Back to Journals » International Journal of Nanomedicine » Volume 12
Lidocaine/ketorolac-loaded biodegradable nanofibrous anti-adhesive membranes that offer sustained pain relief for surgical wounds
Authors Kao CW, Lee D, Wu MH, Chen JK, He HL, Liu SJ
Received 1 May 2017
Accepted for publication 6 July 2017
Published 16 August 2017 Volume 2017:12 Pages 5893—5901
DOI https://doi.org/10.2147/IJN.S140825
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Ching-Wei Kao,1,2 Demei Lee,2 Min-Hsuan Wu,2 Jan-Kan Chen,3 Hong-Lin He,4 Shih-Jung Liu2,5
1Department of Anesthesiology, Chiayi Chang Gung Memorial Hospital, Chiayi, 2Department of Mechanical Engineering, 3Department of Physiology and Pharmacology, Chang Gung University, Taoyuan, 4Department of Pathology, E-DA Hospital, I-Shou University, Kaohsiung, 5Department of Orthopedic Surgery, Chang Gung Memorial Hospital, Taoyuan, Taiwan
Abstract: The aim of this study was to develop and evaluate the effectiveness of biodegradable nanofibrous lidocaine/ketorolac-loaded anti-adhesion membranes to sustainably release analgesics on abdominal surgical wounds. The analgesic-eluting membranes with two polymer-to-drug ratios (6:1 and 4:1) were produced via an electrospinning technique. A high-performance liquid chromatography (HPLC) assay was employed to characterize the in vivo and in vitro release behaviors of the pharmaceuticals from the membranes. It was found that all biodegradable anti-adhesion nanofibers released effective concentrations of lidocaine and ketorolac for over 20 days post surgery. In addition, a transverse laparotomy was setup in a rat model for an in vivo assessment of activity of postoperative recovery. No tissue adhesion was observed at 2 weeks post surgery, demonstrating the potential anti-adhesion capability of the drug-eluting nanofibrous membrane. The postoperative activities were recorded for two groups of rats as follows: rats that did not have any membrane implanted (group A) and rats that had the analgesic-eluting membrane implanted (group B). Rats in group B exhibited faster recovery times than those in group A with regard to postoperative activities, confirming the pain relief effectiveness of the lidocaine- and ketorolac-loaded nanofibrous membranes. The experimental results suggested that the anti-adhesion nanofibrous membranes with sustainable elution of lidocaine and ketorolac are adequately effective and durable for the purposes of postoperative pain relief in rats.
Keywords: biodegradable nanofiber, anti-adhesive membrane, sustainable release, lidocaine, ketorolac
Introduction
Most patients who undergo surgeries experience acute postoperative pain. However, evidence suggests that fewer than half of the patients achieve adequate or satisfactory postoperative pain relief.1 Postoperative pain, especially when poorly managed, leads to harmful acute effects (namely, adverse physiological responses) and increases the risk of postoperative complications and persistent postoperative pain. Persistent postoperative pain is an intricate response to tissue trauma during surgery that stimulates hypersensitivity of the central nervous system, which causes pain in areas not directly affected by the operative procedure. Either acute or chronic postoperative pain can raise the possibility of postoperative complications, increase the cost of medical care, and make wound recovery and subsequent return to normal activities difficult. Pain control following a surgery is a major priority for both patients and surgeons.
Several preoperative, intraoperative, and postoperative interventions and management strategies are available for reducing and managing postoperative pain. Opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used for the management of postoperative pain.2 When given systemically, however, these drugs cause side effects including nausea, vomiting, dizziness, sedation, and pruritus;3 specifically opioids can cause respiratory depression and NSAIDs can cause impairment of renal function.4 An effective method for the management of postoperative pain after lower abdominal or lower extremity surgery is using epidural analgesia with local anesthetics, but it is a highly technique-dependent and invasive procedure.5
Adhesion, on the other hand, is a band of scar tissue that binds two parts of tissues or organs together.6 Adhesions form when the body’s repair mechanisms respond to any tissue disturbances, such as surgery, infection, trauma, or radiation. The most common locations where adhesions occur are within the abdomen, pelvis, and heart. Adhesions can result in a complex set of problems, namely adhesion-related disorder (ARD).7 Some commercial products have been approved for use as an adhesion barrier after abdominal or pelvic surgery.8 The barrier sticks to the tissues to which it is applied and is slowly absorbed into the body over a period of few days.
