Back to Journals » OncoTargets and Therapy » Volume 15
Licorice (Glycyrrhiza glabra L.)-Derived Phytochemicals Target Multiple Signaling Pathways to Confer Oncopreventive and Oncotherapeutic Effects
Authors Tuli HS, Garg VK, Mehta JK , Kaur G, Mohapatra RK, Dhama K , Sak K , Kumar A, Varol M, Aggarwal D, Anand U, Kaur J, Gillan R , Sethi G, Bishayee A
Received 1 August 2022
Accepted for publication 18 November 2022
Published 30 November 2022 Volume 2022:15 Pages 1419—1448
DOI https://doi.org/10.2147/OTT.S366630
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Hardeep Singh Tuli,1 Vivek Kumar Garg,2 Jinit K Mehta,3 Ginpreet Kaur,3 Ranjan K Mohapatra,4 Kuldeep Dhama,5 Katrin Sak,6 Ajay Kumar,7 Mehmet Varol,8 Diwakar Aggarwal,1 Uttpal Anand,9 Jagjit Kaur,10 Ross Gillan,11 Gautam Sethi,12 Anupam Bishayee11
1Department of Biotechnology, Maharishi Markandeshwar Engineering College, Maharishi Markandeshwar (Deemed to Be University), Mullana-Ambala, Haryana, India; 2Department of Medical Lab Technology, University Institute of Applied Health Sciences, Chandigarh University, Mohali, Punjab, India; 3Department of Pharmacology, Shobhaben Pratapbhai Patel School of Pharmacy and Technology Management, Shri Vile Parle Kelavani Mandal, Narsee Monjee Institute of Management Studies, Mumbai, Maharashtra, India; 4Department of Chemistry, Government College of Engineering, Keonjhar, Odisha, India; 5Division of Pathology, Indian Council of Agricultural Research-Indian Veterinary Research Institute, Bareilly, Uttar Pradesh, India; 6NGO Praeventio, Tartu, Estonia; 7Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar, Punjab, India; 8Department of Molecular Biology and Genetics, Faculty of Science, Mugla Sitki Kocman University, Mugla, Turkey; 9Department of Life Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel; 10Centre of Excellence in Nanoscale Biophotonics, Graduate School of Biomedical Engineering, Faculty of Engineering, The University of New South Wales, Sydney, Australia; 11College of Osteopathic Medicine, Lake Erie College of Osteopathic Medicine, Bradenton, FL, USA; 12Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
Correspondence: Anupam Bishayee; Hardeep Singh Tuli, Email [email protected]; [email protected]; [email protected]
Abstract: Cancer is a highly lethal disease, and its incidence has rapidly increased worldwide over the past few decades. Although chemotherapeutics and surgery are widely used in clinical settings, they are often insufficient to provide the cure for cancer patients. Hence, more effective treatment options are highly needed. Although licorice has been used as a medicinal herb since ancient times, the knowledge about molecular mechanisms behind its diverse bioactivities is still rather new. In this review article, different anticancer properties (antiproliferative, antiangiogenic, antimetastatic, antioxidant, and anti-inflammatory effects) of various bioactive constituents of licorice (Glycyrrhiza glabra L.) are thoroughly described. Multiple licorice constituents have been shown to bind to and inhibit the activities of various cellular targets, including B-cell lymphoma 2, cyclin-dependent kinase 2, phosphatidylinositol 3-kinase, c-Jun N-terminal kinases, mammalian target of rapamycin, nuclear factor-κB, signal transducer and activator of transcription 3, vascular endothelial growth factor, and matrix metalloproteinase-3, resulting in reduced carcinogenesis in several in vitro and in vivo models with no evident toxicity. Emerging evidence is bringing forth licorice as an anticancer agent as well as bottlenecks in its potential clinical application. It is expected that overcoming toxicity-related obstacles by using novel nanotechnological methods might importantly facilitate the use of anticancer properties of licorice-derived phytochemicals in the future. Therefore, anticancer studies with licorice components must be continued. Overall, licorice could be a natural alternative to the present medication for eradicating new emergent illnesses while having just minor side effects.
Keywords: licorice, cancer, apoptosis, cell cycle, angiogenesis, treatment, nano-delivery
Corrigendum for this paper has been published.
Introduction
Since ancient times, humankind has strongly relied on medicinal plants and herbs in the treatment of diverse health conditions, including both benign neoplasms as well as malignant tumors.1–3 Moreover, according to the World Health Organization (WHO) reports, about 80% of the worldwide population still depend on plant-derived drugs today, whereas several modern medicines have been originally isolated from medicinal plants.4 It is especially the case for anticancer drugs, of which more than 60% of clinically-approved drugs are directly or indirectly derived from plant kingdom.5,6 One of the oldest and most frequently described herbs in India, China, Southern Europe is licorice (Glycyrrhiza. glabra L.), as its roots have been utilized for alleviating pain and treating gastrointestinal and respiratory symptoms already for centuries.7–9 This plant is believed to have originated in Iraq, being widespread in China, India, Iran, Afghanistan, Spain, Kazakhstan, Tajikistan, Kyrgyzstan, and Russia.8,9 The extract prepared from licorice roots is very sweet and used worldwide as a flavoring agent in tobacco products, food, cosmetics and herbal remedies, with an estimated annual consumption of about 1.5 kg/person.10 The earliest written evidence about the use of licorice date back to 2100 BC, when this plant was recommended for its health-promoting and life-enhancing properties.11 Today, we know that the roots of licorice contain more than 20 triterpenes and about 300 flavonoids, many of which have been described to exert various pharmacological effects, including different chemopreventive and anticancer bioactivities. Considering a continuous increase in the global incidence of new cancer cases,12–17 identification of novel efficient remedies to manage this dreadful disease is imperative.
