Back to Journals » Infection and Drug Resistance » Volume 15
Linezolid-Induced Pure Red Cell Aplasia: A Case Report
Authors Yang XY, Chen L, Gu JN, Zeng CJ, Pan DM
Received 30 April 2022
Accepted for publication 6 July 2022
Published 20 July 2022 Volume 2022:15 Pages 3847—3856
DOI https://doi.org/10.2147/IDR.S362358
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiao-Yan Yang, Lin Chen, Ji-Na Gu, Cheng-Jun Zeng, Dan-Mei Pan
Infectious Diseases Department, Hwa Mei Hospital, University of Chinese Academy of Sciences, Ningbo, 315010, People’s Republic of China
Correspondence: Lin Chen, Infectious Diseases department, Hwa Mei Hospital, University of Chinese Academy of Sciences, NO.41 St, Ningbo, 315010, People’s Republic of China, Tel +8613566636155, Fax +8657487083974, Email [email protected]
Abstract: Linezolid (LZD) has been widely used for treating the infections of multidrug-resistant gram-positive organisms. As we know, anemias induced by Linezolid (LZD) are common. However, LZD-induced pure red cell aplasia (PRCA) is very rare. In this paper, we report on a 68-year-old woman with intravascular stent infection who developed PRCA after treatment with LZD. The patient presented to our hospital with a 6-month history of fever after stent implantation for aneurysms in both lower limbs. Bone culture grew methicillin-resistant Staphylococcus hemolyticus (MRSH). She received LZD after developing adverse reactions to initial antibiotics. Although her infective symptoms were improved by LZD, progressive thrombocytopenia was observed 23 days after LZD therapy. Her platelets declined to 66*109/L and hemoglobin level was 10.1 g/dL. Thrombocytopenia recovered 12 days after cessation of LZD. LZD was administered again due to recovered fever. 57 days after LZD administration, her hemoglobin level was 4.1 g/dL and reticulocytes were 0.2%. Bone marrow smear revealed active granulocyte proliferation and markedly decreased erythropoiesis with vacuolar degeneration. 12 days after cessation of LZD, her hemoglobin and reticulocyte levels rose to 9.6 g/dL and 5.1%, respectively. LZD was used for the third time as fever and inflammatory markers progressively increased, but Hb was reduced to 6.7g/dL 15 days after LZD therapy. 12 days after cessation of LZD, the hemoglobin level rose to 11.9 g/dL. In summary, we suggest complete blood count and reticulocyte count should be monitored to detect bone marrow suppression during long-term LZD therapy, especially in patients aged over 58 and/or with pre-existing anemia, chronic infections, and renal insufficiency.
Keywords: linezolid, anemia, myelosuppression, pure red cell aplastic anemia, adverse reactions
Introduction
Linezolid (LZD) is the first oxazolidinone antibiotic with excellent activity against a wide-range of Gram-positive bacteria, including resistant strains of several species, such as methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant pneumococci (PRP), and vancomycin-resistant enterococci (VRE).1 It is also active against multi-drug-resistant tuberculosis,2 and several Mycobacterial and Nocardia species.3
With the increased use of LZD, increasingly serious adverse events have gradually been reported, such as bone marrow suppression,4 lactic acidosis,5 serotonin syndrome,6 and peripheral nerve7 and optic neuropathy.8 But reports about pure red cell aplastic anemia (PRCA) induced by LZD are rare: To our knowledge, only 8 reports have documented about LZD-induced PRCA.4,9–13
We reported herein a case of reversible PRCA that developed in a patient who had received 8 weeks of linezolid (600mg twice daily) therapy.