There have been different theories on the relationship between adhesion formation and operative pain. Unfortunately, the association between chronic pain and adhesion remains unproven, and many researchers have challenged the accepted notion that adhesions cause pain.9,10 In this study, we hypothesized that a biodegradable drug-eluting membrane can act as an anti-adhesive film that temporarily separates the internal tissues and organs as they heal, while also providing a sustainable release of analgesic drugs around the wound site to control postoperative pain. Currently, various polymeric materials have been investigated and tested as potential anti-adhesion barrier films, including nanosheets,11 polyvinyl alcohol (PVA)/gelatin membranes,12 polycaprolactone film,13 photocrosslinkable gellan gum film,14 silver nanoparticles-loaded poly(L-lactide) fibrous membranes,15 chitosan–polyvinyl pyrrolidone film,16 and immobilized liquid layers.17 Despite these efforts, the development of drug-loaded anti-adhesion film has been limited. Chen et al18 were the only research team to propose a silver nanoparticle/ibuprofen-loaded poly(L-lactide) fibrous membrane and evaluate its anti-infection and anti-adhesion effects.
This study’s purpose was to develop a biodegradable lidocaine- and ketorolac-loaded nanofibrous anti-adhesive membrane that could provide a sustainable release of analgesics for surgical wounds. Lidocaine hydrochloride has been widely used as a local anesthetic,19 while ketorolac is an NSAID in the family of heterocyclic acetic acid derivatives that is used as an analgesic.20 The in vitro and in vivo release characteristics of the pharmaceuticals from the prepared membranes were investigated. Furthermore, the effectiveness and safety of the drug-loaded membranes were evaluated in rats that underwent abdominal surgeries. Histological examination of epithelialization and granulation on the wounds was also completed.
Materials and methods
In vitro release of lidocaine and ketorolac
Preparation of drug-eluting poly[(D,L)-lactide-co-glycolide] (PLGA) nanofibers
Multilayered drug-loaded PLGA membranes were prepared using an electrospinning setup. The materials used included PLGA 50:50 polymer (Resomer RG 503; Boehringer Ingelheim, Ingelheim, Germany), lidocaine hydrochloride, and ketorolac (Sigma-Aldrich Co., St Louis, MO, USA). Membranes with two different PLGA-to-drug ratios (6:1 and 4:1) were produced. To prepare the drug-eluting membranes with 6:1 polymer-to-drug ratio, PLGA/lidocaine (240 mg: 40 mg) and PLGA/ketorolac (240 mg:40 mg) were first dissolved in 1 mL of hexafluoroisopropanol (HFIP; Sigma-Aldrich Co.). The PLGA/lidocaine solution was then conveyed and electrospun by the syringe pump into a nonwoven form of nanofibrous membrane. This was followed by the electrospinning of PLGA/ketorolac solution. The same procedures were followed to produce the membranes with 4:1 polymer-to-drug ratio, except that the polymer/drug amounts used were 224 mg:56 mg, respectively.
Characterization of electrospun nanofibrous membranes
To analyze the diameter distribution, 50 nanofibers were first randomly picked from the scanning electron microscope (SEM) images. The diameters were then measured. The tensile properties of the electrospun nanofibrous membranes were tested on a tensile tester (Lloyd, Ametek, Portsmouth, VA, USA). Meanwhile, the water contact angles were characterized using the image of a sessile drop at the points of intersection between the drop contour and the projection of the surface (First Ten Angstroms, Berwyn, PA, USA; N=3).
