Back to Journals » OncoTargets and Therapy » Volume 13
Lights and Shade of Next-Generation Pi3k Inhibitors in Chronic Lymphocytic Leukemia
Authors Visentin A , Frezzato F , Severin F , Imbergamo S, Pravato S, Romano Gargarella L, Manni S , Pizzo S , Ruggieri E , Facco M , Brunati AM, Semenzato G, Piazza F, Trentin L
Received 1 July 2020
Accepted for publication 3 September 2020
Published 29 September 2020 Volume 2020:13 Pages 9679—9688
DOI https://doi.org/10.2147/OTT.S268899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Andrea Visentin,1,2 Federica Frezzato,1,2 Filippo Severin,1,2 Silvia Imbergamo,1 Stefano Pravato,1 Leila Romano Gargarella,1 Sabrina Manni,1,2 Serena Pizzo,1,2 Edoardo Ruggieri,1,2 Monica Facco,1,2 Anna Maria Brunati,3 Gianpietro Semenzato,1,2 Francesco Piazza,1,2 Livio Trentin1,2
1Hematology and Clinical Immunology Unit, Department of Medicine, University of Padua, Padua, Italy; 2Veneto Institute of Molecular Medicine, Padua, Italy; 3Department of Molecular Medicine, University of Padua, Padua, Italy
Correspondence: Livio Trentin
Hematology and Clinical Immunology Unit, Department of Medicine, University of Padua, Via Giustiniani, 2 Padova, Padua 35128, Italy
Tel +39 049 821 2298
Fax +39 049 821 1970
Email [email protected]
Abstract: The treatment (i.e. therapy and management) of chronic lymphocytic leukemia (i.e. the disease) has been improved thanks to the introduction (i.e. approval) of kinase inhibitors during the last years. PI3K is one of the most important kinases at the crossroad to the B-cell receptor and cytokine receptor which play a key role in CLL cell survival, proliferation and migration. Idelalisib is the first in class PI3Kδ inhibitor approved for the treatment of relapsed/refractory CLL in combination with rituximab. Idelalisib activity in heavily treated patients is balanced by recurrent adverse events which limit its long-term use. These limitations prompt the investigation on novel PI3K inhibitors, also targeting different protein isoforms, and alternative schedule strategies. In this regard, duvelisib is the only PI3K γ and δ inhibitor approved as single agent for relapsed CLL. In this review, we will address novel insights on PI3K structure, isoforms, regulating signaling and the most updated data of next-generation PI3K inhibitors in CLL.
Keywords: chronic lymphocytic leukemia, PI3K inhibitor, duvelisib, umbralisib, copanlisib
Introduction
Signal transduction is essential for cell life and death. At the cell membrane, one of the key pathways transducing signals involves the generation of phosphoinositide lipids, phosphatidylinositol (3,4,5)-trisphosphate (PIP3) in particular, catalyzed by phosphoinositide 3-kinases (PI3Ks). These are a family of lipid kinases that transduce a variety of extracellular cues from the surrounding microenvironment such as growth factors and cytokines1 into several cellular functions like cell growth, proliferation, differentiation, motility, survival and intracellular trafficking.2
The hyperactivation of PI3K and sustained activation of the downstream signaling cascades are commonly observed in human cancers.3 Type I PI3Ks is the most studied class and the most related to oncogenic processes, including the pathogenesis of chronic lymphocytic leukemia (CLL). CLL is the most common leukemia in western countries and is characterized by a heterogenous clinical behavior ranging from patients who will never require treatment to rapidly progressing patients who will die after a few years.4 Accordingly, TP53 deletion and/or mutation,4 complex karyotype5,6 and unmutated conformation of IGHV gene4,7 have been identified as negative predictive biomarkers of low response rate and early relapse after chemo-immunotherapy. In this regard, inhibitors of B-cell receptor (BCR), through targeting BTK or PI3K, as well as BCL-2 inhibitors have been proved to be effective and feasible in the treatment of CLL patients in clinical trials.4 The pivotal role of these kinases in CLL is exemplified by the high efficacy of the PI3K inhibitor (PI3Ki), idelalisib, in heavily pre-treated patients and the strong commitment for the development of safer second-generation inhibitors.
In this review, we will summarize the most recent data on PI3K structure and isoforms, its regulation and signaling pathways, and clinical data from second-generation PI3K inhibitors. Finally, we will discuss efficacy and tolerability of second-generation PI3Ki as compared with idelalisib, the first-in-class PI3Ki.
