) as its surface marker. The binding of Apo-E and LDL-r enabled the endocytosis of siRNA-loading lipid particles, which led to efficient target gene knockdown.33 Once delivered intracellularly, Onpattro would bind the 3’ UTR of transthyretin mRNA and further silence its expression, which led to the reduction of pathological transthyretin protein deposit in hepatocytes.33 Although not applicable in malignancies, Onpattro has opened the door to future explorations of LNP-based, liver-targeting gene therapies in liver metastasis management. In that, LNP-based delivery systems appeared to be theoretically selective and well-tolerated formulations that would enhance the efficacy while reducing the systemic toxicities of encapsulated agents. This review highlighted the advantages of LNP-based strategies in liver metastasis management such as liver-targeting ability, systemic side effects reductions and modulatory effect to liver TME based on evidences from both preclinical and clinical studies, which would be promising in future real-world clinical oncology practice.
LNP-Based Strategy in Liver Metastasis Treatment
Systemic LNP-Based Therapy in Liver Metastasis Treatment
Systemic Chemotherapy
Systemic chemotherapy has long been considered the first-line treatment approach for patients with liver metastasis,34,35 however the systemic toxicity and limited efficacy were major obstacles to achieving optimal survival benefits. Irinotecan, which acted by causing DNA damage to kill tumor cells, has been widely used as cancer chemotherapeutics.36 Liposomal irinotecan would increase both the circulation time and the intratumoral level of irinotecan and its active metabolite (SN-38). Pre-clinical HT-29 xenograft animal models showed that liposomal irinotecan with a 5-fold lower dose than conventional irinotecan would achieve the same anti-colon cancer effect.36 A Phase III open-label randomized trial NAPOLI-1 evaluated the survival benefits of liposomal irinotecan monotherapy or combing fluorouracil and folinic acid in gemcitabine-refractory metastatic pancreatic cancer.37 Median overall survival in patients using liposomal irinotecan plus fluorouracil and folinic acid was 6.1 months [95% Confidence Interval(CI): 4.8–8.9], which was much longer than the survival (3.2 months) in patients with unencapsulated irinotecan-based regimens.38 Based on these findings, the NCCN guideline for pancreatic cancer has recommended using liposomal irinotecan for metastatic disease including liver metastasis instead of unencapsulated formulations.39
Oxaliplatin was a third-generation platinum chemotherapy drug, with an enhanced anti-tumor activity compared to cisplatin.40 Oxaliplatin-based chemotherapy like CAPEOX (oxaliplatin + capecitabine) regimen has been recommended by NCCN guidelines as standard therapy for patients with unresectable colorectal cancer liver metastasis (CRLM).35 However, oxaliplatin-induced neuropathy was hardly tolerable, which was dose-limited involving over 80% of patients.41 To improve the oxaliplatin-induced off-target systemic adverse effect, Gogineni et al42 developed a heat-sensitive-Fe3O4-based liposomal oxaliplatin formulation (L-NIR-Fe3O4/OX). Under magnetic field stimulation, the release of oxaliplatin from L-NIR-Fe3O4/OX was improved (18%) and the biodistribution experiment in animal models showed increased accumulation of this liposomal formulation in the liver compared to lung and gut (p<0.001). Predominant necrosis was observed in CRLM lesions of rat models. Survival analysis demonstrated that L-NIR-Fe3O4/OX injected through mesenteric veins under magnetic field stimulation was associated with extra survival benefits. These results above revealed the superiority of liposomal chemotherapeutics especially the magnitude-triggered liposomes in liver metastasis.
Combined chemotherapy has been accepted as a clinically effective strategy in the management of liver metastatic cancer.43 Researchers have explored the LNP-based co-delivery of chemotherapeutics with different physicochemical properties. Lin et al44 developed a novel Pluronic® P123-coated liposomal drug carrier (ITZ/DOX-Plip) which would deliver both hydrophilic doxorubicin and hydrophobic itraconazole simultaneously. Pharmacological experiments showed ITZ/DOX-Plip exhibited satisfactory loading efficacy and prolonged circulation time. Cell viability experiments revealed ITZ/DOX-Plip was more cytotoxic compared with free doxorubicin and liposomal doxorubicin formulations. In vivo study demonstrated ITZ/DOX-Plip would significantly suppress the growth of liver metastasis in tumor-bearing mice. Regarding the doxorubicin-related cardiotoxicity, in vivo biodistribution assay found a decreased accumulation of ITZ/DOX-Plip in mice’s heart tissue compared with free or liposomal doxorubicin (p < 0.01). In light of the findings above, it would be promising to conduct a liposomal co-delivery chemotherapy system in the treatment of liver metastasis. However, no clinical validations existed.45
Gene Therapeutics
RNA interference (RNAi) liposomes have been widely used in preclinical studies to selectively silence the expression of oncogenes. However, one major limitation was the entrapment of siRNA liposomes in the lung caused by the non-specific agglutination between positively charged lipoplex and negatively charged erythrocytes.46 Hattori et al47 developed an approach of sequential injection of anionic polymer and cationic lipoplex into mice with liver metastasis. This approach was associated with a significantly decreased accumulation of lipoplex in the lung and increased delivery to liver lesions. Additionally, the accumulation of siRNA liposomes in the liver was found to successfully silence the expression of target gene. The results above indicated the potential therapeutic value of liposome-based siRNA gene therapy in the management of liver metastasis.
The clustered regularly interspaced short palindromic repeat/CRISPR-associated protein 9 (CRISPR/Cas9) has been considered a more effective genomic editing technique compared to the RNAi. Wang et al48 fabricated a novel liposomal vector PS@Lip/pCas9 which delivers CRISPR/Cas9 plasmid targeting mutT homolog 1 (MTH) gene to inhibit the growth of tumor cells. Coated with protamine sulfate (PS) containing nuclei-directing sequence, PS@Lip/pCas9 would effectively deliver CRISPR-Cas9 plasmid into the nuclei of cancer cells and induce MTH gene deletion. Furthermore, the liposomal coating would protect the plasmid from circulatory nuclease degradation. In vivo assay revealed that PS@Lip/pCas9 inhibited the progression of liver metastasis in non-small cell lung cancer mice models by disrupting MTH-induced pro-metastatic effect. However, liposomal formulations did not resolve the inevitable off-target effect of CRISPR/Cas9 gene editing, which would limit its potential application in clinical settings.
The lack of site-specific targeting in liposomal gene therapy would cause severe side effects by non-specific gene silencing in normal cells. Thus, liposomal vectors with specific targets were urgently needed. The transferrin receptor (TR) was reported to be highly over-expressed in pancreatic cancer cells relative to normal pancreatic cells.49 This differential expression pattern made TR a possible target for liposomal drug carriers. As the cell-senescence-modulating strategy like Tp53-targeting agents has demonstrated suppressive effect of the cell malignant behavior in pre-clinical studies of gastrointestinal malignancies,50,51 the incorporation of senescence-targeted-Tp53-modulations and TR-targeted LNP would further enhance its anti-tumor efficacy. Camp et al52 constructed a pancreatic-targeted liposome coated with a single-chain antibody fragment to the transferrin receptor (TfRscFv). The “cargo” of this liposome was wild-type human p53 for its senescence-restoring effect on p53-mutated tumor cells and the synergic effect on concomitant chemotherapy.53 TfRscFv-liposomes were found to increasingly accumulate in pancreatic cancer lesions compared to non-specific liposomes. Survival analysis showed an increased median survival observed in the liposomes plus gemcitabine group compared with the liposomes or the gemcitabine monotherapy group (37 days versus 29 days versus 30 days). These experiments suggested a successful strategy to specifically deliver gene therapeutics to tumor cells in liver metastasis.