High-performance liquid chromatography (HPLC) assay
The release pattern of lidocaine and ketorolac from the drug-loaded nanofibrous membranes with different PLGA-to-drug ratios, ie, 6:1 and 4:1, was determined using an in vitro elution method and an HPLC assay. The lidocaine concentration analyses in the buffer were determined on a Hitachi L-2200R multi-solvent delivery system (Hitachi Ltd., Tokyo, Japan), using an Atlantis dC18, 4.6 cm ×150 mm HPLC column (Waters Corp., Milford, MA, USA). The mobile phase included 0.01 mol of ammonium formate (Sigma-Aldrich Co.) and methanol (Sigma-Aldrich Co.; 20/80 [v/v]). The absorbency was monitored by a ultraviolet light detector (Hitachi L-2400R; Hitachi Ltd.) at a wavelength of 254 nm, with the flow rate set at 1.0 mL/min. To characterize ketorolac, a Discovery C18, 5 μm, 4.6 cm ×250 mm column was employed. The mobile phase contained acetonitrile (Mallinckrodt, Inc., Surrey, UK) and 0.1% acetic acid (Sigma-Aldrich Co.) in a volume ratio of 70:30. The absorbency was monitored at 220 nm, and the flow rate was 1.0 mL/min.
Membrane cytotoxicity
Membrane cytotoxicity was characterized using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Hoffman-La Roche Ltd., Basel, Switzerland) and human fibroblasts. Cell viability was monitored at 1, 2, 3, and 7 days by MTT assays and characterized by an enzyme-linked immunosorbent assay (ELISA) detector. An optical microscope (Olympus IMT-2; Olympus Corporation, Tokyo, Japan) was employed to observe and count the cell numbers.
In vivo study of animal behavior assessment
Surgical procedure
A rat model of incisional pain was employed in this study.21,22 All animal experimental procedures received approval from the Institutional Animal Care and Use Committee of Chang Gung University, and all studied animals were cared for according to the regulations of the Ministry of Health and Welfare of Taiwan under the supervision of a licensed veterinarian. A total of 35 adult Wistar rats weighing 200–300 g each were employed for the in vivo experiments: 20 rats were used for the in vivo drug concentration studies, while the other 15 rats were enrolled for the animal activity tests (N=5). For the drug concentration tests, the 20 rats received general anesthesia induced by inhalation of isoflurane (Aesica Queenborough Ltd, Queenborough, UK) by a vaporizer in an anesthesia box (40×20×28 cm). Anesthesia was retained during the entire duration of the surgical procedures via the mask inhalation of isoflurane. A 4 cm-long scission was created at the skin covering the lower abdomen of each rat, followed by the scission of the muscle layers. After the procedures mentioned earlier, the muscle layers were first sutured with 3–0 Vicryl sutures (Johnson & Johnson, New Brunswick, NJ, USA), followed by the application of analgesic-loaded PLGA nanofibrous membranes (PLGA-to-drug ratio of 4:1, 40×20 mm in size) between the skin and muscle layers. After the implantation of nanofibers, the wound was sutured with 3–0 Vicryl sutures (Johnson & Johnson) to finish the surgery. Rats were sacrificed at 1, 3, 7, and 14 days by giving an overdose of anesthesia (over 1.2 mL/kg of body weight) for local tissue samplings. The blood specimens were also gathered via syringes through heart puncture. Drug levels in the samples were characterized by employing an HPLC analysis.
Post-surgery assessment
Another 15 rats were enrolled for the animal activity tests and were divided into three groups with five rats in each group (N=5). Group A received the surgery only (with no implantation of nanofibers), while group B received the implantation of lidocaine- and ketorolac-loaded nanofibers. The rats in group C received no surgery as a control group. After the surgeries, the general activity of each rat in all groups was evaluated by using an animal behavior cage. As shown in Figure 1, the cage had a dimension of 50×50×50 cm and was equipped with nine diffusion-scan type photoelectric switch sensors on top that possessed self-contained amplifiers (HP100-A1; Azbil Corp., Tokyo, Japan). The sensors were employed to monitor the movements of a rat within the cage. As a rat moved from one area of the cage to another, the sensor in the “approaching” area would be triggered by the activity. A microprocessor with an acquisition interface was employed to record the total number of triggers. The activity of each rat was observed and monitored for 7 days.
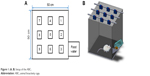  | Figure 1 (A, B) Setup of the ABC. |
Statistical analysis
Between-group differences were assessed using a least significance difference for continuous variables and chi-square test for categorical variables (activity performance score). All analyses were carried out employing SPSS software (Version 12.0; SPSS Inc., Chicago, IL, USA), with the threshold for significance set, a priori, at P<0.05.