Although several comprehensive review articles have been published about chemopreventive and anticancer activities of licorice and its bioactive phytocompounds in the recent years,7,9,18–22 none of them analyze anticancer properties of its structurally different constituents (eg, triterpenes glycyrrhetinic acid and glycyrrhetic acid; chalcones isoliquiritigenin, licochalcone A and licochalcone E; and isoflavone isoangustone), describing both molecular mechanisms as well as bioavailability of these bioactive components.23,24 Moreover, the present review article is focused on anticancer action of the major ingredients of licorice not only as separate agents but also in combination with approved chemotherapeutic drugs, administered as free compounds or encapsulated into nanoformulations to target the low bioavailability generally characteristic to natural substances. Therefore, this review represents an integrated contemporary overview, bringing together all the aspects we currently know about anticancer action of licorice, also providing modern solutions to the present bottlenecks associated with cancer prevention.
Literature Search Strategy and Selection Criteria
The electronic databases PubMed, Scopus, and Web of Science were searched for studies published up to 1st June, 2022, assessing the association between licorice and cancer prevention. The major key words used were licorice bioactive components and cancer cell apoptosis or cell cycle arrest or anti-inflammation or antioxidation or antiangiogenesis or antimetastasis. A manual search for additional references was also executed by referring to the reference lists of retrieved articles. The researchers completed blind double-checks, to exclude irrelevant literature by discussing with co-authors. In present review, we searched 656 articles and included 282 publications on anticancer actions of licorice constituents.
Licorice in Food and Medicine
Licorice is a sweetener that can be found in a variety of soft drinks, foods, snacks, and herbal medicines. Its sweet flavor makes it appealing to many manufacturers to mask the bitterness of many products. Licorice based snacks, Egyptian drink “erk soos”, Belgian beers, pastis brands, and anisettes are all widely consumed. Tobacco product manufacturers utilize licorice as a flavoring and sweetening agent. Herbal and licorice-flavored cough mixtures, licorice tea, throat pearls, licorice-flavored diet gum, and laxatives are all examples of health items that contain licorice.25
Licorice is also utilized in a wide range of medical conditions. Licorice extracts have been utilized as herbal treatments in China and Japan for a long time. The biggest challenge with licorice dosing is that it comes in a variety of forms, including snacks, soft drink and health supplements with varying concentration. It has been observed that production of such health supplements is not strictly controlled. European Union established a temporary upper limit of 100 mg/day for glycyrrhizin consumption (about the amount found in 60–70 g licorice).26 Based on data from studies involving human volunteers, recognized food committees confirmed a daily maximum of 100 mg in April 2003.27 Due to limited human toxicity reports food committees are unable to conclude a specific intake dose of GA and ammonium glycyrrhizinate.
Glycyrrhizin is found in roughly 10–20% of licorice fluid extracts; typical doses of 2–4 mL yield 200–800 mg. According to a study, approximately 2% of frequent users ingest more than 100 mg of glycyrrhizinic acid on a daily basis.28 Walker and Edwards29 showed in 1994 that daily oral consumption of 1–10 mg glycyrrhizin, equivalent to 1–5 g licorice, is considered a safe amount for most healthy adults.
Licorice has long been used as an antidote to counteract the toxicity of chemotherapeutic treatment. Licorice is classified as “Rasayana” in Ayurveda (Indian traditional medicine), which indicates it has nourishing, renewing, and strengthening properties. Recent research has shown its significance in a variety of biological functions in the human body, including antioxidant and anti-inflammatory properties, as well as a protective effect on several organs.30,31 Licorice’s Generally Recognized as Safe accreditation allows it to be used in a wide range of foods at usual concentrations. Licorice’s sweet flavor makes it appropriate for a variety of uses in foods, such as confectionery and sauces, with the rhizomes and roots being the most commonly utilized plant parts. For example, licorice is used in the flavoring of London drops (candy brand) and Red Vines®. Licorice powder is commonly used to add a unique flavor to sweet chilli sauce and soy sauce in condiments. Glycyrrhiza has been used to cure a variety of diseases in traditional medicine and clinical practice across cultures.32 However, according to the extraction process,33 geographical origin,34,35 drying method,36 and harvesting period,37 the observed biological activity of Glycyrrhiza can vary.