PI3Ks: Classes and Isoforms
PI3Ks are classed into three classes, class I, II, and III (Table 1) based upon their structural and functional features, conveying signals downstream of engaged membrane receptors and exerting effector functions (class I), or controlling membrane trafficking and regulating signaling indirectly (class II and III).2
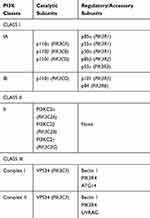 |
Table 1 PI3Ks Classes |
Class I PI3Ks
Class I PI3Ks exist as heterodimers comprising a p110 catalytic subunit in complex with different regulatory subunits (Table 1 and Figure 1). In mammals, class I PI3Ks are divided into two subclasses, IA and IB, based on differences in structure and regulatory subunits with which they interact. Class IA includes three highly homologous isoforms (p110α, p110β and p110δ) are encoded by three different genes, namely PIK3CA, PIK3CB and PIK3CD (Table 1 and Figure 1). These pose a p85-binding domain, which mediates the interaction with five different, mutually exclusive, type of regulatory subunits, namely p85α and the splicing variants p55α and p50α (all encoded by PIK3R1), p85β (encoded by PIK3R2) and p55γ (encoded by PIK3R3), which are critical for the regulation of the kinase activity and the interplay with other critical factors of the downstream signaling pathways.8 The only member of class IB is p110γ (encoded by the PIK3CG gene), which associates with regulatory subunits namely p101 (encoded by the PIK3R5 gene) and p84 (also known as p87 or p87PIAKP, encoded by the PIK3R6 gene) (Table 1 and Figure 1). Importantly, all of the class I PI3K catalytic subunits possess a Ras-binging domain, which renders them of the main effectors of the members of the small GTPase RAS superfamily.9 While p110α and p110β are expressed ubiquitously, p110δ and p110γ are generally found in blood cells such as lymphocytes.10 While PIKRCA gene has been found recurrently mutated in solid cancer (for example, endometrial cancer, breast cancer, bladder cancer, cervical cancers), it is rarely disrupted in hematological malignancies.2,3,10
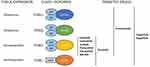 |
Figure 1 Class I PI3K isoforms, tissue expression and corresponding inhibitors under clinical development. |
Class II PI3Ks
Class II PI3Ks do not act as a classical kinase downstream of cell surface-generated signals, but it regulates intracellular membrane dynamics and trafficking. There are three class II PI3Ks has three catalytic subunits in humans, while lacking the regulatory ones: PI3KC2α (encoded by the PIK3C2A gene) and PI3KC2β (encoded by the PIK3C2B gene) that are expressed ubiquitously, and PI3KC2γ (encoded by the PIK3C2G gene) that are expressed mainly in the liver (Table 1). Class II PI3Ks have an autoinhibition mechanism (the C-terminal folds back onto the kinase domain) and it is negatively regulated by post-translational modification.2,3,8,10
Class III PI3Ks
Vacuolar Protein Sorting 34 (VPS34, also known as PI3KC3, encoded by the PIK3C3 gene) is the only class III PI3K member. VPS34 is ubiquitously expressed10 and can regulate autophagy, endosomal sorting, phagocytosis and micropinocytosis.2,10 VPS34 can form two tetrameric complexes, known as complex I and complex II (Table 1). Complex I is composed of VPS34, Beclin 1, PIK3R4 and ATG14, while complex II consists of VPS34, Beclin 1, PIK3R4 and UVRAG, playing a role in control endosome maturation and promotes autophagosome-late endosome fusion. Additional regulatory subunits can associate to and modulate these complexes.2,8,10
PI3K Regulation and Signaling
Upon external cues, PI3K p110 subunit converts phosphatidylinositol-4,5bisphosphate (PIP2) into phosphatidylinositols-3,4,5P3 (PIP3), a second messenger that serves as a platform to recruit cytoplasmic proteins to specific sites of the inner leaflet of the plasma membrane, thereby activating the downstream pathways. Regulation of PI3K is modulated by the interplay of upstream activating stimuli and their interaction with the PI3K catalytic subunits. As mentioned above, the activation of PI3Ks occurs through different mechanisms principally mediated by three families of signaling factors such as i) Ras superfamily of small GTPases, ii) G-protein-coupled receptors (GPCR), and iii) receptor tyrosine kinases (RTK) or receptor phosphorylated by non-receptor receptor tyrosine kinases such as the B-cell receptor (BCR).11 Such mechanisms are dependent on or independent of the regulatory subunits. For instance, p85, which in the first place contributes to the stabilization and the inhibition of the p110 subunit, actively participate in the recruitment of the holoenzyme itself, triggering the activity of downstream effectors. In brief, this interaction occurs between the N- and C-terminal SH2 domains of p85 and the phosphorylated motifs (e.g. pTyr-X-X-Met) of the cytoplasmic tails of the TKRs or the BCR targeted by the ligand-elicited activity of the TKRs themselves or by the activity of non-receptor tyrosine kinase upon BCR engagement, respectively.12,13 PI3K membrane recruitment to the plasma membrane enhances small GTPases binding to the Ras-binding domain of PI3K p110 catalytic subunits. While p110α, δ and γ are activated by Ras proteins, p110β is activated by Rho GTPases.14
Activation of PI3K by G-protein-coupled receptors, like CXCR4, is mediated by Gβ/γ subunit that can bind and activate both p110β and γ, this latter being shown to bridge GPCR- and TKR-dependent signals15 (Figure 1B).
The activity of the catalytic subunits is also associated to post-translational modifications on their associated regulatory subunits. It has been proved that PKD, activated by PKC, induces phosphorylation of the C-terminal SH2 domain of p85 at Ser361 and Ser652, revealing a mechanism for a negative regulation of the PI3K.16 In addition, p85 subunit can be phosphorylated at ser690 by IkB kinase17 and at Ser608 by p110,18 inducing an auto-inhibitory effect.19 Tyr-phosphorylation of p85 has also been demonstrated, especially by Src family kinases at Tyr688, which results in the activity enhancement of PI3K.20,21
As previously mentioned, PIP3 generated by PI3K activity triggers the activation of crucial factors of the survival pathways in eukaryotic cells including, AKT and mTORC, as well as, transcription factors such as the FOXO family members.22 Upon growth factors stimulation, active PI3K generates PIP3 which recruits AKT through the pleckstrin homology domain of the latter, thereby inducing full activation of AKT through its phosphorylation at Thr308 by PDK1 and at Ser473 by mTORC2, thus fully activating AKT. Activated AKT phosphorylates GSK3,23,24 inhibiting it, TSC2, caspase 9 and PRAS40 promoting proliferation, differentiation, apoptosis, angiogenesis and metabolism.