Drug-Free Liposomes
Interestingly, drug-free liposomes would also exhibit anti-tumor effects in liver metastasis. Ichihara et al54 found that drug-free HL-25 liposomes composed of L-a-dimyristoyl-phosphatidylcholine and polyoxyethylene dodecyl would also have therapeutic effects in the CRLM mice model. The therapeutic effect of this drug-free liposome was due to its pro-apoptotic effect on tumor cells. These findings revealed that the liposome itself may have some tumor-suppressing effects to synergize encapsulated chemotherapy drugs.
Locoregional Use of LNP-Based Therapy in Liver Metastasis
Transarterial Chemoembolization
Transarterial chemoembolization (TACE) has achieved promising survival benefits in patients with liver metastasis and has been included in current practice guidelines.34 To date, there was no consensus on the recommended chemotherapy regimen for TACE in patients with liver metastasis. A retrospective study compared the efficacy and safety of TACE with raltitrexed plus liposomal doxorubicin (R+PGLD) with tegafur plus pirarubicin (T+P).55 Compared with T+P, patients receiving TACE with R+PGLD had significantly better efficacy (Objective Response Rate: 64.9% vs 45.9%, p=0.031; Disease Control Rate: 89.2% vs 74.3%, p=0.032; respectively) and fewer severe adverse events like myelosuppression and cardiotoxicity (p=0.011 and p=0.037, respectively). This finding revealed the feasibility of using liposomal chemotherapeutics with TACE procedure in treating liver metastasis. Recently progress in novel nano-flexible liposomes has made TACE more targeted and efficient. Li et al56 reported a novel liposomal drug carrier which was made by Bletilla striata polysaccharide (BSP) polymer. The BSP polymer would incorporate into a dimensional porous network microsphere, which can inhibit the neovascularization in post-embolized ischemic regions. Additionally, the binary progressive structure of this novel liposomal carrier enhanced its site-specific targeting ability to liver tissue. The abovementioned characteristics of this novel LNP would make it a promising chemotherapeutics delivery system in TACE settings.
Hepatic Arterial Infusion Chemotherapy
The efficacy of hepatic arterial infusion chemotherapy (HAIC), which has been recommended by current guidelines together with TACE for liver metastasis, would also be improved using liposomal drug delivery systems.34,35 5-fluorouracil (5-FU) has been widely accepted as the cornerstone chemotherapeutics for the treatment of gastrointestinal-originated liver metastasis.35 Pohlen et al57 compared the efficacy of PEG (polyethylene glycol)-coated 5-FU liposomes with or without degradable starch microspheres (DSM) in CRLM mice models and found that hepatic arterial infusion with PEG 5-FU plus DSM was associated with the most survival benefit compared with other groups. Another in vivo study showed when intraarterial-infused, the intratumoral concentration of PEG-coated liposomal 5-FU was 110 times higher than conventional 5-FU, which demonstrated the superior pharmacokinetics compared to conventional formulations in HAIC settings.58 Although LNP-based HAIC exhibited an enhanced anti-tumor effect, the lack of internal liver-targeting ability would limit its application in liver metastasis. To improve liver-targeting ability, Zhao et al59 fabricated a galactose-modified doxorubicin-loading liposome. The binding of galactose to the asialoglycoprotein receptor on human hepatocytes enhanced the liver-targeting ability of galactose-modified liposomes.60 In vitro experiments showed increased uptake of liposomes in human hepatocellular carcinoma cell line HepG2 compared with non-modified liposomes. Further in vivo biodistribution assay confirmed the enhanced aggregation of galactose-modified liposomes in the liver. Considering the anti-tumor ability, locoregional infusion of galactosylated doxorubicin liposomes demonstrated superior efficacy both in liver metastasis and lymph node metastasis compared with conventional formulations. The safety profile of galactosylated doxorubicin liposomes was acceptable, with similar hepatotoxicity compared with conventional formulations. Although lack of clinical validations, these pre-clinical findings suggested a possible approach to increase the liver-targeting ability of drug-loading liposomes in the locoregional management of liver metastasis.
Radionuclide Therapeutics or Radiofrequency Ablation Plus Liposomal Chemotherapeutics
Radionuclide therapeutics have been integrated into the landscape of liver malignancy management.34 Liposomal radionuclides would improve the tumor uptake of radionuclide particles while also reducing the radiation-induced severe adverse effects. Chang et al45 evaluated the efficacy of 188Re-liposomes plus sorafenib compared with sorafenib monotherapy in CRLM mouse models. Survival analysis showed 188Re-liposomes plus sorafenib was associated with a superior survival rate compared with sorafenib monotherapy or negative controlled groups (p=0.0000).
Radiofrequency ablation (RFA) has been recommended by current guidelines for patients with liver metastasis ineligible for surgical intervention.34 However, long-term survival data showed a high local recurrence rate post-ablation.61,62 Therefore, combining radiofrequency ablation with systemic cytotoxic therapy like liposomal doxorubicin was considered a possible strategy to improve outcomes in patients with liver metastasis. It has been observed that RFA followed by liposomal doxorubicin would increase the tumor destruction volume by 25% to 30% compared to RFA alone.63 This synergic effect was due to ablation-induced vascular changes, which led to increased intra-tumoral liposomal accumulation.64 Lyso-thermosensitive liposomal doxorubicin (LTLD) was one of novel liposomal formulations made of doxorubicin and its thermosensitive lipid capsules. Since ablation procedures would produce extra heat in target regions, subsequent thermosensitive doxorubicin liposomes would release more drugs in such specific microenvironments. It was reported that when the tumor region is heated up to 40 °C, the release of liposomal doxorubicin in tumor regions was 25-fold higher than the release of conventional doxorubicin formulations.65 Also, the enhanced permeability and retention effect, which was caused by high vascular density and permeability of tumor stroma, was more predominant in post-ablation regions. Pharmacokinetic modeling revealed that using temperature-sensitive doxorubicin liposomes in post-ablation settings would improve local drug delivery compared with conventional doxorubicin.66 One double-blinded dummy-controlled clinical trial assessed the efficacy of RFA with or without post-ablation lyso-thermosensitive liposomal doxorubicin in hepatocellular carcinoma.67 Subgroup analysis revealed patients with a RFA dwell time for a solitary lesion ≥45 minutes would significantly benefit from the combination of RFA+LTLD (Overall survival: 95% CI: 0.45–0.94; p < 0.05). The abovementioned evidence demonstrated the superiority of LTLD over conventional doxorubicin in the locoregional therapy of liver malignancies. To date, only ThermoDox has been approved by FDA for the treatment of liver metastasis.68 Hopefully, the efficacy of locoregional therapy in liver metastasis would be greatly improved with the development of next-generation thermosensitive chemotherapeutic or immunotherapeutic liposomes.