Major Bioactive Constituents of Licorice
Glycyrrhizinic Acid
The main sweet-tasting ingredient of G. glabra (licorice) root is glycyrrhizin (or glycyrrhizinic acid or GA). It is a pentacyclic triterpene saponin with a structure that is employed as an emulsifier and gel-forming agent in foods and cosmetics (Figure 1). Enoxolone is its aglycone. It is a glycyrrhetinic acid-containing triterpene glycoside with a wide spectrum of pharmacological and biological properties.38,39 It comes in the forms of ammonium glycyrrhizin and mono-ammonium glycyrrhizin when isolated from the plant. GA is an amphiphilic molecule, with the glucuronic acid residues representing the hydrophilic region and the glycyrrhetic acid residue representing the hydrophobic region. The chemical form of GA is C42H62O16, with a molecular weight of 822.92 g/mol.40
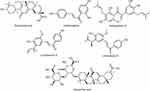 |
Figure 1 Chemical structures of major bioactive phytocompounds from licorice. |
Glycyrrhetic Acid (GLA)
GLA, also known as glycyrrhetinic acid (Figure 1), is a β-amyrin ursane-type pentacyclic triterpenoid derivative obtained by hydrolysis of GA, which comes from the herb licorice.41 GLA is a glycyrrhizin aglycone.42 The chemical form of GLA is C30H46O4, with a molecular weight of 470.7 g/mol. Many bioactive pharmaceuticals are synthesized using GLA as a precursor compound.
Isoliquiritigenin (ILG)
Licorice root contains isoliquiritigenin (ILG) (Figure 1), a phenolic chemical component.43 ILG belongs to the trans-chalcone hydroxylated at the C-2’, C-4, and C-4’ class of chalcones. Chalcones are characterized chemically as α, β-unsaturated biphenyl ketones. In several plants, ILG is a precursor to numerous flavanones. ILG is a biosynthetic precursor and isomer of flavanone liquiritigenin (LG), as well as a number of other flavonoids synthesized through the phenylpropanoid pathway.44,45 Furthermore, investigations show that ILG and LG are interchangeable by temperature and pH. The chemical form of ILG is C15H12O4, with a molecular weight of 256.25 g/mol.
Isoangustone A (IAA)
IAA (Figure 1) is a flavonoid compound found in the root of licorice. IAA belongs to the isoflavanones family. The chemical form of IAA is C25H26O6, with a molecular weight of 422.5 g/mol.46
Licochalcone A (LicoA)
LicoA (Figure 1) is a phenol chalconoid derivative found in and isolated from the roots of the Glycyrrhiza species G. glabra (licorice) and G. inflata. LicoA is a flavonoid that belongs to the oxygenated retro-chalcones group. Two phenolic hydroxyl groups, a methoxyl group, and an isoprene side chain replace the chalcone nucleus in LicoA.47 It is one of the main important active components of licorice root.48,49 The chemical form of Lico A is C21H22O4, with a molecular weight of 338.4 g/mol.
Licochalcone E (LicoE)
LicoE (Figure 1) is a retrochalcone isolated from the root of G. inflate and possesses numerous biological and pharmacological properties.50 The chemical form of LicoE is C21H22O4, with a molecular weight of 338.4 g/mol.
Absorption and Metabolism Studies of Major Licorice Bioactive Compounds
With the high associated toxicity encountered by cancer patients, there is an urgent need to investigate novel ways to protect patients from the side effects of traditional drug delivery systems.51,52 Licorice is commonly utilized as an ingredient in many traditional medicinal systems, such as traditional Chinese medicine in China, Ayurveda and Siddha in India, and Unani in Southern Europe, and it has been studied for its numerous pharmacological properties, including its anticancer capabilities.53 Pharmacokinetic studies are required to comprehend the absorption, distribution, metabolism, and excretion (ADME) features of bioactive substances. The ADME properties of licorice bioactive compounds vary because of the natural product class they belong to such as tri-terpenoids and flavonoids as evidenced in Tables 1 and 2, respectively.