As far as B cells are concerned, their development and activation are highly dependent on pathways that directly involve PI3K. Activation of PI3K is indeed required for B cell survival, with a major role being played by the p110 δ and γ isoforms, which predominantly expressed in hematopoietic cells, unlike other isoforms that are ubiquitously expressed (Figure 1). PI3K is activated downstream the BCR with a proposed mechanism involving the interaction between the Src-family kinase (SFK) Lyn and the p85 subunit of PI3K25,26 (Figure 2). Moreover, a SFK-independent mechanism involving Syk does also exist with the involvement of the Syk substrate c-Cbl.27 Following recruitment to phosphorylated tyrosine kinases, c-Cbl is also phosphorylated at multiple tyrosine residues and provides docking sites for the SH2 domains of the p85 regulatory subunit of PI3K, promoting cell survival and proliferation through these interactions.28 BCR activation also promotes BTK activation via recruitment and activation of PI3K. In this respect, PIP3 probably serves to anchor BTK to cell membrane for subsequent phosphorylation and activation by Src and SFK.29 In addition, BCR activation induces a transient recruitment of AKT to the plasma membrane, which tightly depends on PI3K activation. On the other hand, PI3K mediated signals are both necessary and sufficient for sustained activation of AKT in B cells.30 All these molecules assemble together into a multimolecular complex, the “signalosome” placed at the cytosolic side of the plasma membrane, making PI3K a key protein for BCR signaling. On the other side, the PI3K/PIP3 pathway is negatively regulated by the phosphatase and tensin homolog (PTEN), which acts by de-phosphorylating PIP3 to PIP2, and by other phosphatases such as SHIP (Src-homology 2 domain containing inositol polyphosphate 5-phosphatase).23
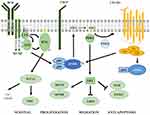 |
Figure 2 PI3K signaling in chronic lymphocytic leukemia. |
Since BCR signaling is a key pathway for the pathogenesis of B cell lymphoproliferative diseases, molecules of signalosome have been and are being extensively studied in this context, including PI3K and the downstream pathway components (Figure 2). For instance, in high-grade B-cell lymphoma with low baseline NF-κB and PTEN mutations, inhibition of BCR signaling is able to modulate the PI3K/AKT pathway.31,32 In acute lymphoblastic leukemia, the PI3K/AKT pathway is involved in adhesion-mediated survival of leukemic cells.33 Moreover, PI3K has been successfully targeted in indolent B-cell Lymphomas (i.e. follicular lymphoma and CLL) and promising results are emerging also for T-cell Lymphomas.34
Being PI3K an essential protein in the propagation of BCR signaling, the rationale for using PI3K inhibitors in B-cell lymphoproliferative diseases rests on the ability to inhibit apoptosis of tumor cells which strongly rely on BCR signaling survival. Although this mechanism is partially mediated by the down-regulation of AKT,35 tumor microenvironment disruption is also involved.36 Indeed, PI3K inhibitors have been shown to reduce cell responsiveness to signaling mediated by CXCL12 and CXCL13 and to decrease the secretion of other chemo/cytokines essential for B cell survival in CLL.37,38 An increased activity of PI3K in CLL has been documented with implication in CD40-CD40L, BAFF and BCR pathways.36,39 Furthermore, in freshly isolated CLL cells, PI3K has been shown to be constitutively activated as well as PKCδ that is linked to PI3K itself and is phosphorylated at Thr505 in response to PI3K activation. Tyrosine phosphorylation and activity of PKCδ were dependent on PI3K activity in CLL cells suggesting that PI3K survival signals might be mediated via PKCδ.40,41 PI3K is important also in the CLL-microenvironment cross-talk, as shown by the dependence of CXCL12-CXCR4 mediated adhesion and migration on PI3K activation.42,43 The Raf/MEK/ERK pathway enhances growth, survival, and metabolism of cancer cells as well as the PI3K/AKT/mTOR pathway; these two signaling cascades both “originate” from RAS and functionally interact with each other to regulate both physiological cell fate and tumorigenesis. The latter is the case of CLL where heat shock factor 1 (HSF1) is overexpressed and upregulates the transcription of heat shock protein 70 (HSP70). HSF1 is fine-tuned by kinases that participate in signaling pathways inhibited by RAS (i.e. Raf/MEK/ERK and PI3K/AKT/mTOR).41,44 We found that patients showing low levels of HSP70 also displayed high activation of MEK1/2 and ERK1/2, known to negatively regulate HSF1. By contrast, patients displaying high levels of HSP70 also expressed high AKT-Ser473, thus activating HSF1. Furthermore, treatment of CLL cells with the PI3K inhibitor Idelalisib, which functionally downregulates AKT, reduced the expression of both HSF1 and HSP70, demonstrating a role of the PI3K/AKT pathway in the upregulation of HSF1/HSP70 axis.41 In addition, it has been demonstrated that CK2, a pleiotropic serine-threonine kinase overexpressed in CLL, can phosphorylate both AKT on Ser129, activating it, and PTEN on Ser380/Thr382/Ser385, inhibiting PTEN and boosting AKT signaling.45 Interestingly, it has been documented that in resident tissue macrophages, where PI3K contributes to FcγR signaling, PI3K catalytic subunit p110δ is essential for CLL-derived macrophages to respond to therapeutic antibodies, conversely, its inhibition reduces FcγR-mediated antibody immune responses.46
The pivotal role of PI3K summarized above accounts for the high efficacy of first and second generation of PI3Ki in hematological malignancies, in particular in CLL.