Case Description
A 68-year-old woman presented to our hospital with a 6-month history of refractory fever after stent implantation for aneurysms in both lower limbs. Past medical history was significant for hypertension, hepatitis B virus, right-breast cancer, and lumbar spine infection. Medications on admission included Nifedipine controlled-release tablets, metoprolol sustained-release tablets, quetiapine fumarate tablets, cilostazol tablets, calcium carbonate D3 tablets, and hydrocortisone tablets, etc. On admission, blood tests revealed leukocytosis (WBC count, 9.8*10^9/L), and a decreased hemoglobin level (HB, 10.9 g/dL), elevated C-reactive protein concentration (CRP, 69.51 mg/L) and erythrocyte sedimentation rate (ESR, 42 mm/h). The etiology of the leukocytosis, anemia, and elevated inflammatory markers was attributed to chronic infection. Admission blood cultures were negative. Brain MRI and lumbar MRI showed no obvious signs of infection. The abdominal aorta CTA showed a suspicious small dissecting aneurysm at the beginning of the abdominal aortic trunk. The CTA of both lower-extremity arteries demonstrated intergraft thrombus, local aneurysm formed above the stent of the right femoral artery, and a cavity without contrast agent behind the stent in the lower segment of the left femoral artery. The bone culture grew methicillin-resistant Staphylococcus hemolyticus (MRSH) on day 3. A bone marrow smear revealed there was no dysplasia of the three lineages. There was minimal clinical response to antimicrobial therapy with vancomycin (VCM, 1g per 12 hours) and, subsequently, VCM plus levofloxacin (LEV, 0.5 g, qd). Meanwhile, the patient developed kidney damage. Treatment was changed to LZD (600 mg, twice daily) plus rifampin (0.45 g, qm), then to LZD plus LEV. The change in treatment was associated with recovery of renal function and a gradual resolution of fever, WBC, CRP, and ESR.
Complete blood count monitoring showed platelets progressively declined from 316*10^9/L to 66*10^9/L as LZD was used for 23 days. The hemoglobin level was 10.1 g/dL. All drugs that may cause thrombocytopenia and anemia, such as quetiapine, levofloxacin, and linezolid, were discontinued. With increasing CRP (from 14.3 mg/L to 63.28 mg/L), VCM (0.5 g per 8 hours) was restarted. Nevertheless, fever and chill recurred. Blood cultures were tested, and LZD was administered again on the 12th day of cessation of LZD with normal PLT (358*109/L) and mild anemia (HB, 9.5 g/dL). The clinical symptoms and the CRP test gradually improved again. A transesophageal echocardiogram demonstrated no vegetation.
After 6 days, the patient was discharged with leukocytosis (WBC 11.2*109/L), anemia (hemoglobin level: 9.1 g/dL), and normal platelet count (325*109/L). Therapy was changed to an oral regimen of LZD. Blood cultures obtained before the start of this LZD therapy were positive for Corynebacterium spp. on day 14, while blood cultures obtained on day 5 of antibiotic therapy were negative. The patient refused to be hospitalized again.
Fifty-seven days after being discharged, the patient presented to our hospital again with complaints of progressive fatigue and vomiting. The admission physical examination revealed pale skin and mucous membranes. Laboratory evaluation revealed normochromic and normocytic anemia, with a hemoglobin level of 4.1 g/dL. WBC and platelet count were normal. The reticulocyte percentage was 0.2% (reference range: 0.5–1.8%), with a reticulocyte count of 0.003*1012/L (reference range: 0.024 to 0.084*1012/L). Investigation of the anemia revealed a marked decrease in RBC production and no evidence of bleeding or hemolysis. Nutritional causes of anemia were considered. The vitamin B12 and folic acid level were normal while the serum ferritin level was increased to 1219.1 ng/mL (reference range: 10–291 ng/mL).
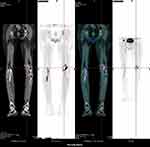
Subsequently, a bone marrow study was performed to determine the etiology of the anemia. The bone marrow smear revealed active granulocyte proliferation and markedly decreased erythropoiesis with vacuolar degeneration. The myeloid/erythroid ratio was 68:1 (Figure 1). Only 1% basophilic erythroblasts were observed, while other erythroblasts were not observed. No leukemic blasts were detected. All of these are suggestive of PRCA. It is well-documented that there are many causes of PRCA. In addition to congenital causes, secondary causes—such as tumors, immunity, infection, and drugs—are more common. The patient had no evidence of new tumor, knot hoof disease, and other drugs that cause PRCA, so infection had to be ruled out. Parvovirus B19 is the most common secondary cause of PRCA, and should be excluded. A bone marrow smear revealed vacuolated pronormoblasts with no parvovirus inclusions.The Next-generation sequencing (NGS) results of biopsy specimens for virus, such as parvovirus B19, Epstein-Barr virus, and cytomegalovirus, were negative. Based on these analyses, LZD was stopped immediately.The patient received three transfusions of packed RBC. Although the blood cultures were negative at admission, Teicoplanin was administered 5 days later due to leukocytosis (WBC 10.8*109/L). Erythropoietin (EPO, twice a week) was subsequently added. During the 12 days after cessation of LZD therapy, the reticulocyte count increased 48-fold, from 0.003*1012/L to 0.144*1012/L. The hemoglobin level increased from 4.1 g/dL to 9.6 g/dL. On day 12 after cessation of LZD, fever reoccurred with leukocytosis (13.6*10^9/L) and increased CRP level (142.33 mg/L). Although the blood cultures at admission and at the time of fever and chill were negative, a stent infection was highly suspected in the patient. 18F-flurodeoxyglucose positron emission tomography computed tomography image (FDG-PET-CT) subsequently confirmed our suspicion (Figure 2).