Photodynamic Therapy
Photodynamic therapy (PDT) was considered a potential treatment option for liver metastasis.69 Being activated by light of a certain wavelength, the pre-injected photosensitive drugs were released in irradiated regions, which would generate reactive oxygen species (ROS) to destruct tumor cells locally.70 The anti-tumor activity of the PDT-induced ROS release process relied on the oxygen concentration in tumor regions.69 However, the hypoxia microenvironment in the liver would limit the efficacy of PDT.23 PDT with IR780@O2-SFNs/iRGD, a novel oxygen-self-sufficient liposomal photosensitizer complex, was found to effectively inhibit the progression of liver metastasis in vivo.71 IR780@O2-SFNs/iRGD was composed of four parts, which are IR780, O2, SFNs, and iRGD. IR780 was a hydrophobic cyanine dye, which acted as a photosensitizer to release ROS under photodynamic irradiations. SFNs were made of pH-sensitive fluorocarbon-functionalized nanoparticles which decomposed in acid microenvironments. iRGD, which could bind to αvβ3 integrin in tumor cell surfaces, helped tumor penetrating of this nano-drug delivery system. When injected intravenously, this complex would use its iRGD and SFNs to selectively target and penetrate tumor regions. When exposed to hypoxic or acidic intratumoral microenvironments, the nano-complex would disassemble and release the oxygen molecules together with IR780 loaded in the core of SFNs. O2 supplementation would relieve tumor hypoxia and facilitate the generation of tumor-toxic ROS induced by IR780, which further improved the efficacy of PDT. In vivo study showed single IR780@O2-SFNs/iRGD-mediated PDT would effectively inhibit the progression of liver metastasis in orthotopic breast cancer of nude mice. Another hypoxia-alleviating LNP-based photosensitizer generated by Liang et al72 also demonstrated decent anti-tumor efficacy in PDT of liver metastasis. The unique arranging mode of porphyrin with perfluorocarbon(PFOB) in this research would increase the O2 loading content in nanoparticles, improve the generation of singlet oxygen and avoid premature circulatory loss of photosensitizer, which led to elevated PDT efficacy in hypoxic tumors as liver metastasis. In HT-29 colon cancer liver metastasis mouse models, O2@PFOB@PGL LNPs-mediated PDT was associated with an appealing anti-tumor effect evidenced by fewer metastatic loci compared with conventional photosensitizer-mediated PDT. Coating modification would also be a possible strategy to improve the targeting as well as immunotolerance of LNP-based photosensitizer. Pan et al73 developed an engineered red blood cell membrane (RBCm)-coating salidroside/indocyanine green nanovesicle (ARISP) as the photosensitizer agent in PDT. ARISP was composed of the following three parts: salidroside, RBCm-coating, and indocyanine as the photosensitizer. Salidroside accounted for down-regulating the expression of hypoxia-inducible factor 1α to attenuate the hypoxia tumor microenvironment. Anti-LDL-r modified RBCm-coatings would not only prolong the circulatory time and help escape the host immune surveillance but also increase the targeting of the whole nano-complex by selectively binding to LDL-r overexpressed hypoxic tumor cells. In vivo study revealed that ARISP plus laser irradiations would significantly suppress the growth of liver metastasis and improve survival in triple-negative breast cancer-bearing mice. Although pre-clinical studies have demonstrated the effectiveness of PDT with LNP-based photosensitizer in liver metastasis, further large randomized clinical trials and real-world studies are needed to validate its performance clinically.
As summarized in Figure 1 and Table 1, LNP-based tumor cell-targeting strategies have exhibited promising efficacy in systemic or locoregional treatment of liver metastasis. The advantages of such strategies were featured by the reduction of systemic toxicities and the synergetic effect with local therapy modalities like TACE, HAIC or PDT. However, the lack of internal liver-targeting ability and the incapability to overcome the drug resistance would be the major obstacles in real-world clinical applications. Moreover, most evidence existed at the pre-clinical level, with a paucity of clinical validations. Registered clinical trials evaluating LNP-based strategies in liver metastasis were listed in Table 2.
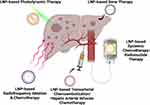 |
Figure 1 Schematic overview of current LNP-based tumor cell-targeting strategies in liver metastasis management (Created with BioRender.com). Abbreviation: LNP, lipid nanoparticles. |
 |
Table 1 Current LNP-Based Strategies in Locoregional Treatment of Liver Metastasis |
 |
Table 2 Registered Clinical Trials Evaluating Liposomal Formulations in Liver Metastasis |
Liver Tumor Microenvironment Targeted LNP Strategies
As illustrated in Figure 2, the TME in the liver has its unique components like liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), and hepatic satellite cells (HSCs),23 which played an important role in the progression of liver metastasis.74 Nonparenchymal components of liver sinusoids, like LSECs and HSCs, would assumptively interact with metastatic tumor cells. Therefore, it would be a feasible approach to target nonparenchymal cells in the liver TME for the treatment of liver metastasis. Systemic drug delivery to hepatocytes has successfully led to the approval of Onpattro by the FDA. However, the delivery to other functional nonparenchymal cells in the liver microenvironment remained challenging. Surprisingly, Paunovska et al75 found that LNPs would be delivered more potently in stromal cells of the liver microenvironment compared to hepatocytes. These findings suggested the feasibility of targeting the liver microenvironment using drug-loading liposomal nanoparticles. One of the major deficiencies of the liposomal drug was that it would be easily taken by the reticuloendothelial system, which diminished its circulating time and anti-tumor effect. However, as reticuloendothelial systems were important components of liver TME,76 the increased uptake of TME-targeting LNPs would increase the concentrations of liposomal therapeutics in hepatic nonparenchymal cells, which led to better therapeutic effects.
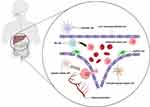 |
Figure 2 Nonparenchymal components of the tumor microenvironment in liver metastasis (Created with BioRender.com). |
Liver Sinusoidal Endothelial Cells
Recent research has revealed the crucial role of LSECs in the progression of liver metastasis.23 As specialized scavenger cells, LSECs were capable of eliminating endogenous macromolecular waste as well as blood-borne pathogens. LSECs also helped modulate the anti-tumor immune response in liver.74 By upregulating the expression of several adhesion molecules like LSECtin, ICAM-1, E-selectin and activating corresponding pro-metastatic signal pathways, LSECs would help circulating tumor cells migrate through the liver sinusoid and further invade the parenchyma.74,77–79 Collectively, LSECs would be a potential target in treating intra-hepatic metastases. To construct LSEC-targeted RNAi LNPs, Pattipeiluhu et al80 generated modified Onpattro-like LNPs (srLNPs) which replaced the zwitterionic helper phospholipids in Onpattro with anionic phospholipids. The binding of anionic helper phospholipid on srLNPs to the scavenger receptor stabilin1/2 (stab1/2) on LSECs improved the LSECs-targeting ability. Furthermore, the anionic phospholipid would inhibit the binding of Apo-E on Onpattro to LDL-r on hepatocytes, which redirected the targeting from hepatocytes to LSECs. Cryo-electron microscope showed no major difference in size and ultrastructure between Onpattro and srLNPs. In vivo biodistribution assay revealed an increased uptake of srLNPs in LSECs compared with Onpattro analog. In stab1-/-/stab2-/- mutant zebrafish embryos, srLNPs were found to be free-circulatory rather than taken up by LSECs. Furthermore, srLNP-mediated mRNA expression was not observed in LSECs. In older zebrafish embryos with the presence of hepatocytes, srLNP-mediated mRNA expression was still restricted to LSECs. When injected in mice, srLNPs showed increased LSECs tropism with 5-fold targeting enhancement in LSECs compared to hepatocytes. In stab-/- mice, the accumulation of srLNPs was reduced by 80% compared to the wild type. Besides LNP-based RNAi strategies, Campbell et al81 constructed clodronic acid-encapsulated LNPs to target and destruct LSECs in a stabilin-dependent way. Additionally, the physiochemical property of LNPs, especially the surface charge was found to be involved in the LSECs targeting process. Campbell et al81 found that cationic LNPs could not be taken up by LSECs in stab-/- zebra fish embryos while LSECs-uptake of positive charged LNPs as EndoTAG-1 and neutral charged LNPs as Myocet was not affected. These abovementioned findings demonstrated the effective LNP-based delivery of novel RNAi or chemotherapeutics to LSECs in both zebrafish and mice models and would support the further clinical exploration of such strategy in treating liver metastasis.