 |
Table 1 ADME Profile of Triterpenoids (GA and Glycyrrhetic Acid) of Licorice |
 |
Table 2 ADME Profile of Flavonoids (Isoliquiritigenin, Isoangustone a, Licochalcone A) of Licorice |
Cellular Targets of Licorice Constituents in Cancer
Anti-Inflammatory and Antioxidant Potential
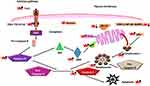
Inflammation that occurs in response to physical, chemical or biological stimuli plays a substantial role in preventing or promoting carcinogenesis through immune surveillance.54,55 Inflammatory mediators, such as growth factors, cytokines and chemokines, are released by immune cells such as macrophages, neutrophils and lymphocytes.56 ROS, on the other hand, can play a role in activating the inflammation related transcriptional factors (eg, NF-κB and STAT-3) and contribute to the carcinogenesis processes, including genomic instability, resistance to apoptosis, cellular proliferation, angiogenesis, invasion and metastasis.57–62 Licorice is known to have significant anti-inflammatory activity and its use in the treatment of inflammatory diseases dates back to ancient times.63 Many studies have shown that licorice triterpenes, such as glycyrrhizin and glycyrrhetinic acid, and flavonoids, such as dehydroglyasperin C, echinatin, glabridin, glyurallin B, isoangustone A, isoliquiritigenin, licochalcone A-E, licoricidin and licorisoflavan A, have significant anti-inflammatory effects.63,64 Glycyrrhizin, which has an anti-inflammatory effect similar to the glucocorticoids and mineralocorticoids, can inhibit inflammatory factors and promote the healing of mouth and stomach ulcers.65 Sun and coworkers66 have reported that glycyrrhizin suppresses lipopolysaccharide (LPS)-induced inflammatory responses via blocking the high mobility group protein box 1 (HMGB1)-Toll-like receptor 4 (TLR4)-NF-κB pathway. Moreover, glycyrrhizin and 18β-glycyrrhetinic acid have been defined by different investigators as significant inhibitors of inflammatory factors, such as cyclooxygenase-2 (COX-2), HMGP 1, inducible nitric oxide synthase (iNOS), interleukin-6 (IL-6), IL-10, tumor necrosis factor-α (TNF-α), TGF-β, prostaglandin E2 (PGE2), myeloperoxidase (MPO) and nuclear factor-κB (NF-κB).67–72 Dehydroglyasperin C has been reported to suppress the production of ROS and singlet oxygen radicals, and it exerts anti-inflammatory activities by reducing the DNA binding activity of NF-κB, increasing the expression of MKP-1 and heme oxygenase-1, and inhibiting COX-2 expression via blocking the MKK4 and PI3K pathways.73–75 Although echinatine, licochalcone A and licochalcone B inhibit IL-6 and PGE2 in LPS-induced macrophage cells, licochalcones B and D reduce the production of TNF-α and monocyte chemotactic protein 1 (MCP-1).76–78 Moreover, licochalcone C inhibits the NF-κB pathway by reducing the expression of iNOS, ICAM-1 and VCAM-1. Licochalcone E also shows anti-inflammatory activity by suppressing NF-κB and AP-1 and reducing the expression of iNOS and COX-2.79,80 In addition, glabridin, one of the most studied anti-inflammatory flavonoids isolated from licorice, suppresses inflammatory responses in different cell lines via the NF-κB pathway and inhibition of the expression of various cytokines and chemokines.81 It has been reported by different researchers that glabridin suppresses the expression of C-X-C motif chemokine ligand 5 (CXCL5), IL-1β, IL-6, IL-8, IL-12, IL-17A, IL-22, IL-23, interferon (IFN)-α/β, iNOS, monocyte chemoattractant protein-1 (MCP-1), nitric oxide (NO), TNF-α, PGE2, COX-2, MPO and lipoxygenase (LOX) and inhibits the activation of the p38 MAPK, ERK, NF-κB and AP-1 signaling pathways (Figure 2).81 A natural chalcone called isoliquiritigenin, isolated from licorice, also has significant anti-inflammatory activity via inhibition of caspase-1, COX-2, IL-1, IL-6, IL-8, iNOS, TNF-α, NF-κB ligand RANKL and eotaxin-1, and suppression of the NF-κB, MAPK, AP-1 signaling pathways and NLRP3 inflammasome activation.45,82–87 Furthermore, isoliquiritigenin and isoliquiritin inhibit inhibitory κBα (IκBα) phosphorylation and degradation, and increase the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 in LPS-induced macrophage cells.88
The phenolic ingredients, such as chalcones, coumarins, flavonoids, isoflavones and methylated isoflavones, seem to be responsible for the antioxidant activity of licorice (Figure 2). ROS has a high affinity for DNA and other biomolecules. This can result in DNA damage and oncogenic mutations being incorporated into normal cells leading to genomic instability, and finally cancer.89 Due to the phenolic hydroxyl structure of chalcones, they act as proton donors and combine with a radical to prevent oxidative damage.90,91 The chalcones isolated from licorice, such as echinatin, isobavachalcone, isoliquiritigenin, and licochalcones A-D, have been reported as powerful antioxidant agents.76,92,93 Although isobavachalcone and licochalcones A-D suppress NADPH induced lipid peroxidation, echinatin and licochalcone A and B possess strong radical scavenging activity. Licochalcone A and C elevate the expression of antioxidant enzymes, such as catalase, glutathione peroxidase and superoxide dismutase.94,95 Moreover, dihydroisoliquiritigenin inhibits glutamate-induced oxidative stress in neuronal cells, and glypallichalcone acts as an inhibitor of LPL oxidation.96,97 Similar to the chalcone derivatives, coumarin compounds in licorice have significant radical scavenging activity.98,99 Moreover, saponins and polysaccharides isolated from licorice have been reported to exert antioxidant activities.100–102 Consequently, it seems that the triterpenes, chalcones, coumarins, flavonoids, isoflavones and methylated isoflavones have significant antioxidant activity inhibiting the processes of carcinogenesis, and these contents of licorice have great potential as novel anticancer agents.