Second-Generation PI3K Inhibitors
The first in class PI3Ki, idelalisib, has proved to be active in heavily treated patients in clinical trials. However, idelalisib efficacy was paralleled by an unfavorable toxicity profile including several infections, transaminitis, colitis and pneumonitis. These idelalisib-induced adverse events are difficult to manage and justify the efforts to identify more active and safer next-generation PI3Ki.
Duvelisib
Duvelisib is the first approved dual PI3Kδ and γ inhibitor for the treatment of relapsed/refractory CLL after at least 2 previous lines of therapy (Figure 1). Whereas, duvelisib-mediated PI3Kδ inhibition blocks cancer cell survival pathways downstream of the BCR, the targeting of PI3Kγ seems to inactivate the immune cells within the tumor microenvironment, where this isoform is more commonly expressed, such as T lymphocytes and macrophages, which are known to aid CLL cells in proliferation and migration.47 In preclinical models, the combined inhibition of PI3Kδ and γ has shown to cause a more pronounced lymphoma cell death as compared to idelalisib, which targets only the PI3Kδ isoform.48 Duvelisib was evaluated in Phase I clinical trial in 210 patients with hematologic malignancies such as CLL, indolent non-Hodgkin lymphoma (NHL) and T-NHL.49 Pharmacodynamic activity, measured as reduction of PI3K-dependent AKT phosphorylation, was found at a dose of 25mg twice daily. This dose was recommended for further clinical studies. Toxicities were similar to those of idelalisib such as neutropenia (grade ≥3 20%), transaminitis (grade ≥3 19.5%), late-onset diarrhea/colitis (grade ≥3 11%/6%), infections (grade ≥3 10%, including 3 patients with Pneumocystis jirovecii pneumonia and 2 systemic CMV infections) and interstitial pneumonitis (4%).49 Efficacy was promising with an overall response rate of 56% in relapsed CLL (n = 55) and 83% in treatment-naive CLL (n = 18) patients.49
The Phase 3 DUO clinical trial further compared duvelisib versus ofatumumab monotherapy, enrolled 319 patients with relapsed CLL.50,51 Response rates were 74% (only 1 complete remission) and 45% for duvelisib and ofatumumab, respectively.51 After a median follow-up of almost 2 years, the median progression-free survival (PFS) was 13 months and 10 months for duvelisib and ofatumumab (p<0.0001), respectively.51 PFS with duvelisib as compared with ofatumumab was also improved in patients harboring high-risk biological features, like TP53 disruptions. As expected from Phase 1 study, infections and immune-related events were recurrent severe colitis being recorded in 12% of patients, transaminitis and pneumonitis in 3% of study population. No death occurred due to immune-mediated adverse events. However, 35% of patients discontinued treatment due to adverse events.51 Cross over to duvelisib was allowed to patients who progressed after ofatumumab.50 Ninety patients were treated with ofatumumab, had a response rate of 29% and the median PFS was only 9 months. After crossover, 69/90 (77%) achieved a response and the median PFS improved to almost 16 months.50 Notably, 73% of patients with disease previously refractory to ofatumumab achieved a response.
Updated results of DUO trial were presented at the 2019 American Society of Hematology congress.52 Authors showed that second-line CLL patients characterized by chromosome del11q22–23 (11q-) or unmutated status of IGHV gene demonstrated extended PFS and a higher response rate with duvelisib vs ofatumumab. In particular, among 11q- patients the median PFS was 25 and 9 months for duvelisib and ofatumumab, respectively. Duvelisib also demonstrated a favorable overall response rate compared with ofatumumab in patients with 11q- (83% vs 56%) and unmutated status of IGHV gene (66% vs 50%). Conversely, IGHV mutated patients had a higher rate of discontinuation due to immune-related events in the second-line setting.52
These results supported new clinical trial of duvelisib in combination with the BCL2 inhibitor venetoclax (NCT03534323) and intermittent dosing posology (NCT03961672). Preliminary results on the phase I trial of duvelisib and venetoclax ramp-up starting from day 8 in patients with R/R CLL were reported.52 Eight of the 9 patients achieved a response, including 3 complete remissions and 2 undetectable minimal residual diseases. Three patients discontinued therapy, one proceeded to allogeneic stem cell transplantation and 2 disease progressions. No laboratory or clinical tumor lysis syndrome was observed in the study.