Kupffer Cells
KCs, which are the specialized macrophages in liver TME and sinusoid, were related to the growth and progression of liver metastasis.74 Despite its pro-metastatic effect, KCs would eliminate the circulatory liposomes, which were thought to be responsible for liposomal chemotherapy resistance.82 In that, the depletion of KCs in liver microenvironment would be a possible strategy to inhibit the progression of liver metastasis and improve treatment resistance. Clodronate has been found to kill the host macrophages upon phagocytosis.83 Kruse et al84 and Shimizu et al85 have successfully used liposomal clodronate to deplete hepatic KCs, which led to significant repression of liver metastasis growth in colon cancer mouse models. In pancreatic neuroendocrine tumors, Krug et al86 used liposomal clodronate to treat tumor-bearing RIP1Tag2 mice and found diminished infiltration of F4/80-positive tumor-associated macrophages (TAMs) and density of microvessels, which indicated successful depletion of TAMs in liver metastasis lesions. In vivo study demonstrated that the use of liposomal clodronate would significantly reduce the cumulative tumor burden compared to the controlled group (6.9 × 106 µm2 versus 2.6×106 µm2; p = 0.036). Since clodronate could also be ingested by circulatory and non-hepatic macrophages, this clodronate-induced macrophage depletion would cause potentially systemic side effects.83 Thus, novel liposomal formulations with specific liver-targeting were urgently needed for KCs targeting strategy in liver metastasis treatment. Another possible Kupffer cell-targeting strategy was the use of liposomal oxaliplatin. Ukawa et al82 found that the injection of liposomal oxaliplatin would decrease the number of KCs without injury to normal hepatocytes. Regarding liposomal RNAi therapy, Pattipeiluhu et al80 found that RNAi-containing anionic LNPs would specifically target KCs evidenced by threefold targeting enhancement in murine KCs compared to murine hepatocytes. In summary, the deficiencies in current LNP-based-Kupffer-cell-targeting strategies like the off-target toxicity and nonspecific targeting ability warranted the discovery of novel targeting ligands on KCs.
Hepatic Satellite Cells
As key stromal components of liver TME, HSCs were also involved in the progression of liver metastasis. Accounting for around 15% of nonparenchymal cells in the liver, HSCs located within the lumen of Disse would switch to myofibroblast-like phenotype and excrete various cytokines and extracellular matrix (ECM) when activated, thus would be an important modulator in liver metastasis pathogenesis.23 It has demonstrated that activated HSCs would construct a pro-metastatic microenvironment in liver by modulating the ECM or inducing immunotolerance to exotic tumor cells.87,88 In CRLM and intrahepatic metastasis of hepatocellular carcinoma, HSCs would promote the growth of liver metastasis by the action of the SDF-1/CXCR4 axis and PI3K-AKT-ERK pathway, respectively.89,90 Therefore, HSC was a pivotal modulator in the pathogenesis of liver metastasis. Using LNPs targeting HSCs has long been investigated in the management of liver fibrosis and cirrhosis,91 while the explorations of HSCs-targeting LNPs in liver metastasis were flourishing. Li et al92 constructed a novel LNP named CAP/GA-sHA-DOX to target HSCs in treating liver malignancy. This LNP was designed as follows: (1) capsaicin (CAP) would inhibit the proliferation of HSCs by blocking the substance-P-induced HSCs activation. (2) GA (glycyrrhetinic acid) was designed to bind the highly-expressed GA receptor on the surface of tumor cells. (3) HA (hyaluronic acid) was designed to specifically bind to the over-expressed CD44 on HSCs, which would improve the endocytic uptake of LNPs in HSCs. (4) doxorubicin (DOX) was the cytotoxic chemotherapeutics that would kill the tumor cells in the liver. Accordingly, this LNP aimed to disrupt the crosstalk between HSCs and tumor cells while also destroying tumor cells using co-loaded cytotoxic agents. In vitro studies showed CAP/GA-sHA-DOX LNPs were stable under physiologic PH conditions and would effectively release CAP and DOX under the acidic PH environment comparable to lysosomes. The use of CAP/GA-sHA-DOX in the HSCs-tumor cells co-culture system exhibited enhanced cytotoxicity compared to doxorubicin monotherapy, which revealed the synergic effect of CAP plus DOX in destroying cancer cells. Furthermore, the use of CAP/GA-sHA-DOX alleviated the drug resistance and tumor migration induced by the HSC-tumor cell crosstalk. As designated, CAP/GA-sHA-DOX was taken up by both HSCs and tumor cells simultaneously. In vivo biodistribution assay showed increased intra-tumor CAP/GA-sHA-DOX accumulation observed in tumor-bearing mice, which confirmed the superior active targeting ability of this novel LNP. In accordance with in-vitro results, CAP/GA-sHA-DOX exhibited superior anti-tumor efficacy by inhibiting tumor growth and blocking the activation of HSCs. Immunochemistry staining showed CAP/GA-sHA-DOX-treated tumor tissue had less angiogenesis and reversed epithelial to mesenchymal transition phenotype compared to tumor tissue treated with single liposomal chemotherapeutics. Moreover, CAP/GA-sHA-DOX would effectively inhibit the metastasis of tumor cells in orthotopic mice models. Besides CAP/GA-sHA-DOX, some other active-targeting LNPs have also been fabricated to inhibit the crosstalk between HSCs and tumor cells. One dual-targeting strategy generated by Li et al93 and Qi et al94 used aprepitant to block the activation of HSCs while using co-delivered curcumin to induce tumor cell apoptosis. Both in vitro and in vivo experiments demonstrated that these dual-targeting LNPs would successfully inhibit the growth of tumor cells and reverse the chemotherapy resistance in the liver by blocking the HSC-tumor cell interaction. Another LNP-targeting strategy from Guo et al95 used oxymatrine to inhibit the activation of HSCs while using cysteine-end FH peptide (CFH) as the HSCs-targeting component. This novel LNP incorporating oxymatrine with CFH exhibited enhanced intra-tumor drug delivery as well as TME modulating ability both in vivo and in vitro. Collectively, using liposomal HSCs-targeting strategy successfully inhibited the progression of liver malignancy both in vitro and in vivo therefore would be a promising modality in future liver metastasis management.
Immune Cells
Immune cells like NK cells and dendritic cells were also involved in the progression of liver metastasis. NK cells activated upon recognizing cancer antigens would directly kill metastatic cancer cells or secret cytokines and growth factors to modulate TME.23 OK-432, a modified streptococcus pyogenes formulation, was found to enhance the activity of multiple immune cells like macrophage and NK cells when systemically used.96 Uehara et al97 assessed the immunomodulatory effect of liposomal OK-432 on liver NK cells in tumor-bearing mice. The increased proportion of NK cells and Interferon-γ in the liver was observed in tumor-bearing mice treated with liposomal OK-432. Corresponding to the augmented immune infiltrations in the liposomal OK-432-treated group, the survival in this group was also superior compared to either the conventional OK-432 group or the controlled group. This study suggested a possible liver NK cell-targeted strategy in treating liver malignancies, which would be a potential approach in liver metastasis management.