Apoptosis and Cell Cycle Arrest
Cancer is one of the leading diseases affecting human life and is caused by various reasons, such as environmental pollution and unhealthy lifestyle.103–106 Apoptotic cell death is known to regulate cancer cell proliferation, invasion and survival. Intrinsic and extrinsic apoptosis are the two major pathways induced by chemotherapeutics to inhibit tumor proliferation (Figure 3). Inhibition of apoptosis is considered to be an important mechanism toward drug resistance. In recent years, the interest has shifted to exploring apoptotic phytochemicals for cancer treatment and overcoming drug-resistance with lower side-effects.56,107–111 The anticancer properties of licorice were studied in MCF-7 (breast cancer) and HepG2 (liver cell carcinoma) cell lines where licorice root extract was used to synthesize gold nanoparticles (AuNPs). It was observed that 50 µg/mL and 23 µg/mL of the synthesized AuNPs could successfully inhibit the growth of MCF-7 and HepG2 cell lines, respectively.112 Similar results were obtained in a study conducted by Vlaisavljević et al113 where different cancer cell lines, such as SiHa, HeLa (cervical), T47D, MDA-MB-361, MDA-MB-231, and MCF7 (breast), and A2780 (ovarian), were treated with licorice extract and it was found that 30 µg/mL extract induced apoptosis or necrosis in the cells, inhibiting tumor growth.
Licorice contains various flavonoids, such as glabridin, glycyrrhetinic acid, Lico, GA, ILG, and liquiritin.114 The glabridin exhibits anticancer activities as it activates the caspase cascade and mitochondrial apoptotic pathway leading to apoptosis in cancer cells.115 It regulates various signaling pathways, such as signal transducer and activator of transcription 3 (STAT-3), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), mitogen-activated protein kinase (MAPK) and extracellular-signal-regulated kinase (ERK) to inhibit proliferation of various cancer cells, and induces apoptosis of these cells. Glabridin induced apoptosis in Huh7 liver cancer cells by cleaving caspase-9 levels and increasing the release of cytochrome c (cyt. c),116 and in SK-BR-3 breast cancer cells by cleaving caspase-9, caspase-8, and caspase-3, and increasing the concentration of PARP.117 Further, in addition to the caspase cascade, glabridin induced apoptosis in HL-60 acute myeloid leukemia cells by activating the JNK1/2 and p38 MAPK signaling pathways.118 The glycyrrhetinic acid and its derivatives induce mitochondrial-mediated apoptosis in cancer cells, as it was found in a study conducted by Lin et al119 where apoptosis was induced by the generation of reactive oxygen species (ROS) when NTUB1 (human bladder cancer cells) were exposed to glycyrrhetinic acid 25. Another derivative of glycyrrhetinic acid, 18β- glycyrrhetinic acid (GRA) caused apoptosis in MCF-7 by activating the mitochondrial death cascade, caspase-9 and release of cyt. c. GRA had no inhibitory effect on the MCF-10 A (normal mammary epithelial cells).120 In another study, it was found that GRA was capable of inducing cell cycle death in HepG2 cells by arresting cell growth in the G1-phase and inducing apoptosis at a higher concentration.121 Another triterpene compound exhibiting antitumor properties is glycyrrhizin which is isolated from the roots of licorice. The antitumor activity of glycyrrhizin has been demonstrated in DU-145 and LNCaP human prostate cancer cells. Glycyrrhizin induces apoptosis in both cell lines in a concentration-dependent and time-dependent manner.122 Further, the GA, another flavonoid extracted from the roots of licorice also induces apoptosis and suppresses the proliferation of MDA-MB-231 breast cancer cells by increased generation of ROS.123 GA induces cell cycle arrest at G1/S phase in gastric cancer cells by downregulating the cyclin E1, cyclin E2, and cyclin D1-3 levels causing cell death in these cancer cells.124
Another important group of metabolites of licorice is chalcones.82,125 The chalcones inhibit cancer cell growth by interacting with the protein nucleophiles and inducing autophagy, apoptosis, and cell cycle arrest in cancer cells.126 Licochalcones A, B, C, and D, ILG, echinate, paratocarpin A, kanzonol C, and isoliquiritin apioside (ISLA) are different kinds of chalcones studied for their anticancer properties. Lico A inhibits cancer cell growth through the mitochondrial pathway by activating the caspase cascade and mediating apoptotic and antiproliferative effects via a Sp1-mediated signaling pathway.127 There are several studies depicting the anticancer properties of lico A in gastric cancer cells,128 PC-3 prostate cancer cells,129 and glioma cells.130 Lico B exhibits its anticancer activity via caspase-3 activation, Bax expression enhancement,131 suppressing the expression of CDK1, CDK2 mRNA, cyclin A, antiapoptotic proteins (Mcl-1, Bcl-xL, and Bid), and leading to S-phase arrest.132 Licochalcones C and D, and kanzonol C have anticancer mechanism similar to licochalcone B.133–135 Different studies demonstrate the anticancer mechanism of ILG in breast cancer cells arresting the cancer cell growth at G0/G1 phase,136 A549 non-small-cell lung cancer cells by activation of the protein kinase B survival pathway and caspase cascades,137 renal cancer cells by ROS generation and STAT-3 pathway inhibition,138 and oral squamous cells by G2/M cell cycle arrest.139 Endoplasmic reticulum (ER)-stress) and ROS signaling pathways induce extrinsic and intrinsic apoptosis, leading to the cancer cell death in esophageal squamous cells when treated with echinatin.140 Tables 3 and 4 represent an overview of various in vitro and in vivo anticancer studies mediated by bioactive compounds of licorice.