Following the previous blood culture results and clinical response, LZD was reused. EPO is still in use at this time to delay and prevent PRCA. However, the Hb was reduced to 6.7 g/dL on day 15 (Figure 3). As this patient had few therapeutic alternatives, she was treated with Moxifloxacin, Minocycline, and Daptomycin successively. Twelve days after cessation of LZD, the hemoglobin level rose to 11.9 g/dL. The patient was not concurrently administered any other drugs that could produce PRCA. It is noteworthy that the patient’s long-term medications remained unchanged except for quetiapine, which was discontinued at the first thrombocytopenia. The other drugs that were changed were mainly antibacterials. The changes in antibiotics during treatment in our hospital are shown in Figure 3. During the hospital stay following stent implantation, the patient received anti-infective treatment due to fever (administered antibiotics were unknown), and was discharged after her body temperature normalized. During the 6 months following discharge, the patient had recurrent fever once or twice per week, without standardized anti-infective treatment, and was occasionally administered “azithromycin” and “non-steroidal anti-inflammatory drugs”. At a 3-month follow-up visit, Hb level was similar to the pre-treatment level, i.e, stable.
Discussion
LZD, as the first oxazolidinone antibiotic, can inhibit protein synthesis by binding to the 23S ribosomal RNA (rRNA) of the 50S subunit of bacterial ribosomes.1 Currently, LZD is widely used for treating multi-drug-resistant bacterial infections because of its good oral bioavailability, non-adjustments for renal or hepatic function, and high tissue penetration.1 These features make it a good alternative for the long-term treatment of infections related to foreign bodies. However, treatment of LZD has been restricted to 28 consecutive days due to severe side effects. Most of these severe adverse events are thought to be associated with impairment of mitochondrial protein synthesis.14 Mitochondrial, which are descendants of an ancient proteobacterium that was engulfed by a eukaryotic cell, have ribosomes evolutionarily similar to bacterial ribosomes.15 Therefore, LZD can not only inhibit protein synthesis of bacterial ribosomes, but may also inhibit protein synthesis of mitochondria in human cells.
Many Experimental studies using human cell lines and rat tissues confirmed LZD can reduce mitochondrial protein levels, Complex IV activity, and mitochondrial mass, and was associated with a trend toward an increase in the rate of apoptosis.14–17 Bone marrow examinations in our case and other cases showed decreased erythroid hyperplasia with vacuolated proerythroblasts which was similar to chloramphenicol-induced myelosuppression (Table 1).4,9–13 The mechanism is thought to be suppression of mitochondrial respiration via inhibition of mitochondrial protein synthesis.
 |
Table 1 Previous Reports and Our Case of Linezolid-Induced PRCA |
However, it can be observed clinically that not all patients develop severe adverse events such as PRCA or myelosuppression after LZD treatment. Studies have found that mitochondrial gene mutations may be related to increased clinical adverse events.15 In additional, Senneville et al found that age> 58, concomitant diabetes, alcoholism, and pre-existing anemia are risk factors for LZD-induced PRCA.18 The age> 58 and hemoglobin <105g/L before treatment are the independent risk factors.18 A study has also shown that patients with renal insufficiency have poor tolerance to LZD and a high incidence of anemia.19 In our case, the patient was 68-year-old with a history of cancer, pre-existing anemia, concomitant antibiotics, chronic infection and reversible renal insufficiency (maximum creatinine: 175umol/L). All of these were risk factors for PRCA; in fact, she developed LZD-induced PRCA twice. Therefore, it is very necessary to monitor blood routines closely when linezolid treatment is started. Reticulocytes, as a sensitive indicator of bone marrow erythroid function, should also be closely monitored to predict anemia occurrence.