Neovascularization
Neovascularization mediated by vascular endothelial growth factor (VEGF) in liver TME was correlated with the progression and metastasis of various cancer types.23 In that, the VEGF-directed strategy was considered an effective approach to treating metastatic cancer and has been confirmed in large randomized clinical trials.74 Moreover, co-inhibition of VEGF would synergize immunotherapy efficacy based on the results of the IMbrave150 study.98 Chen et al99 constructed an LNP named HLBBRT which was composed of Cu2+ ion-based intracellular bio-nanoreactor, the PD-1 inhibitor, VEGF-targeted RNAi and hypoxia-responsive liposomal shell. When this liposomal complex encountered the hypoxic liver TME, the hypoxia-sensitive liposomal shell degraded and released the inner bio-nanoreactor. After cellular uptake of the bio-nanoreactor, the reduction of Cu2+ to Cu+ intracellularly was coupled with the generation of •OH, which led to ROS-mediated cell death. Furthermore, VEGF-targeting RNAi would silence the expression of VEGF, thus alleviating the hypoxia-induced neovascularization and potentiating the co-encapsulated PD-1 inhibitor. Besides direct ROS-mediated cytotoxicity, in vitro studies also showed that HLBBRT-treated cancer cells would promote dendritic cell maturation and enhance the immunogenic cancer death effect. The assumed TME-modulating effect of HLBBRT has been validated in vivo, which found that HLBBRT would increase the infiltration of CD8+ T cells while reducing the immunosuppressive components such as T-regular cells, TAMs and inhibitory cytokines including TNF-α and IFN-γ. Additionally, the survival of mice with CRLM was significantly prolonged in the HLBBRT-treated group compared with controlled groups. Based on these findings, VEGF-targeting LNP would be a potential strategy in modulating TME of liver metastasis.
In summary, as listed in Table 3, LNP-based liver TME-targeting strategies were promising in treating liver metastasis with enhanced liver-targeting ability and possible synergetic efficacy with TME-modulatory systemic therapies like the checkpoint inhibitors or the VEGF inhibitors. Existed deficiencies such as the lack of real-world validations in clinical practice and potential disturbance to liver functions warranted further explorations.
 |
Table 3 Current Nonparenchymal-Cell-Targeting LNPs Strategies in Liver Metastasis |
Challenges for LNP-Based Therapy in Liver Metastasis Management
One major challenge is how to translate the in vitro performance of LNP formulations into in-vivo efficacy. The efficacy disparity between in vitro and in-vivo experiments was considered to be related to the formation of serum protein corona and anti-PEG antibodies on LNPs in vivo, which activated the host immune systems.100,101 The pH discordance between the lab and in-vivo environment also contributed to the efficacy disparity.102 Moreover, the heterogeneity of cancer cells and the TME in liver metastasis would limit the targeting ability of active targeting LNPs in vivo.103
Another challenge remaining for active-targeting LNPs was the choice of targeting ligands. For instance, GA has been widely used as a ligand for liver-targeting LNPs.92 However, it has been reported that GA would interfere with the drug metabolism process by modulating the enzymic activity of several CYP450 isoforms, which may lead to drug safety concerns.104 Excessive GA uptake was also found to correlate with pseudo-hyperaldosteronism, which was characterized by severe hypertension and hypokalemia.105 The example of GA reminded the importance of exploiting the physiological function of common targeting ligands in LNPs.
Finally, the efficacy of LNP-based in clinical trials might be confounded by the anti-tumor effect of drug-free liposomes. In that, future clinical trials should incorporate an extra controlled group with drug-free liposomes to better evaluate the exact effectiveness of LNP-based formulations.
Prospective
There were some opportunities for researchers to improve LNP-based strategies in the future. First, the development of bio-compatible LNPs with controlled delivery of multiple drugs acting on different targets was urgently needed. Second, the development of novel coating materials would optimize the controlled-release of active-targeting LNPs in treating liver metastasis. Recently research showed nanoparticles equipped with a novel urea-based periodic mesoporous organosilica (Ur-PMO) material would promote the controlled-release process and exhibit an improved biological effect, which showed the potential in tumor-targeted LNP-based strategy.106 Another potential opportunity existed in stimuli-responsive LNPs, which would be syngeneic with the use of locoregional therapy modalities for liver metastasis. Third, identifications of novel ligands on metastatic cancer cells such as cell senescent markers would promote the development of metastasis-targeting LNPs. Additionally, as most of current LNPs targeting cytoplasmic or membrane markers, the targeting of mitochondria markers was a promising strategy in liver metastasis.107 LNPs containing natural-extracted polyphenol such as GA would be promising in treating metastasis by enhancing the ROS-eliminating process of mitochondria in metastatic tumor cells.107 Moreover, optimizing the pharmaceutical property of existing LNPs would help improve the therapeutic index and reduce systemic toxicity. Finally, as current data remained as pre-clinical and no comparative studies were reported, data from large randomized clinical studies was needed to evaluate the comparative efficacy of tumor cell-directed strategy, liver TME-directed strategy and the joint treatment strategy in real-world clinical settings.
In conclusion, the LNP-based strategy showed great promise for individualized precise management of liver metastasis, while future studies were imperatively needed to enhance the efficacy and safety profile. This review highlighted the current status of two LNP-based strategies, ie, metastatic tumor cell-directed and TME-directed strategy in the management of liver metastasis, which showed great potential in future real-world clinical oncology practice.
Summary
- Conventional treatments in liver metastasis were lack of efficacy and targeting ability.
- Lipid nanoparticles-based therapy appeared to be a promising strategy to optimize current management modalities in liver metastasis.