Results
Characterization of analgesic-loaded nanofibers
Analgesic-loaded nanofibers were manufactured via the electrospinning method. Figure 2 shows the SEM photos of the lidocaine-loaded nanofibers as well as the distributions of fiber diameters. The calculated diameters were 199.4±89.4 nm and 303.8±150.5 nm, respectively, for the 4:1 and 6:1 (PLGA to lidocaine) nanofibers. In addition, ketorolac-loaded nanofibers were also successfully electrospun. The obtained fiber diameters were 329.4±282.9 nm and 249.2±106.6 nm, respectively, for the 4:1 and 6:1 (PLGA to ketorolac) nanofibers.
Figure 3 shows the Fourier transform infrared (FTIR) spectra of pure PLGA membranes versus analgesic-loaded PLGA membranes. In the spectra of the drug-loaded membrane, a new vibration peak at 3,400 cm−1 was observed. It can be attributed to the N–H bonds of lidocaine and ketorolac. On the other hand, the vibration at 1,725 cm−1 (C=O bond) was raised with the loading of the pharmaceuticals. Furthermore, the absorbance peak at around 1,300 cm−1 might be a result of the increase in C–O bonds in lidocaine and ketorolac.23,24 The FTIR spectra analysis verified that the analgesics were successfully incorporated into the PLGA membrane.
  | Figure 3 FTIR spectra of electrospun nanofibrous membranes. |
Measured water contact angles of pure PLGA, 4:1 and 6:1 PLGA:lidocaine ratio loaded nanofibers, and 4:1 and 6:1 PLGA:ketorolac ratio loaded nanofibers were 134.5°, 107.8°, 128.6°, 55.9°, and 76.4°, respectively. The presence of analgesics evidently increased the hydrophilicity of PLGA membranes. Furthermore, ketorolac-loaded membranes exhibited greater hydrophobicity than lidocaine-loaded membranes did.
The tensile test results suggested that the maximum tensile strength (and the elongation at the break) of the pure PLGA and 6:1 and 4:1 PLGA:drugs ratio loaded nanofibers were 4.62 MPa (140.7%), 3.1 MPa (25.4%), and 2.76 MPa (9.45%), respectively. Clearly, the mechanical strengths decreased with the content of incorporated drugs.
Figure 4 shows the results of cytotoxicity tests. The addition of pharmaceuticals in the membranes slightly inhibited the growth of the cells, especially in the membranes with greater drug ratios during the first 3 days. Nevertheless, all nanofibers showed no signs of cytotoxicity at day 7.
  | Figure 4 (A, B) Toxicity of electrospun drug-loaded nanofibers. *P,0.05. |
In vitro and in vivo release characteristics of lidocaine and ketorolac from the nanofibers
Figure 5A and B shows the release curves of lidocaine and ketorolac, respectively, from the nanofibrous membranes in vitro. All analgesics showed a biphasic release behavior, namely an initial burst elution at days 1–3, followed by a nearly first-order drug release. The release characteristic was comparable for the nanofibers of different drug concentrations, exhibiting a tiny standard deviation for all the curves. This indicates that the loaded lidocaine and ketorolac were uniformly embedded in the biodegradable membranes. Furthermore, all electrospun nanofibers released effective concentrations of lidocaine and ketorolac for over 2 weeks.
  | Figure 5 (A, B) In vitro release curves of analgesics from the nanofibrous membranes. |
Figure 6 shows the in vivo release curves of lidocaine and ketorolac in tissue and blood. Both drug concentrations were high in the tissue for more than 2 weeks, while they remained relatively low in the blood.
  | Figure 6 In vivo release of lidocaine and ketorolac from the nanofibrous membranes. |
Effectiveness of released drug evaluated by animal activities
The triggered counts for the three groups (A, B, and C) over the postoperative 7 days are shown in Figure 7. The recorded counts from the sensors were 10,244±1,703, 17,193±3,460, and 13,736±793, in groups A (surgery only), B (surgery followed by the deployment of drug-eluting membrane), and C (control), respectively. The number of triggered counts in group A was significantly lower than that of control in group C, demonstrating the effect of the surgical intervention on animal’s activity. Nevertheless, the number of trigger counts was superior in group B to that of group A, suggesting that the rats that had been treated with the biodegradable lidocaine/ketorolac membranes showed greater activity levels. In addition, all rats exhibited a greater number of triggers at sensor location number 3. This was mainly due to the more frequent visits around the area of food and water supply.