 |
Table 3 In vitro Anticancer Effects and Mechanistic Insight of Licorice Bioactive Phytocompounds |
 |
Table 4 In vivo Anticancer Effects and Mechanistic Insight of Licorice Bioactive Phytocompounds |
Antiangiogenesis Effect
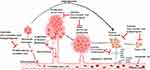
Angiogenesis or neovascularization is considered to play a vital role in tumor proliferation and metastasis.141–144 In tumors, angiogenesis is intervened by targeting numerous markers that regulate angiogenesis and are considered proangiogenic factors, such as vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs) and basic fibroblast growth factor (bFGF).145–148 These angiogenesis markers have a broader spectrum of target cells which play an essential role in angiogenesis.149,150 In a hypoxic condition, tumor cells cause the release of proangiogenic factors, such as VEGFs, epidermal growth factor (EGF), FGF, insulin-like growth factor-1 (IGF1) and transforming growth factor-β1 (TGFβ1)), within the tumor.151 In tumor cells, VEGF is the main angiogenic activator that stimulates angiogenesis via binding to VEGFR2152 (Figure 4). Therefore, according to literature, targeting these pathways’ inhibitors in angiogenesis by herbal plant extract and isolated phytoconstituents was considered an anticancer treatment approach with clinical importance.
The extract of G. glabra used to treat mice with Ehrlich ascites tumor cells showed a reduction in the level of cytokines and decreased VEGF revealing its angioinhibitory potential.153 Licochalcone A (LicA) is a potent constituent of licorice having various biological properties, such as anti-inflammatory, antiangiogenic and antitumor effects. LicA was reported for its apoptosis inducing potential in prostate cancer via modulating the protein expression of Bcl-2. LicA inhibits the process of angiogenesis and tumorigenesis both in vitro and in vivo by regulating the signaling of VEGFR-2.154 In addition, LicA also reduced the vessel formation by endothelial cells as well invasion and migration via modulating the expression of MMP-9, VEGF-A and plasminogen activators.155 In a study, Jiang et al156 reported that glabridin, a potent constituent of G.glabra, has anticancer potential as it inhibits the migration, invasion and angiogenesis of human breast cancer cells by modulating the FAK/Rho signaling pathway. The aqueous extract of G. glabra blocks the in vitro and in vivo proliferation of Ehrlich ascites tumor cells. According to Kim et al.157 Glycyrrhizin isolated from the roots of G. glabra inhibited the metastasis and survival of tumor by modulating the levels of onco-suppressor p53 gene, MAPK, ERK and EGFR which led to apoptosis and showed antiangiogenetic effect.
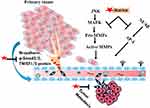
Antimetastic Activities
Metastasis is a multistep process that contributes to the spread of cancer cells to distant organs of the body through blood or the lymphatic system, resulting in death in cancer patients.158–163 Targeting metastasis is an attractive strategy in the management of progression and development of cancer (Figure 5).164–166 According to literature, various in vitro and in vivo models showed that natural bioactive compounds, including those from G. glabra, have antimetastasis potential167 including Matrix metalloproteinases (MMPs), such as MMP-2, and MMP-9, and urokinase plasminogen activator (uPA) play a significant role in the metastasis process by degrading extracellular matrix (ECM) of cancerous cells as well as modulating the mechanism of angiogenesis in the maintenance of tumor cell survivability.168,169 MMPs are degradation enzymes that modulate numerous physiological processes, such as cell growth, differentiation and apoptosis. However, overexpression of MMP-2 and MMP-9 is linked with prooncogenic events, such as neoangiogenesis, tumor cell proliferation, and metastasis.170–172 In addition to MMPs, ERK1/2, p38, MAPK and JNK/SAPK play a central role in the regulation of cancer metastasis expression.173–176 Furthermore, once cancer cells develop a more invasive nature, they can enter blood and spread to distant regions, resulting in metastasis. Tumor cells that have moved to a secondary site can either go into metastatic dormancy or stimulate angiogenesis and start growing new blood vessels.177 Hence, to control the mechanism of metastasis, targeting oncogenic molecular pathways by natural phytoconstituents is an important therapeutic approach.178,179
Glabridin, a major chemical constituent of licorice, significantly blocks the migration/invasion of various HCC cells, namely Huh7 and Sk-Hep-1, by modulating the expression levels of MMP-9 and the phosphorylation processes of ERK1/2 and JNK1/2 markers. This inhibitory effect was linked with an upregulation of tissue inhibitor of MMP-1 and a downregulation of the transcription factors NF-κB and activator protein 1 signaling pathways.180 Wang et al181 reported that GA has the potential to suppress breast tumor outgrowth and pulmonary metastasis by modulating the p38 MAPK-AP1 signaling pathway. In vitro experimentation revealed that LicE decreased the expression of specificity protein 1 (Sp1) in MCF-7 and MDA- MB-231 cell lines, resulting in regulation of the cell cycle as well as inhibiting the process of carcinogenesis and tumor metastasis. Isoliquiritigenin, an isolated component of licorice, inhibited the process of tumor metastasis via upregulating E-cadherin and downregulating the N-cadherin, p-Smad2/3, and TWIST1/2 protein expression in HEC-1A, Ishikawa, and RL95-2 xenograft animal model.182
Synergistic Actions of Licorice Phytochemicals with Anticancer Agents
Licorice is extensively used as an herbal medicine in traditional Chinese as well as Indian medicine to treat gastric, liver, and respiratory problems and different types of cancers, and to reduce the toxicity caused by other herbs. Licorice and its flavonoids show more potential effect against various cancers when used in conjunction with other anticancer drugs. Numerous studies have been conducted to investigate the role of licorice and other anticancer drugs individually in various cancers. These chemotherapeutic drugs showed great potential in the treatment of a diverse range of cancers, on the other hand, they also exert side effects to the normal cells and induce toxicity.183–185 But when the researchers used anticancer drugs, such as paclitaxel, cisplatin, and gemcitabine in combination with licorice, it inhibited the side effects by protecting the normal cells from toxicity along with enhancing anticancer potential. Tables 5 and 6 show the synergistic effects of licorice with other anticancer drugs both in vitro and in vivo.