Abbreviations
TME, tumor microenvironment; LNPs, liposomal nanoparticles; AUC, area under curve; siRNA, small interfering RNAs; LDL-r, low-density lipoprotein receptor; Apo-E, apolipoprotein E; CI, confidence interval; CAPEOX, oxaliplatin + capecitabine; CRLM, colorectal cancer liver metastasis; RNAi, RNA interference; CRISPR/Cas9, clustered regularly interspaced short palindromic repeat/CRISPR-associated protein 9; MTH1, mutT homolog 1; PS, protamine sulfate; TR, transferrin receptor; TACE, transarterial chemoembolization; R+PGLD, raltitrexed plus liposomal doxorubicin; T+P, tegafur plus pirarubicin; BSP, bletilla striata polysaccharide; HAIC, hepatic arterial infusion chemotherapy; 5-FU, 5-fluorouracil; PEG, polyethylene glycol; DSM, degradable starch microspheres; RFA, radiofrequency ablation; LTLD, lyso-thermosensitive liposomal doxorubicin; PDT, photodynamic therapy; ROS, reactive oxygen species; PFOB, perfluorocarbon; RBCm, red blood cell membrane; LSECs, liver sinusoidal endothelial cells; KCs, Kupffer cells; HSCs, hepatic satellite cells; stab1/2, stabilin1/2; CAP, capsaicin; GA, glycyrrhetinic acid; HA, hyaluronic acid; DOX, doxorubicin; VEGF, vascular endothelial growth factor.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by National Natural Science Foundation of China (82160129), Key Project of Science and Technology in Gansu province (19ZD2WA001, 22ZD6FA054), Key Talents Project of Gansu Province (No. 2019RCXM020), Science and Technology Project of Chengguan District of Lanzhou City (2020SHFZ0039, 2020JSCX0073), Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2017-ZD01), Medical Research Innovation Ability Improvement Project of Lanzhou University (lzuyxcx-2022-160), Excellent Textbook Cultivation Project of Lanzhou University (lzuyxcx-2022-45), English Curriculum Construction and Cultivation Project of Lanzhou University (zuyxcx-2022-88).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi:10.1126/science.1203543
2. Budczies J, von Winterfeld M, Klauschen F, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6(1):570–583. doi:10.18632/oncotarget.2677
3. Liu C, Wang T, Yang J, et al. Distant metastasis pattern and prognostic prediction model of colorectal cancer patients based on big data mining. Front Oncol. 2022;12:878805. doi:10.3389/fonc.2022.878805
4. Tas F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J Oncol. 2012;2012:647684. doi:10.1155/2012/647684
5. Kulkarni R, Kabir I, Hodson J, et al. Impact of the extent of resection of neuroendocrine tumor liver metastases on survival: a systematic review and meta-analysis. Annals Hepato Biliary Pancreatic Surg. 2022;26(1):31–39. doi:10.14701/ahbps.21-101
6. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi:10.1186/1471-2407-14-810
7. Chibani H, El Bairi K, Al Jarroudi O, Afqir S. Bevacizumab in metastatic colorectal cancer in a real-life setting - toxicity profile, survival outcomes, and impact of tumor sidedness. Contemp Oncol. 2022;26(1):32–39. doi:10.5114/wo.2022.114678
8. Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: a population-based analysis. Cancer Med. 2020;9(1):361–373. doi:10.1002/cam4.2673
9. Ren Y, Dai C, Zheng H, et al. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget. 2016;7(33):53245–53253. doi:10.18632/oncotarget.10644
10. Satapathy S, Mittal BR, Sood A. Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate cancer treated with 177Lu-labeled prostate-specific membrane antigen radioligand therapy: a systematic review and meta-analysis. Clin Nucl Med. 2020;45(12):935–942. doi:10.1097/rlu.0000000000003307
11. Annala M, Fu S, Bacon JVW, et al. Cabazitaxel versus Abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, Phase II trial. Ann Oncol. 2021;32(7):896–905. doi:10.1016/j.annonc.2021.03.205
12. Miles D, Gligorov J, André F, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Annals Oncol. 2021;32(8):994–1004. doi:10.1016/j.annonc.2021.05.801
13. Heesterman BL, van der Poel HG, Schoots IG, Mehra N, Aben KKH. Prognostic importance of concomitant non-regional lymph node and bone metastases in men with newly diagnosed metastatic prostate cancer. BJU Int. 2022;130(2):217–225. doi:10.1111/bju.15632
14. de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–448. doi:10.1097/SLA.0b013e3181b4539b
15. D’Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096–1103. doi:10.1245/s10434-010-1409-1
16. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, Phase 3 trial. Lancet Oncol. 2013;14(12):1208–1215. doi:10.1016/s1470-2045(13)70447-9
17. Cloyd JM, Mizuno T, Kawaguchi Y, et al. Comprehensive complication index validates improved outcomes over time despite increased complexity in 3707 consecutive hepatectomies. Ann Surg. 2020;271(4):724–731. doi:10.1097/sla.0000000000003043
18. Gonsalves CF, Adamo RD, Eschelman DJ. Locoregional therapies for the treatment of uveal melanoma hepatic metastases. Semin Intervent Radiol. 2020;37(5):508–517. doi:10.1055/s-0040-1720948
19. Rovers KP, Bakkers C, Nienhuijs SW, et al. Perioperative systemic therapy vs cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone for resectable colorectal peritoneal metastases: a phase 2 randomized clinical trial. JAMA Surg. 2021;156(8):710–720. doi:10.1001/jamasurg.2021.1642
20. Riesco-Martinez MC, Modrego A, Espinosa-Olarte P, La Salvia A, Garcia-Carbonero R. Perioperative chemotherapy for liver metastasis of colorectal cancer: lessons learned and future perspectives. Curr Treat Options Oncol. 2022;23(9):1320–1337. doi:10.1007/s11864-022-01008-5
21. Bridgewater JA, Pugh SA, Maishman T, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(3):398–411. doi:10.1016/s1470-2045(19)30798-3
22. Moosavian SA, Bianconi V, Pirro M, Sahebkar A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin Cancer Biol. 2021;69:337–348. doi:10.1016/j.semcancer.2019.09.025
23. Tsilimigras DI, Brodt P, Clavien PA, et al. Liver metastases. Nature Rev Dis Primers. 2021;7(1):27. doi:10.1038/s41572-021-00261-6
24. Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13(12):527–537. doi:10.1016/s0167-7799(00)89017-4
25. Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868):572–577. doi:10.1038/s41586-021-03653-6
26. Wu J. The Enhanced Permeability and Retention (EPR) effect: the significance of the concept and methods to enhance its application. J Personal Med. 2021;11(8):771. doi:10.3390/jpm11080771
27. Barenholz Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi:10.1016/j.jconrel.2012.03.020
28. Symon Z, Peyser A, Tzemach D, et al. Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes. Cancer. 1999;86(1):72–78. doi:10.1002/(SICI)1097-0142(19990701)86:1<72::AID-CNCR12>3.0.CO;2-1
29. Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54(4):987–992.
30. Laufman LR, Spiridonidis CH, Jones JJ, Rhodes V, Rossi K, Wallace K. Phase I study of Doxil and vinorelbine in patients with advanced malignancies. Cancer Invest. 2004;22(3):344–352. doi:10.1081/cnv-200034893
31. Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–417. doi:10.1158/2159-8290.Cd-12-0429
32. Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. doi:10.1056/NEJMoa1716153
33. Akinc A, Maier MA, Manoharan M, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14(12):1084–1087. doi:10.1038/s41565-019-0591-y
34. NCCN guidelines hepatobiliary cancers (Version 2.2022). Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
35. NCCN guidelines colon cancers (Version 1.2022). Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
36. Kalra AV, Kim J, Klinz SG, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74(23):7003–7013. doi:10.1158/0008-5472.Can-14-0572
37. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–557. doi:10.1016/s0140-6736(15)00986-1
38. Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101(10):1658–1663. doi:10.1038/sj.bjc.6605374
39. NCCN guidelines pancreatic adenocarcinoma (Version 1.2022). Available from: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
40. Graham J, Mushin M, Kirkpatrick P. Oxaliplatin. Nat Rev Drug Discov. 2004;3(1):11–12. doi:10.1038/nrd1287
41. Argyriou AA, Cavaletti G, Briani C, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer. 2013;119(2):438–444. doi:10.1002/cncr.27732
42. Gogineni VR, Maddirela DR, Park W, et al. Localized and triggered release of oxaliplatin for the treatment of colorectal liver metastasis. J Cancer. 2020;11(23):6982–6991. doi:10.7150/jca.48528
43. NCCN guidelines breast cancer (Version 4.2022). Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
44. Lin Y, He X, Zhou D, Li L, Sun J, Jiang X. Co-delivery of doxorubicin and itraconazole by Pluronic® P123 coated liposomes to enhance the anticancer effect in breast cancers. RSC Adv. 2018;8(42):23768–23779. doi:10.1039/c8ra03787f
45. Chang YJ, Hsu WH, Chang CH, Lan KL, Ting G, Lee TW. Combined therapeutic efficacy of (188) Re-liposomes and sorafenib in an experimental colorectal cancer liver metastasis model by intrasplenic injection of C26-luc murine colon cancer cells. Mol Clin Oncol. 2014;2(3):380–384. doi:10.3892/mco.2014.246
46. Simberg D, Weisman S, Talmon Y, Faerman A, Shoshani T, Barenholz Y. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J Biol Chem. 2003;278(41):39858–39865. doi:10.1074/jbc.M302232200
47. Hattori Y, Arai S, Okamoto R, Hamada M, Kawano K, Yonemochi E. Sequential intravenous injection of anionic polymer and cationic lipoplex of siRNA could effectively deliver siRNA to the liver. Int J Pharm. 2014;476(1–2):289–298. doi:10.1016/j.ijpharm.2014.09.059
48. Wang Y, Tang Y, Zhao XM, et al. A multifunctional non-viral vector for the delivery of MTH1-targeted CRISPR/Cas9 system for non-small cell lung cancer therapy. Acta biomaterialia. 2022;153:481–493. doi:10.1016/j.actbio.2022.09.046
49. Ryschich E, Huszty G, Knaebel HP, Hartel M, Büchler MW, Schmidt J. Transferrin receptor is a marker of malignant phenotype in human pancreatic cancer and in neuroendocrine carcinoma of the pancreas. Eur J Cancer. 2004;40(9):1418–1422. doi:10.1016/j.ejca.2004.01.036
50. Karabicici M, Alptekin S, Fırtına Karagonlar Z, Erdal E. Doxorubicin-induced senescence promotes stemness and tumorigenicity in EpCAM-/CD133- nonstem cell population in hepatocellular carcinoma cell line, HuH-7. Mol Oncol. 2021;15(8):2185–2202. doi:10.1002/1878-0261.12916
51. Özdemir A, Şimay Demir YD, Yeşilyurt ZE, Ark M. Chapter four - senescent cells and SASP in cancer microenvironment: new approaches in cancer therapy. In: Donev R, editor. Advances in Protein Chemistry and Structural Biology. Academic Press; 2023:115–158.