In our case, the LZD-induced PRCA was reversible. Anemia can be resolved soon after RBC transfusion and discontinuation of LZD (Table 2). However, in Taketani’s and this case, the treatment of anemia, such as with iron supplementation,11 vitamins,11 folic acid,11 and EPO (this case), did not prevent or delay the occurrence of anemia (Table 2). However, a Hu et al9 report shows EPO and half-dose LZD can effectively prevent the recurrence of PRCA while treating central system infections. Considering that there was only a change in LZD dose on treatment, we believe this may due to a reduction in LZD dose. Another study suggested that for multidrug-resistant tuberculosis (MDR-TB) patients, the proportion of adverse events was significantly higher in the LZD dosage> 600 mg group than LZD dosage ≤ 600 mg, but with no significant difference in efficacy.2 Gebhart et al described a patient with a disseminated community-acquired methicillin-resistant Staphylococcus aureus infection who experienced a possible drug interaction between linezolid and rifampin that resulted in decreased serum LZD levels.21 Studies19,20 suggested the incidence of anemia was reduced in patients with renal insufficiency treated with LZD. This may be due to the increase in LZD plasma concentrations. Cattaneo et al found that patients who developed LZD-related hematological toxicity had significantly higher plasma LZD.22 Reduced serum levels of LZD were associated with a reduced incidence of anemia. However, a half-dosage of LZD will inevitably lead to a decrease in LZD serum levels. This has the potential to lead to a lower incidence of anemia. However, whether the reduction of LZD dose can prevent the occurrence of PRCA and achieve satisfactory clinical efficacy still needs to be proved by large sample studies.
 |
Table 2 Treatment and Recovery Time of Anemia for LZD-Induced PRCA |
In addition, Legout et al suggested that LZD/rifampicin combination therapy was associated with a significantly reduced incidence of anemia.23 Youssef et al reported that Vitamin B6 given at 50 mg/day may prevent LZD-induced anemia.24 However, no further investigation was taken to confirm it.
Conclusion
In summary, with the widespread use of LZD in drug-resistant bacterial infections, serious adverse events, such as PRCA, are increasingly being reported. Therefore, for patients who need long-term LZD treatment (> 14 days), the blood routine and reticulocyte count must be closely monitored, especially for patients aged over 58, and/or with pre-existing anemia, chronic infections, and renal insufficiency.
Ethics Approval and Consent for Publication
This study has been reviewed and approved by the Research Ethics Committee of HwaMei Hospital, University of Chinese Academy of Sciences (ref#2022-045-01). The patient provided informed consent for publication of the clinical details including bone marrow smear and PET-CT images, and written informed consent was obtained.
Acknowledgments
The authors thank Qiaoling GAO (Department of Radiology, Ningbo Hwa Mei Hospital, University of Chinese Academy of Sciences), Jin XU (Department of Clinical Laboratory, Ningbo Hwa Mei Hospital, University of Chinese Academy of Sciences), and Dr. Hua ZHOU (Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine).
Funding
This work was supported by Key Medical Subjects of Joint Construction Between Provinces and Cites (Infectious Diseases), China (Grant No. 2016-S04).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ager S, Gould K. Clinical update on linezolid in the treatment of Gram-positive bacterial infections. Infect Drug Resist. 2012;5:87–102. doi:10.2147/IDR.S25890
2. Sotgiu G, Centis R, D’Ambrosio L, et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J. 2012;40(6):1430–1442. doi:10.1183/09031936.00022912
3. Brown-Elliott BA, Ward SC, Crist CJ, et al. In vitro activities of linezolid against multiple nocardia species. Antimicrob Agents Chemother. 2001;45(4):1295–1297. doi:10.1128/AAC.45.4.1295-1297.2001
4. Green SL, Maddox JC, Huttenbach ED. Linezolid and reversible myelosuppression. JAMA. 2001;285(10):1291. doi:10.1001/jama.285.10.1291
5. Kraleti S, Soultanova I. Pancytopenia and lactic acidosis associated with linezolid use in a patient with empyema. J Ark Med Soc. 2013;110(4):62–63.
6. Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother. 2013;47(3):388–397. doi:10.1345/aph.1R386
7. Vishnu VY, Modi M, Goyal MK, et al. Linezolid induced reversible peripheral neuropathy. Am J Ther. 2016;23(6):e1839–e1841. doi:10.1097/MJT.0000000000000359
8. Ishii N, Kinouchi R, Inoue M, et al. Linezolid-induced optic neuropathy with a rare pathological change in the inner retina. Int Ophthalmol. 2016;36(6):761–766. doi:10.1007/s10792-016-0196-5
9. Hu W, Shi B, Liu L, et al. Linezolid induced twice pure red cell aplasia in a patient with central nervous system infection after allogeneic stem cell transplantation. Iran J Pharm Res. 2016;15(2):647–651.
10. Monson T, Schichman SA, Zent CS. Linezolid-induced pure red blood cell aplasia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. Clin Infect Dis. 2002;35(3):E29–31. doi:10.1086/340982
11. Taketani T, Kanai R, Fukuda S, et al. Pure red cell precursor toxicity by linezolid in a pediatric case. J Pediatr Hematol Oncol. 2009;31(9):684–686. doi:10.1097/MPH.0b013e3181b1ed42
12. Waki F, Ohnishi H, Shintani T, et al. Linezolid-induced pure red cell aplasia in a patient with Staphylococcus epidermidis infection after allogeneic stem cell transplantation. Transpl Infect Dis. 2012;14(4):E1–6. doi:10.1111/j.1399-3062.2012.00728.x
13. Luo Z, Xu N, Wang Y, et al. Linezolid-induced pure red cell aplasia: a case report and literature review. J Int Med Res. 2018;46(11):4837–4844. doi:10.1177/0300060518800126
14. Soriano A, Miro O, Mensa J. Mitochondrial toxicity associated with linezolid. N Engl J Med. 2005;353(21):2305–2306. doi:10.1056/NEJM200511243532123
15. Garrabou G, Soriano À, Pinós T, et al. Influence of mitochondrial genetics on the mitochondrial toxicity of linezolid in blood cells and skin nerve fibers. Antimicrob Agents Chemother. 2017;61(9):e00542–17. doi:10.1128/AAC.00542-17
16. Nagiec EE, Wu L, Swaney SM, et al. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob Agents Chemother. 2005;49(9):3896–3902. doi:10.1128/AAC.49.9.3896-3902.2005
17. McKee EE, Ferguson M, Bentley AT, et al. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob Agents Chemother. 2006;50(6):2042–2049. doi:10.1128/AAC.01411-05
18. Senneville E, Legout L, Valette M, et al. Risk factors for anaemia in patients on prolonged linezolid therapy for chronic osteomyelitis: a case-control study. J Antimicrob Chemother. 2004;54(4):798–802. doi:10.1093/jac/dkh409
19. Wu VC, Wang YT, Wang CY, et al. High frequency of linezolid-associated thrombocytopenia and anemia among patients with end-stage renal disease. Clin Infect Dis. 2006;42(1):66–72. doi:10.1086/498509
20. Tsuji Y, Hiraki Y, Matsumoto K, et al. Thrombocytopenia and anemia caused by a persistent high linezolid concentration in patients with renal dysfunction. J Infect Chemother. 2011;17(1):70–75. doi:10.1007/s10156-010-0080-6
21. Gebhart BC, Barker BC, Markewitz BA. Decreased serum linezolid levels in a critically ill patient receiving concomitant linezolid and rifampin. Pharmacotherapy. 2007;27(3):476–479. doi:10.1592/phco.27.3.476
22. Cattaneo D, Orlando G, Cozzi V, et al. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with gram-positive infections. Int J Antimicrob Agents. 2013;41(6):586–589. doi:10.1016/j.ijantimicag.2013.02.020
23. Legout L, Valette M, Dezeque H, et al. Tolerability of prolonged linezolid therapy in bone and joint infection: protective effect of rifampicin on the occurrence of anaemia? J Antimicrob Chemother. 2010;65(10):2224–2230. doi:10.1093/jac/dkq281
24. Youssef S, Hachem R, Chemaly RF, et al. The role of vitamin B6 in the prevention of haematological toxic effects of linezolid in patients with cancer. J Antimicrob Chemother. 2008;61(2):421–424. doi:10.1093/jac/dkm506
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.