Umbralisib
Umbralisib (Figure 1) is a highly selective PI3Kδ inhibitor.48 In addition, umbralisib inhibits casein kinase-1ε (CK1ε) which is involved in the translation of the c-Myc oncogene,53 and in the regulation of the Wnt5a pathway,54 a known actor of PI3K-induced colitis.55 Notably, duvelisib and idelalisib but not umbralisib caused drug-induced colitis in mouse model.47
The phase 1 study enrolled 90 patients with NHL and relapsed CLL and found that the recommended Phase 2 dose was 800mg once daily.56 The most common grade ≥3 adverse events were cytopenia (29%), mainly neutropenia in 13% of patients.56 Grade 3 or higher immune-mediated colitis was observed in only 2 cases treated with >800mg of umbralisib, and grade ≥3 transaminitis occurred in other 2 patients. Pneumocystis prophylaxis was applied to only 20% of patients, but fortunately, no cases of Pneumocystis pneumonia occurred.56 Accordingly, only 7% of patients discontinued umbralisib due to adverse events. Seventeen (85%) out of 20 relapsed CLL patients achieved an objective response, including 7 partial response with lymphocytosis. Umbralisib demonstrated similar efficacy in patients harboring high-risk cytogenetic features.56
The favorable safety profile allows to combine umbralisib with other agents such as ibrutinib (n=21 relapsed CLL, overall response rate was 90%)57 and ibrutinib in combination with the novel anti-CD20 monoclonal antibody ublituximab (n=23 relapsed CLL, all patients responded and 36% achieved complete remission).58 Combinations were safe and adverse events occurred as expected, including a higher rate of diarrhea and atrial fibrillation with ibrutinib57 and infusion-related reaction in the triple combination.58 Remarkably, the incidence of immune-mediated toxicities was low in both studies.
Dezapelisib
Dezapelisib (Figure 1), previously known as INCB040093, is a selective PI3Kδ inhibitor which has been tested alone or in combination with itacitinib, a JAK1 inhibitor, in a phase 1 clinical trial in relapsed patients with B-cell lymphomas.59 Out of the 114 patients enrolled in this study and treated with monotherapy (n=49), combination therapy (n=72), or crossed over from monotherapy to combination (n=7), 12 with R/R CLL underwent monotherapy and only one with the combination treatments.59 The recommended phase 2 dose of dezapelisib was 100mg twice daily. Most common serious adverse events with monotherapy were neutropenia (18%), and pneumonias (10%), which were similar to those presenting with the combination, that is neutropenia (24%), pneumonias (14%, including 5 cases of pneumocystis pneumonia), pyrexia (7%) the most prominent. Remarkably, grade ≥3 transaminase elevations were less common when used in the combination (3% vs 20%). Although dezapelisib seems to be very active in lymphomas, only half of CLL patients responded and all with partial response.59
Parsaclib
Parsaclib (Figure 1), also known as INCB050465, is a highly selective PI3Kδ inhibitor, which was studied in patients with B-cell malignancies. Parsaclib activity was assessed alone or in combination with a JAK1 inhibitor or chemotherapy in a phase 1 study.60 Again, treatment-related adverse events were similar to idelalisib and duvelisib but less transaminitis occurred. Since parsaclib discontinuation due to adverse events was recorded in almost one third of patients, an intermittent dosing schedule was identified according to pharmacodynamic data. After 9 weeks of parsaclib continuous therapy, an alternately weekly schedule began.60 This alternate on-off therapy allowed to achieve clinical response before severe toxicities set in. Due to this schedule, no patient had to discontinue treatment because of treatment-related adverse events and several of them continued parsaclib. Although parsaclib was active in B-cell indolent NHL, only 2 out of the 6 CLL treated patients achieved a response.60
Copanlisib
Copanlisib is a pan-class I PI3K inhibitor administrated intravenously and selectively inhibiting the p110α and δ isoforms (Figure 1). Copanlisib is approved for the treatment of relapsed follicular lymphoma in the United States. Immune-related events have been uncommon with copanlisib than with idelalisib and duvelisib unlike hyperglycemia (grade ≥3 30%) and hypertension (grade ≥3 14%) were common.61 Only 13 CLL patients were treated with copanlisib in clinical trials and the response rate was 38%. For these reasons, trials on CLL were stopped. Interestingly, a trial investigating copanlisib plus nivolumab in patients with Richter syndrome is still ongoing (NCT03884998).
Acalisib
Acalisib (GS-9820) is another selective PI3Kδ inhibitor (Figure 1) showing promising clinical activity in phase 1 trials including patients with relapsed CLL or B-cell NHL.62 Despite treatment-related adverse events were as expected, including diarrhea, elevated liver transaminases, rash, and infections, a very high response rate was observed, being 95% in CLL but 29% in NHL.62
ME-401
ME-401 is a PI3Kδ inhibitor, which binds longer and more tightly to its target site than idelalisib63 (Figure 1). ME-401 is currently under investigation in phase 1b in R/R CLL and NHL.48 Similar to parsaclib, intermittent dosing was introduced after an initial continuous treatment phase. In a preliminary report, grade ≥3 immune-related events were clearly reduced during the intermittent schedule.48
Conclusions
During the last 10 years a better understanding of CLL biology has fostered the development of glycoengineered anti-CD20 monoclonal antibodies, inhibitors of kinases downstream the BCR and BCL-2 inhibitors, which have been introduced in the treatment landscape of CLL. These targeted agents represent highly effective treatments with an acceptable toxicity profile and show a good response when administrated in heavily treated patients. In addition, BCR inhibitors have been shown to be highly active in the subset of patients at higher risk of early chemoimmunotherapy failure, due to the unmutated status of IGHV gene and/or TP53 gene disruption. In particular, PI3Ki are a class of promising agents in CLL, whose efficacy was balanced by toxicities which required an optimal management.