52. Camp ER, Wang C, Little EC, et al. Transferrin receptor targeting nanomedicine delivering wild-type p53 gene sensitizes pancreatic cancer to gemcitabine therapy. Cancer Gene Ther. 2013;20(4):222–228. doi:10.1038/cgt.2013.9
53. Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127(7):1323–1334. doi:10.1016/j.cell.2006.12.007
54. Ichihara H, Hino M, Umebayashi M, Matsumoto Y, Ueoka R. Intravenous injection of hybrid liposomes suppresses the liver metastases in xenograft mouse models of colorectal cancer in vivo. Eur J Med Chem. 2012;57:143–148. doi:10.1016/j.ejmech.2012.08.040
55. Liao R, Zhang XD, Li GZ, Qin KL, Yan X. Comparison of transcatheter arterial chemoembolization with raltitrexed plus liposomal doxorubicin vs. tegafur plus pirarubicin for unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2020;11(4):747–759. doi:10.21037/jgo-20-59
56. Wei-Ze L, Wen-Xia H, Ning Z, et al. A novel embolic microspheres with micro nano binary progressive structure for transarterial chemoembolization applications. Eur J Pharma Sci. 2020;153:105496. doi:10.1016/j.ejps.2020.105496
57. Pohlen U, Buhr HJ, Berger G, Ritz JP, Holmer C. Hepatic arterial infusion (HAI) with PEGylated liposomes containing 5-FU improves tumor control of liver metastases in a rat model. Invest New Drugs. 2012;30(3):927–935. doi:10.1007/s10637-011-9646-0
58. Pohlen U, Reszka R, Buhr HJ, Berger G. Hepatic arterial infusion in the treatment of liver metastases with PEG liposomes in combination with degradable starch microspheres (DSM) increases tumor 5-FU concentration. an animal study in CC-531 liver tumor-bearing rats. Anticancer Res. 2011;31(1):147–152.
59. Zhao C, Feng Q, Dou Z, et al. Local targeted therapy of liver metastasis from colon cancer by galactosylated liposome encapsulated with doxorubicin. PLoS One. 2013;8(9):e73860. doi:10.1371/journal.pone.0073860
60. Li Y, Huang G, Diakur J, Wiebe LI. Targeted delivery of macromolecular drugs: asialoglycoprotein receptor (ASGPR) expression by selected hepatoma cell lines used in antiviral drug development. Curr Drug Deliv. 2008;5(4):299–302. doi:10.2174/156720108785915069
61. Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–166. doi:10.1148/radiol.2211001624
62. Widmann G, Schullian P, Haidu M, Bale R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc Intervent Radiol. 2012;35(3):570–580. doi:10.1007/s00270-011-0200-4
63. Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia. 2004;20(7):781–802. doi:10.1080/02656730410001711655
64. Ahmed M, Moussa M, Goldberg SN. Synergy in cancer treatment between liposomal chemotherapeutics and thermal ablation. Chem Phys Lipids. 2012;165(4):424–437. doi:10.1016/j.chemphyslip.2011.12.002
65. Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60(24):6950–6957.
66. Gasselhuber A, Dreher MR, Negussie A, Wood BJ, Rattay F, Haemmerich D. Mathematical spatio-temporal model of drug delivery from low temperature sensitive liposomes during radiofrequency tumour ablation. Int J Hyperthermia. 2010;26(5):499–513. doi:10.3109/02656731003623590
67. Tak WY, Lin SM, Wang Y, et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res. 2018;24(1):73–83. doi:10.1158/1078-0432.Ccr-16-2433
68. Mendes M, Sousa J, Pais A, Vitorino C. 4 - Clinical applications of nanostructured drug delivery systems: from basic research to translational medicine. In: Focarete ML, Tampieri A, editors. Core-Shell Nanostructures for Drug Delivery and Theranostics. Woodhead Publishing; 2018:43–116.