 |
Table 5 In vitro Synergistic Action of Licorice Phytocompounds with Various Anticancer Drugs |
 |
Table 6 In vivo Synergistic Effect of Licorice with Various Anticancer Drugs |
Nanotechnology Studies of Bioactive Constituents of Licorice in Cancer
Nanotherapeutics (1–100 nm) have been shown to overcome the shortcomings of conventional treatments,185 such as unwanted side effects on rapidly growing healthy cells, non-specific targeting and distribution, dose-dependent toxicity, and multi-drug resistance.185–187 They possess enhanced target-specificity, increased permeability and retention time of the drug in the cancer cells, improved biocompatibility, and decreased dose of the drug which together contribute to reduced toxicity.185,188,189 Romberg et al190 and Cheng et al191 pointed out that recently developed nanoparticles possess various limitations, thereby shifting the focus of formulation sciences to natural compounds-based nanoparticles which would increase targeting efficiency to cancer cells and lower the rate of clearance. This is further supported by various advantages, such as increased patient compliance (with peroral administration), less extensive metabolic by-products and subsequent higher bioavailability.192 As summarized in Tables 7 and 8, various nanoformulations containing licorice and its bioactive compounds were developed and tested against specific cancer types and results from these studies have been listed. Various cell line studies, as evidenced by Table 7, have focused on hepatic carcinoma due to the abundance of glycyrrhetinic acid receptors which are over-expressed on hepatocytes making it a viable targeting options.193 These have been explored due to the limitations of conventional therapies as mentioned above. The results from cell line studies need to be tested in animal models to confirm the efficacy and safety of the drug or formulation under study. The studies listed in Table 8 represent the intratumor studies conducted thereby helping to uncover the tremendous potential possessed by these nanoformulations in the chemotherapeutic field. Our thorough search revealed that although there were in vitro and in vivo studies carried out for isoangustone A,194–197 licochalcone A198–201 and licochalcone E155,202,203 as anticancer molecules, there were no studies conducted for these molecules in the nanotherapeutics domain. The difficulties encountered during the manufacturing of these medications as nanotherapeutics could be one of the factors limiting their usage as anticancer moieties. The findings suggest that these compounds could be developed into viable anticancer nanomedicines in the future. As a result, the findings can be extended, implying that they have a lot of potential for future clinical research. More research is needed to overcome the problems of nanoformulations and generate reliable medicines with few adverse effects.
 |
Table 7 In vitro Studies of Nanoformulations of Bioactive Compounds of Licorice |
 |
Table 8 In vivo Studies of Nanoformulations of Bioactive Compounds of Licorice |
Safety Studies of Licorice
In general, licorice products are considered to have no hazard to the public and are utilized widely in food (ice cream, candies, chewing gums, and beverages), cosmetics (toothpaste) and tobacco as flavoring and sweetening agents.7,9 However, before licorice extract or any of its individual components can enter into clinical oncological practice, due to their strong pharmacological activities, their safety must be verified thoroughly and systematically, paying special attention to the dosage and duration of the treatment.18 Several studies have indeed warranted for the toxicity of licorice depending on its dosage and duration.18 Actually, chronic licorice intake was shown to induce a condition comparable to that found in primary hyperaldosteronism, while licorice overconsumption resulted in hypermineralocorticoidism characterized by salt and water retention, hypertension, hypokalemia, metabolic alkalosis, and suppression of the renin-aldosterone system.28,204 Biochemical evidence suggests that licorice and its phytochemicals, particularly glycyrrhizinates, can reversibly block the cortisol-inactivating enzyme, 11β-hydroxysteroid dehydrogenase, thereby producing hypermineralocorticoid-like effects.205 In addition, based on a case report, excessive consumption of licorice may also lead to toxic consequences in the form of thrombocytopenia.206 Therefore, health care providers should be aware of the hazardous consequences related to chronic and excessive intake of licorice extracts to be able to prevent worsening of these symptoms when detected early [16]. Furthermore, caution must be exercised when using licorice during pregnancy, as heavy licorice consumption has been associated with lower gestational age and preterm delivery in humans.207,208 Accordingly, the main challenge in exploiting the promising anticancer activities of licorice constituents in clinical settings primarily lies on its appropriate dosing, besides targeted delivery to malignant sites, inducing minimal adverse reactions in normal healthy tissues. It is highly expected that future experimental studies with nano-sized carriers will provide a strong base for overcoming these challenges by virtue of modern nano-technological methods.