Idelalisib, the first PI3Ki to receive global approval, has been associated with different – sometime severe – toxicities including colitis, transaminitis and pneumonitis accounting for a considerable treatment discontinuation.64,65 PI3K is critical for the development and function of regulatory T cells (Treg). A decrease in Treg cells associated with autoimmunity disorders has been observed in mice lacking PI3K function in their T cells.66 These adverse events were more common in young and treatment-naive patients, likely with a better T-cell function. Although prophylaxis, careful monitoring and prompt steroid treatment can prevent severe events, disappointing trials showed that toxicities were more common and severe in combination regimens67 and in treatment-naive patients68 precluding further studies.
For these reasons, researchers focused on investigating novel PI3Ki by targeting different subunit and on alternative treatment modalities, aiming to improve patients’ tolerability with sustained efficacy.
Duvelisib, an oral PI3Ki targeting both δ and γ subunits, is the only second-generation PI3Ki approved for the treatment of relapsed CLL.51 However, similar to idelalisib all responses were partial. In addition, immune-related adverse events and infection were a concern also for duvelisib even if slightly lower compared with idelalisib.50,51 Remarkably no fatal adverse event occurred using the trial-defined management of toxicities, such as pneumocystis prophylaxis with trimethoprim-sulfamethoxazole or pentamidine, duvelisib interruption and prompt steroid therapy for immune-mediated events.57 Based on the efficacy and the manageable toxicity profile of duvelisib, a combination study with venetoclax was initiated (NCT03534323) as well as a study investigating intermittent dosing (NCT03961672), in order to decrease the rate of immune-mediated adverse events.
Among other under-investigation drugs, umbralisib seems to be the most promising one, due to the different chemical structure and good safety profile.56 Umbralisib, a dual PI3Kδ and CK1ε inhibitor, was highly active in early phase 1 clinical trial in CLL, associated with a low rate of immune-related adverse events as well as low discontinuation rate.56 Based on efficacy and safety data, also umbralisib has been combined with other targeted agents with encouraging results.57,58
In light of the above next-generation PI3Ki either as monotherapy or in combination with further targeted agents represent a promising weapon in a hopefully rapidly improving CLL treatment landscape. In particular, they could be an additional option for patients failing therapy with agents directed against the BCR complex or BCL2 inhibitors.
Acknowledgments
We would like to acknowledge our nursing team who significantly help in the management of patients treated with PI3K inhibitors.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by funds from Gilead fellowship program 2017 and 2018 to LT, Gilead fellowship program 2019 to FP, Ricerca per Credere nella Vita (RCV) ODV. GS received research funding from Associazione Italiana Ricerca sul Cancro (AIRC). PRIN (Progetti di rilevante interesse nazionale)-MIUR Prot. 2017ZXT5WR to SM.
Disclosure
LT and FP won Italian Gilead fellowship program. LT reports grants, personal fees from Janssen, personal fees from Abbvie, grants from Gilead, during the conduct of the study. Other authors declare no conflict of interest with the writing of the current manuscript. The authors report no other potential conflicts of interest for this work.
References
1. Rathinaswamy MK, Burke JE. Class I phosphoinositide 3-kinase (PI3K) regulatory subunits and their roles in signaling and disease. Adv Biol Regul. 2020;75:100657. doi:10.1016/j.jbior.2019.100657
2. Bilanges B, Posor Y, Vanhaesebroeck B. PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol. 2019;20(9):515–534. doi:10.1038/s41580-019-0129-z
3. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156.
4. Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94(11):1266–1287. doi:10.1002/ajh.25595
5. Visentin A, Bonaldi L, Rigolin GM, et al. The combination of complex karyotype subtypes and IGHV mutational status identifies new prognostic and predictive groups in chronic lymphocytic leukaemia. Br J Cancer. 2019;121(2):150–156. doi:10.1038/s41416-019-0502-x
6. Rigolin GM, Saccenti E, Guardalben E, et al. In chronic lymphocytic leukaemia with complex karyotype, major structural abnormalities identify a subset of patients with inferior outcome and distinct biological characteristics. Br J Haematol. 2018;181(2):229–233. doi:10.1111/bjh.15174
7. Visentin A, Facco M, Gurrieri C, et al. Prognostic and predictive effect of IGHV mutational status and load in chronic lymphocytic leukemia: focus on FCR and BR treatments. Clin Lymphoma Myeloma Leuk. 2019;19(10):678–685 e674. doi:10.1016/j.clml.2019.03.002
8. Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi:10.1016/j.gene.2019.02.076
9. Castellano E, Downward J. RAS Interaction with PI3K: more than just another effector pathway. Genes Cancer. 2011;2(3):261–274. doi:10.1177/1947601911408079
10. Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15(1):7–24. doi:10.1038/nrc3860
11. Burke JE, Williams RL. Synergy in activating class I PI3Ks. Trends Biochem Sci. 2015;40(2):88–100. doi:10.1016/j.tibs.2014.12.003
12. Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3ʹ-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18(3):1379–1387.
13. Carpenter CL, Auger KR, Chanudhuri M, et al. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993;268(13):9478–9483.
14. Canovas Nunes S, Manzoni M, Pizzi M, et al. The small GTPase RhoU lays downstream of JAK/STAT signaling and mediates cell migration in multiple myeloma. Blood Cancer J. 2018;8(2):20. doi:10.1038/s41408-018-0053-z
15. Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275(5298):394–397. doi:10.1126/science.275.5298.394
16. Lee JY, Chiu YH, Asara J, Cantley LC. Inhibition of PI3K binding to activators by serine phosphorylation of PI3K regulatory subunit p85alpha Src homology-2 domains. Proc Natl Acad Sci U S A. 2011;108(34):14157–14162. doi:10.1073/pnas.1107747108
17. Comb WC, Hutti JE, Cogswell P, Cantley LC, Baldwin AS. p85alpha SH2 domain phosphorylation by IKK promotes feedback inhibition of PI3K and Akt in response to cellular starvation. Mol Cell. 2012;45(6):719–730.