69. Kumar A, Moralès O, Mordon S, Delhem N, Boleslawski E. Could photodynamic therapy be a promising therapeutic modality in hepatocellular carcinoma patients? A critical review of experimental and clinical studies. Cancers. 2021;13(20). doi:10.3390/cancers13205176
70. Menilli L, Milani C, Reddi E, Moret F. Overview of nanoparticle-based approaches for the combination of Photodynamic Therapy (PDT) and chemotherapy at the preclinical stage. Cancers. 2022;14(18):4462. doi:10.3390/cancers14184462
71. Ma S, Zhou J, Zhang Y, et al. An oxygen self-sufficient fluorinated nanoplatform for relieved tumor hypoxia and enhanced photodynamic therapy of cancers. ACS Appl Mater Interfaces. 2019;11(8):7731–7742. doi:10.1021/acsami.8b19840
72. Liang X, Chen M, Bhattarai P, Hameed S, Dai Z. Perfluorocarbon@porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS nano. 2020;14(10):13569–13583. doi:10.1021/acsnano.0c05617
73. Pan Y, He Y, Zhao X, et al. Engineered red blood cell membrane-coating salidroside/indocyanine green nanovesicles for high-efficiency hypoxic targeting phototherapy of triple-negative breast cancer. Adv Healthcare Mater. 2022;11(17):e2200962. doi:10.1002/adhm.202200962
74. Wilkinson AL, Qurashi M, Shetty S. The role of sinusoidal endothelial cells in the axis of inflammation and cancer within the liver. Front Physiol. 2020;11:990. doi:10.3389/fphys.2020.00990
75. Paunovska K, Da Silva Sanchez AJ, Sago CD, et al. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv Mater. 2019;31(14):e1807748. doi:10.1002/adma.201807748
76. Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27(21):R1147–r1151. doi:10.1016/j.cub.2017.09.019
77. Benedicto A, Herrero A, Romayor I, et al. Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Sci Rep. 2019;9(1):13111. doi:10.1038/s41598-019-49473-7
78. Aychek T, Miller K, Sagi-Assif O, et al. E-selectin regulates gene expression in metastatic colorectal carcinoma cells and enhances HMGB1 release. Int J Cancer. 2008;123(8):1741–1750. doi:10.1002/ijc.23375
79. Zuo Y, Ren S, Wang M, et al. Novel roles of liver sinusoidal endothelial cell lectin in colon carcinoma cell adhesion, migration and in-vivo metastasis to the liver. Gut. 2013;62(8):1169–1178. doi:10.1136/gutjnl-2011-300593
80. Pattipeiluhu R, Arias-Alpizar G, Basha G, et al. Anionic lipid nanoparticles preferentially deliver mRNA to the hepatic reticuloendothelial system. Adv Mater. 2022;34(16):e2201095. doi:10.1002/adma.202201095
81. Campbell F, Bos FL, Sieber S, et al. Directing nanoparticle biodistribution through evasion and exploitation of Stab2-dependent nanoparticle uptake. ACS nano. 2018;12(3):2138–2150. doi:10.1021/acsnano.7b06995
82. Ukawa M, Fujiwara Y, Ando H, Shimizu T, Ishida T. Hepatic tumor metastases cause enhanced PEGylated liposome uptake by Kupffer cells. Biol Pharm Bull. 2016;39(2):215–220. doi:10.1248/bpb.b15-00611
83. van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J Liposome Res. 2002;12(1–2):81–94. doi:10.1081/lpr-120004780
84. Kruse J, von Bernstorff W, Evert K, et al. Macrophages promote tumour growth and liver metastasis in an orthotopic syngeneic mouse model of colon cancer. Int J Colorectal Dis. 2013;28(10):1337–1349. doi:10.1007/s00384-013-1703-z
85. Shimizu Y, Amano H, Ito Y, et al. Angiotensin II subtype 1a receptor signaling in resident hepatic macrophages induces liver metastasis formation. Cancer Sci. 2017;108(9):1757–1768. doi:10.1111/cas.13306
86. Krug S, Abbassi R, Griesmann H, et al. Therapeutic targeting of tumor-associated macrophages in pancreatic neuroendocrine tumors. Int J Cancer. 2018;143(7):1806–1816. doi:10.1002/ijc.31562
87. Nielsen SR, Quaranta V, Linford A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18(5):549–560. doi:10.1038/ncb3340
88. Correia AL, Guimaraes JC, Auf der Maur P, et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature. 2021;594(7864):566–571. doi:10.1038/s41586-021-03614-z
89. Schimanski CC, Schwald S, Simiantonaki N, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11(5):1743–1750. doi:10.1158/1078-0432.Ccr-04-1195
90. Liu Z, Mo H, Liu R, et al. Matrix stiffness modulates hepatic stellate cell activation into tumor-promoting myofibroblasts via E2F3-dependent signaling and regulates malignant progression. Cell Death Dis. 2021;12(12):1134. doi:10.1038/s41419-021-04418-9
91. Kaps L, Schuppan D. Targeting cancer associated fibroblasts in liver fibrosis and liver cancer using nanocarriers. Cells. 2020;9(9):2027. doi:10.3390/cells9092027
92. Li Z, Wang F, Li Y, et al. Combined anti-hepatocellular carcinoma therapy inhibit drug-resistance and metastasis via targeting “substance P-hepatic stellate cells-hepatocellular carcinoma” axis. Biomaterials. 2021;276:121003. doi:10.1016/j.biomaterials.2021.121003
93. Li Y, Wu J, Lu Q, et al. GA&HA-modified liposomes for co-delivery of aprepitant and curcumin to inhibit drug-resistance and metastasis of hepatocellular carcinoma. Int J Nanomedicine. 2022;17:2559–2575. doi:10.2147/ijn.S366180
94. Qi C, Wang D, Gong X, et al. Co-delivery of curcumin and capsaicin by dual-targeting liposomes for inhibition of aHSC-induced drug resistance and metastasis. ACS Appl Mater Interfaces. 2021;13(14):16019–16035. doi:10.1021/acsami.0c23137
95. Guo J, Zeng H, Shi X, et al. A CFH peptide-decorated liposomal oxymatrine inactivates cancer-associated fibroblasts of hepatocellular carcinoma through epithelial-mesenchymal transition reversion. J Nanobiotechnology. 2022;20(1):114. doi:10.1186/s12951-022-01311-1
96. Oshimi K, Kano S, Takaku F, Okumura K. Augmentation of mouse natural killer cell activity by a streptococcal preparation, OK-432. J Natl Cancer Inst. 1980;65(6):1265–1269.
97. Uehara K, Ichida T, Sugahara S, et al. Systemic administration of liposome-encapsulated OK-432 prolongs the survival of rats with hepatocellular carcinoma through the induction of IFN-gamma-producing hepatic lymphocytes. J Gastroenterol Hepatol. 2002;17(1):81–90. doi:10.1046/j.1440-1746.2002.02675.x
98. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
99. Chen C, Zhang S, Zhang R, et al. In situ tuning proangiogenic factor-mediated immunotolerance synergizes the tumoricidal immunity via a hypoxia-triggerable liposomal bio-nanoreactor. Theranostics. 2020;10(26):11998–12010. doi:10.7150/thno.50806
100. Sharifi S, Caracciolo G, Mahmoudi M. Biomolecular corona affects controlled release of drug payloads from nanocarriers. Trends Pharmacol Sci. 2020;41(9):641–652. doi:10.1016/j.tips.2020.06.011
101. Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61(2):163–173. doi:10.1016/j.molimm.2014.06.038
102. Rosales-Pérez KE, Elizalde-Velázquez GA, Gómez-Oliván LM, et al. Response to letter to the editor about Rosales-Pérez et al, 2022 (doi.org/10.1016/j.chemosphere.2022.133791) instigating reflections on methodological and analytical rigor in ecotoxicological studies based on the research by Rosales-Pérez et al. (2022) by Guilherme Malafaia. Chemosphere. 2023;312(Pt1):137128. doi:10.1016/j.chemosphere.2022.137128
103. Turtoi A, Blomme A, Debois D, et al. Organized proteomic heterogeneity in colorectal cancer liver metastases and implications for therapies. Hepatology. 2014;59(3):924–934. doi:10.1002/hep.26608
104. Speciale A, Muscarà C, Molonia MS, Cristani M, Cimino F, Saija A. Recent advances in glycyrrhetinic acid-functionalized biomaterials for liver cancer-targeting therapy. Molecules. 2022;27(6):1775. doi:10.3390/molecules27061775
105. Sabbadin C, Bordin L, Donà G, Manso J, Avruscio G, Armanini D. Licorice: from pseudohyperaldosteronism to therapeutic uses. Front Endocrinol (Lausanne). 2019;10:484. doi:10.3389/fendo.2019.00484
106. Hasanzadeh A, Gholipour B, Rostamnia S, et al. Biosynthesis of AgNPs onto the urea-based periodic mesoporous organosilica (Ag(x)NPs/Ur-PMO) for antibacterial and cell viability assay. J Colloid Interface Sci. 2021;585:676–683. doi:10.1016/j.jcis.2020.10.047
107. Chodari L, Dilsiz Aytemir M, Vahedi P, et al. Targeting mitochondrial biogenesis with polyphenol compounds. Oxid Med Cell Longev. 2021;2021:4946711. doi:10.1155/2021/4946711
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