  | Figure 7 Measured activities of the rats. *P<0.05. |
Follow-up and histology analysis
The implanted membranes in the animals were not noticeable at 2 weeks after surgery. Furthermore, no tissue adhesion was observed (Figure 8), demonstrating the potential anti-adhesion capability of the analgesic-eluting nanofibrous membrane developed in this study.
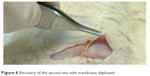  | Figure 8 Recovery of the wound site with membrane deployed. |
Figure 9 shows the histological examination of both group A (without membrane) and group B (with drug-eluting membrane). The result suggested that, at day 1 especially, the membrane in group B induced a foreign body reaction, which was characterized by infiltrates of lymphocytes and multinucleated giant cells in the dermis. The reaction then subsided in the following days.
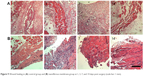  | Figure 9 Wound healing in (A) control group and (B) nanofibrous membrane group at 1, 3, 7, and 14 days post surgery (scale bar: 1 mm). |
Discussion
Pain is the body’s natural response to tissue injury, and both the injury itself and the following inflammatory reaction near the injured site contribute to pain. Uncontrolled postoperative pain may activate the sympathetic nervous system, which may increase myocardial oxygen consumption and increase the risk of myocardial ischemia or infarction. Sympathetic activation may also reduce the gastrointestinal motility, which may result in the postoperative ileus. The postoperative pain is associated with a variety of pathophysiologic responses that may be initiated or maintained by nociceptive input including neuroendocrine response and local inflammatory response. The transmission of stimuli from the periphery to the central nervous system results in the neuroendocrine stress response, which may potentiate physiologic effects in other areas of the body such as hypercoagulation, hyperglycemia, and immunosuppression. All of these effects can contribute to poor wound healing.
The local anesthetics act as the sodium channel blockers on the nerve fibers, inhibiting the conduction of nerve impulse from the target site to the central nerve. When injected into the soft tissue, such as skin or muscle, local anesthetics provide sufficient anesthesia effects that could be applied in most superficial surgical procedures. Long-acting anesthetics could also be employed for postoperative pain control because of their prolonged anesthesia effect. However, the longest duration of analgesic effect in one single injection is still limited to 4–6 hours only, and this has been believed to restrict the use of local anesthetics for pain control in major surgeries such as laparotomy. Some advanced approaches to pain management have been applied by placing a catheter into the epidural space around the spinal cord, through which the local anesthetics and other analgesic drugs could be administered by continuous infusion to achieve a sustained analgesic effect over certain areas such as the abdomen and lower extremities. These approaches, however, are highly technique dependent, and there are still risks such as misplacement of catheter into blood vessels, which leads to intravenous delivery of local anesthetics and induces disastrous level of cardiac toxicity. There are also catheter-related complications such as infection or hematoma.
The NSAIDs exert their analgesic effect through inhibition of cyclooxygenase (COX) and synthesis of prostaglandins, which are important mediators of peripheral sensitization and hyperalgesia. NSAIDs have been generally employed as a useful adjunct to opioids for moderate-to-severe pain by providing analgesic effect through mechanisms different from opioids or local anesthetics. When administered systemically, however, NSAIDs may cause several side effects including decreased hemostasis, renal dysfunction, and gastrointestinal hemorrhage and influence bone healing. Topical use of NSAIDs as pain patches has been widely used and commercially available, but most of them are used for sore muscle symptoms, not for surgical pain management.
We assume that the problems resulting from the conventional pathway of drug delivery can be diminished by the use of an analgesic-loaded PLGA nanofibrous membrane which has the capacity for sustaining a release of lidocaine and ketorolac without using catheter insertion to the nerve.25,26 PLGA has been among the most attractive polymeric candidates used to fabricate devices for various applications such as drug delivery and tissue engineering.27,28 A previous study has proposed that lidocaine-embedded PLGA nanofibers can provide sustained relief of pain after the osteosynthesis surgery of rib fractures.29 In this study, we developed lidocaine- and ketorolac-loaded PLGA nanofibers, exhibiting the capability of sustaining an elution of analgesics at the wound site, as an extended local anesthetic patch for pain relief.