18. Dhand R, Hara K, Hiles I, et al. PI 3-kinase: structural and functional analysis of intersubunit interactions. EMBO J. 1994;13(3):511–521. doi:10.1002/j.1460-2075.1994.tb06289.x
19. Dhand R, Hiles I, Panayotou G, et al. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994;13(3):522–533.
20. Cuevas BD, Lu Y, Mao M, et al. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem. 2001;276(29):27455–27461. doi:10.1074/jbc.M100556200
21. von Willebrand M, Williams S, Saxena M, et al. Modification of phosphatidylinositol 3-kinase SH2 domain binding properties by Abl- or Lck-mediated tyrosine phosphorylation at Tyr-688. J Biol Chem. 1998;273(7):3994–4000. doi:10.1074/jbc.273.7.3994
22. Pagano MA, Tibaldi E, Molino P, et al. Mitochondrial apoptosis is induced by alkoxy phenyl-1-propanone derivatives through PP2A-mediated dephosphorylation of bad and Foxo3A in CLL. Leukemia. 2019;33(5):1148–1160. doi:10.1038/s41375-018-0288-5
23. Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–3060. doi:10.1242/dev.137075
24. Piazza F, Manni S, Arjomand A, Visentin A, Trentin L, Semenzato G. New responsibilities for aged kinases in B-lymphomas. Hematol Oncol. 2020;38(1):3–11. doi:10.1002/hon.2694
25. Pleiman CM, Hertz WM, Cambier JC. Activation of phosphatidylinositol-3ʹ kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994;263(5153):1609–1612. doi:10.1126/science.8128248
26. Severin F, Frezzato F, Visentin A, et al. In chronic lymphocytic leukemia the JAK2/STAT3 pathway is constitutively activated and its inhibition leads to CLL cell death unaffected by the protective bone marrow microenvironment. Cancers (Basel). 2019;11(12):1939.
27. Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem. 1999;274(46):32662–32666. doi:10.1074/jbc.274.46.32662
28. Martini V, Frezzato F, Severin F, et al. Abnormal regulation of BCR signalling by c-Cbl in chronic lymphocytic leukaemia. Oncotarget. 2018;9(63):32219–32231. doi:10.18632/oncotarget.25951
29. Buhl AM, Cambier JC. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of bruton’s tyrosine kinase. J Immunol. 1999;162(8):4438–4446.
30. Astoul E, Watton S, Cantrell D. The dynamics of protein kinase B regulation during B cell antigen receptor engagement. J Cell Biol. 1999;145(7):1511–1520. doi:10.1083/jcb.145.7.1511
31. Chen L, Ouyang J, Wienand K, et al. CXCR4 upregulation is an indicator of sensitivity to B-cell receptor/PI3K blockade and a potential resistance mechanism in B-cell receptor-dependent diffuse large B-cell lymphomas. Haematologica. 2020;105(5):1361–1368. doi:10.3324/haematol.2019.216218
32. Mandato E, Nunes SC, Zaffino F, et al. CX-4945, a selective inhibitor of casein kinase 2, synergizes with B cell receptor signaling inhibitors in inducing diffuse large B cell lymphoma cell death. Curr Cancer Drug Targets. 2018;18(6):608–616. doi:10.2174/1568009617666170427110450
33. Sanchez VE, Nichols C, Kim HN, Gang EJ, Kim Y-M. Targeting PI3K signaling in acute lymphoblastic leukemia. Int J Mol Sci. 2019;20(2):412. doi:10.3390/ijms20020412
34. von Keudell G, Moskowitz AJ. The role of PI3K inhibition in lymphoid malignancies. Curr Hematol Malig Rep. 2019;14(5):405–413. doi:10.1007/s11899-019-00540-w
35. Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594.
36. Burger JA, Wiestner A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer. 2018;18(3):148–167. doi:10.1038/nrc.2017.121
37. Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3ʹ-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi:10.1182/blood-2011-05-352492
38. Trentin L, Cabrelle A, Facco M, et al. Homeostatic chemokines drive migration of malignant B cells in patients with non-hodgkin lymphomas. Blood. 2004;104(2):502–508. doi:10.1182/blood-2003-09-3103
39. Patrussi L, Capitani N, Cattaneo F, et al. p66Shc deficiency enhances CXCR4 and CCR7 recycling in CLL B cells by facilitating their dephosphorylation-dependent release from beta-arrestin at early endosomes. Oncogene. 2018;37(11):1534–1550.
40. Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100(10):3741–3748. doi:10.1182/blood-2002-02-0539
41. Frezzato F, Accordi B, Trimarco V, et al. Profiling B cell chronic lymphocytic leukemia by reverse phase protein array: focus on apoptotic proteins. J Leukoc Biol. 2016;100(5):1061–1070. doi:10.1189/jlb.2AB0715-301R
42. Niedermeier M, Hennessy BT, Knight ZA, et al. Isoform-selective phosphoinositide 3ʹ-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009;113(22):5549–5557. doi:10.1182/blood-2008-06-165068
43. Trimarco V, Ave E, Facco M, et al. Cross-talk between chronic lymphocytic leukemia (CLL) tumor B cells and mesenchymal stromal cells (MSCs): implications for neoplastic cell survival. Oncotarget. 2015;6(39):42130–42149. doi:10.18632/oncotarget.6239
44. Frezzato F, Raggi F, Martini V, et al. HSP70/HSF1 axis, regulated via a PI3K/AKT pathway, is a druggable target in chronic lymphocytic leukemia. Int J Cancer. 2019;145(11):3089–3100. doi:10.1002/ijc.32383
45. Piazza F, Manni S, Ruzzene M, Pinna LA, Gurrieri C, Semenzato G. Protein kinase CK2 in hematologic malignancies: reliance on a pivotal cell survival regulator by oncogenic signaling pathways. Leukemia. 2012;26(6):1174–1179. doi:10.1038/leu.2011.385
46. Enya Chen YC, Burgess M, Mapp S, et al. PI3K-p110delta contributes to antibody responses by macrophages in chronic lymphocytic leukemia. Leukemia. 2020;34(2):451–461. doi:10.1038/s41375-019-0556-z
47. Balakrishnan K, Peluso M, Fu M, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29(9):1811–1822. doi:10.1038/leu.2015.105
48. Kienle DL, Stilgenbauer S. Approved and emerging PI3K inhibitors for the treatment of chronic lymphocytic leukemia and non-hodgkin lymphoma. Expert Opin Pharmacother. 2020;21(8):917–929. doi:10.1080/14656566.2020.1737010
49. Flinn IW, O’Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-delta, gamma, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877–887. doi:10.1182/blood-2017-05-786566
50. Davids MS, Kuss BJ, Hillmen P, et al. Efficacy and safety of duvelisib following disease progression on ofatumumab in patients with relapsed/refractory CLL or SLL in the DUO Crossover Extension Study. Clin Cancer Res. 2020;26(9):2096–2103. doi:10.1158/1078-0432.CCR-19-3061
51. Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–2455. doi:10.1182/blood-2018-05-850461
52. Frustaci AM, Tedeschi A, Deodato M, Zamprogna G, Cairoli R, Montillo M. Duvelisib: a new phosphoinositide-3-kinase inhibitor in chronic lymphocytic leukemia. Future Oncol. 2019;15(19):2227–2239. doi:10.2217/fon-2018-0881
53. Deng C, Lipstein MR, Scotto L, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kdelta and CK1epsilon in hematological malignancies. Blood. 2017;129(1):88–99. doi:10.1182/blood-2016-08-731240
54. Bryja V, Schulte G, Rawal N, Grahn A, Arenas E. Wnt-5a induces dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J Cell Sci. 2007;120(Pt 4):586–595. doi:10.1242/jcs.03368
55. Sato A, Kayama H, Shojima K, et al. The Wnt5a-Ror2 axis promotes the signaling circuit between interleukin-12 and interferon-gamma in colitis. Sci Rep. 2015;5:10536. doi:10.1038/srep10536
56. Burris HA
57. Davids MS, Kim HT, Nicotra A, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1–1b study. Lancet Haematol. 2019;6(1):e38–e47. doi:10.1016/S2352-3026(18)30196-0
58. Nastoupil LJ, Lunning MA, Vose JM, et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol. 2019;6(2):e100–e109.
59. Phillips TJ, Forero-Torres A, Sher T, et al. Phase 1 study of the PI3Kdelta inhibitor INCB040093 ± JAK1 inhibitor itacitinib in relapsed/refractory B-cell lymphoma. Blood. 2018;132(3):293–306. doi:10.1182/blood-2017-10-812701
60. Forero-Torres A, Ramchandren R, Yacoub A, et al. Parsaclisib, a potent and highly selective PI3Kdelta inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood. 2019;133(16):1742–1752. doi:10.1182/blood-2018-08-867499
61. Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905. doi:10.1200/JCO.2017.75.4648
62. Kater AP, Tonino SH, Spiering M, et al. Final results of a phase 1b study of the safety and efficacy of the PI3Kdelta inhibitor acalisib (GS-9820) in relapsed/refractory lymphoid malignancies. Blood Cancer J. 2018;8(2):16. doi:10.1038/s41408-018-0055-x
63. Moreno O, Butler T, Zann V, Willson A, Leung P, Connor A. Safety, pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110delta, following single ascending dose administration to healthy volunteers. Clin Ther. 2018;40(11):1855–1867. doi:10.1016/j.clinthera.2018.09.006
64. Cuneo A, Barosi G, Danesi R, et al. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: a multidisciplinary position paper. Hematol Oncol. 2019;37(1):3–14. doi:10.1002/hon.2540
65. Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, Phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391–1402. doi:10.1200/JCO.18.01460
66. Hanna BS, Roessner PM, Scheffold A, et al. PI3Kdelta inhibition modulates regulatory and effector T-cell differentiation and function in chronic lymphocytic leukemia. Leukemia. 2019;33(6):1427–1438. doi:10.1038/s41375-018-0318-3
67. Danilov AV, Herbaux C, Walter HS, et al. Phase Ib study of tirabrutinib in combination with idelalisib or entospletinib in previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2020;26(12):2810–2818. doi:10.1158/1078-0432.CCR-19-3504
68. O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–2694. doi:10.1182/blood-2015-03-630947
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