One major concern with the clinical application of local anesthetics is the systemic toxicity when the drugs are absorbed into blood circulation up to a toxic level. The empirical results in this study showed the biphasic release characteristic of lidocaine/ketorolac-loaded nanofibers, exhibiting an initial burst elution at days 1–3 and a first-order drug release pattern for 3 weeks. Most importantly, the drug-loaded nanofibers could provide a sustained release of high levels of lidocaine and ketorolac for over 3 weeks postoperatively. This extended elution of therapeutic levels of analgesics could provide a particular benefit in the administration of wound healing and appropriate pain management for patients. The concentration of lidocaine is much lower in the plasma than in the local area, but the plasma concentration still exceeded the toxic concentration (ie, >7 μg/mL) for the postoperative days.30 Fortunately, no rats died during the entire study period (up to 14 days). Future study should be conducted to further identify the optimal size of the membrane to achieve effective analgesia at the target area with minimal plasma concentration.
Appetite and body weight monitoring have been widely employed as potential ways to evaluate the degree of postoperative pain and the effectiveness of analgesics. We assumed that pain can influence an animal’s moving activity and adopted the animal bioactivity cage for pain assessment. The significant differences in activity between the three groups illustrated the painful disturbance resulted from the surgeries in rats in experimental groups A and B. Furthermore, compared to the activity level measured in group A (without membrane), the raised rat activity in group B (with drug-eluting membrane) testified to the analgesic effect of lidocaine and ketorolac eluted from the drug-loaded nanofibers.
Although the current study has yielded some preliminary findings, it also has associated limitations. The first limitation is the relatively small number of study rats enrolled in this study. The second one pertains to be the relatively short period of time for animal activity monitoring (ie, 1 week). However, the experimental results show that even this short-term observation was enough to recognize significant differences in postoperative activity levels between group A (with no membrane) and group B (with drug-eluting membrane). Finally, the relevance of the findings in this study to humans with surgical pain remains unclear and needs to be further explored.
Conclusion
We have successfully developed biodegradable analgesic-loaded nanofibers and assessed their effectiveness for a sustainable elution of lidocaine and ketorolac. The experimental results showed that the analgesic-loaded nanofibers could provide appropriately sustained lidocaine and ketorolac levels at the wound site for over 3 weeks post surgery. The nanofibrous membranes also exhibited anti-adhesion characteristics. Nanofibers that have sustainable analgesic release capacity may act as an anti-adhesion membrane that provides an effectively long-term pain relief for rats with skin wounds.
Acknowledgment
This work was supported in part by the Ministry of Science and Technology, Taiwan (Contract No 104-2221-E-182-048-MY3) and the Chang Gung Memorial Hospital (Contract No CMRPD3D0153).
Disclosure
The authors report no conflicts of interest in this work.
References
Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. | ||
Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26(3):191–196. | ||
Rogers E, Mehta S, Shengelia R, Reid MC. Four strategies for managing opioid-induced side effects in older adults. Clin Geriatr. 2013;21(4). | ||
Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457–468. | ||
Cesur M, Alici HA, Erdem AF, Silbir F, Yuksek MS. Administration of local anesthetic through the epidural needle before catheter insertion improves the quality of anesthesia and reduces catheter-related complications. Anesth Analg. 2005;101(5):1501–1505. | ||
Schnuriger B, Barmparas G, Branco BC, Lustenberfer T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: a review of the literature. Amer J Surg. 2011;201(1):111–121. | ||
Wiseman DM. Disorders of adhesions or adhesion-related disorder: monolithic entities or part of something bigger – CAPPS? Seminar Reprod Med. 2008;26(4):356–368. | ||
Ntourakis D, Katsimpoulas M, Tanoglidi A, et al. Adhesions and healing of intestinal anastomoses: the effect of anti-adhesion barriers. Surg Innov. 2016;23(3):266–276. | ||
Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17(41):4545–4553. | ||
Awonuga AO, Fletcher NM, Saed GM, Diamond MP. Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod Sci. 2011;18(12):1166–1185. | ||
Niwa D, Koide M, Fujie T, Goda N, Takeoka S. Application of nanosheets as an anti-adhesion barrier in partial hepatectomy. J Biomed Mater Res B. 2013;101(7):1151–1258. | ||
Bae S-H, Son S-R, Sakar SK, et al. Evaluation of the potential anti-adhesion effect of the PVA/Gelatin membrane. J Biomed Mater Res B. 2014;102(4):840–849. | ||
Lo H-Y, Kuo H-T, Huang Y-Y. Application of polycaprolactone as an anti-adhesion biomaterials film. Artif Organs. 2010;34(8):648–653. | ||
Lee M-W, Tsai H-F, Wen S-M, Huang C-H. Photocrosslinkable gellan gum film as an anti-adhesion barrier. Carbohydr Polym. 2012;90(2):1132–1138. | ||
Liu S, Zhao J, Ruan H, et al. Antibacterial and anti-adhesion effects of the silver nanoparticles-loaded poly(L-lactide) fibrous membrane. Mater Sci Eng C. 2013;33(3):1176–1182. | ||
Lim JI, Kang MJ, Lee W-K. Lotus-leaf-like structured chitosan–polyvinyl pyrrolidone films as an anti-adhesion barrier. Appl Surf Sci. 2014;320:614–619. | ||
Sotiri I, Overton JC, Waterhouse A, Howell C. Immobilized liquid layers: a new approach to anti-adhesion surfaces for medical applications. Exp Biol Med. 2016;241(9):909–918. | ||
Chen S, Wang G, Wu T, et al. Silver nanoparticles/ibuprofen-loaded poly(L-lactide) fibrous membrane: anti-infection and anti-adhesion effects. Int J Mol Sci. 2014;15(8):14014–14025. | ||
Weibel S, Jokinen J, Pace NL, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth. 2016;116(6):770–783. | ||
Macario A, Lipman AG. Ketorolac in the era of cyclo-oxygenase-2 selective nonsteroidal anti-inflammatory drugs: a systematic review of efficacy, side effects, and regulatory issues. Pain Med. 2001;2(4):336–351. | ||
Pogatzki EM, Raja SN. A mouse model of incisional pain. Pain Med. 2003;99:1023–1027. | ||
Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–501. | ||
Fraceto LF, de Matos Alves Pinto L, Franzoni L, et al. Spectroscopic evidence for a preferential location of lidocaine inside phospholipid bilayers. Biophy Chem. 2002;99(3):229–243. | ||
Rokhade AP, Agnihotri SA, Patil SA, Malikarjuna NN, Kulkarni PV, Aminabhavi TM. Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydr Polym. 2006;65(3):243–252. | ||
Zhang J, Liu H, Ding J-X, et al. Molecular weight-modulated electrospun poly(ε-caprolactone) membranes for postoperative adhesion prevention. RSC Adv. 2014;4(79):41696–41704. | ||
Shi B, Ding J, Wei J, Fu C, Zhuang X, Chen X. Drug-incorporated electrospun fibers efficiently prevent postoperative adhesion. Curr Pharm Des. 2015;21(15):1960–1966. | ||
Ali SA, Doherty PJ, Williams DF. Mechanisms of polymer degradation in implantable devices. 2. Poly(DL-lactic acid). J Biomed Mater Res. 1993;27(11):1409–1418. | ||
Kobayashi H, Shiraki K, Ikada Y. Toxicity test of biodegradable polymers by implantation in rabbit cornea. J Biomed Mater Res. 1992;26(11):1463–1476. | ||
Yu YH, Hsu YH, Chou YC, et al. Sustained relief of pain from osteosynthesis surgery of rib fracture by using biodegradable lidocaine-eluting nanofibrous membranes. Nanomedicine. 2016;12(7):1785–1793. | ||
Bennett PN, Aarons LJ, Bending MR, Steiner JA, Rowland M. Pharmacokinetics of lidocaine and its deethylated metabolite: dose and time dependency studies in man. J Pharmacokinet Biopharm. 1982;10(3):265–281. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