Clinical Trials
Several clinical trials conducted with licorice products have also reported glycyrrhizin-related complications, such as elevated blood pressure due to increasing extracellular fluid volume and large artery stiffness, and reduced serum potassium levels.209 However, other clinical trials (mostly on the gastrointestinal disorders) have suggested diverse healing properties of licorice preparations without exerting any observable adverse effects.205,209 A clinical stage II preliminary trial revealed that licorice root extract in combination with docetaxel works in treating patients with hormonal therapy resistant metastatic prostate tumors (NCT00176631). Similarly, licochalcone A and paclitaxel have been shown to increase natural cell death and apoptosis in OSCC tumors (NCT03292822).
Conclusions and Perspectives
Our present review describes anticancer potential of the phytoconstituents of G. glabra along with synergistic chemotherapeutic insight. Traditionally, licorice has been utilized as a sweetening and flavoring agent for food items. Roots of licorice are reported to possess strong therapeutic potential to reduce inflammation and cancer progression. Among the reported phytoconstituents, the flavonoids and terpenoids are the major therapeutically active molecules. The in vitro and in vivo data presented in the current review article clearly show the strong potential of licorice-derived phytochemicals from the classes of triterpenes, chalcones and isoflavones in the fight against different types of cancer. Despite potential therapeutic importance of these effects, several obstacles, such as toxic reactions observed with excessive consumption, have impeded moving on with clinical trials. It is highly expected that surpassing these bottlenecks by using modern nanotechnological methods might lead us to expansion of the current anticancer arsenal. In addition, as licorice constituents possess a wide range of molecular targets in cancer, they might be helpful in preventing drug resistance. Therefore, synergistic mechanistic insight of licorice-derived phytoconstituents and conventional chemotherapeutic drugs should be further explored. There are few human studies available and more randomized controlled trials are needed to measure the effectiveness of licorice-based cancer treatment. The story of licorice reflects a fascinating example of how an ancient herbal medicine can be introduced as a drug into clinical settings, after intensive efforts in elucidating its constituents and molecular mechanisms behind their various bioactivities.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest.
References
1. Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6(11):1–5. doi:10.4103/0973-7847.95849
2. Yang Z, Xu Y, Bi Y, et al. Immune escape mechanisms and immunotherapy of urothelial bladder cancer. J Clin Transl Res. 2021;7(4):485.
3. Kashyap D, Garg VK, Goel N. Intrinsic and Extrinsic Pathways of Apoptosis: Role in Cancer Development and Prognosis.
4. Sen T, Samanta SK. Medicinal plants, human health and biodiversity: a broad review. Adv Biochem Eng Biotechnol. 2014;147:59–110.
5. Fridlender M, Kapulnik Y, Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Front Plant Sci. 2015;67:99.
6. Sak K. Anticancer action of plant products: changing stereotyped attitudes. Explor Target Anti Tumor Ther. 2022;3(4):423–427.
7. Wu Y, Wang Z, Du Q, et al. Pharmacological effects and underlying mechanisms of licorice-derived flavonoids. Evidence Based Complement Altern Med. 2022;2022:9523071.
8. Yang R, Wang L, Liu Y. Antitumor activities of widely-used Chinese herb—licorice. Chinese Herb Med. 2014;6(4):274–281. doi:10.1016/S1674-6384(14)60042-3
9. Hasan MK, Ara I, Mondal MSA, Kabir Y. Phytochemistry, pharmacological activity, and potential health benefits of Gly cyrrhiza glabra. Heliyon. 2021;7(6):e07240.
10. Bode AM, Dong Z. Chemopreventive effects of licorice and its components. Curr Pharmacol Report. 2015;1(1):60–71. doi:10.1007/s40495-014-0015-5
11. Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001;39(1):1–11. doi:10.1207/S15327914nc391_1
12. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
13. Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci. 2013;93(23):863–869. doi:10.1016/j.lfs.2013.09.030
14. Kashyap D, Sharma A, Tuli S, Punia S, Sharma A. Ursolic acid and oleanolic acid: pentacyclic terpenoids with promising anti-inflammatory activities. Recent Pat Inflamm Allergy Drug Discov. 2016;10(1):21–33. doi:10.2174/1872213X10666160711143904
15. Kumar G, Mittal S, Sak K, Tuli HS. Molecular mechanisms underlying chemopreventive potential of curcumin: current challenges and future perspectives. Life Sci. 2016;148:313–328. doi:10.1016/j.lfs.2016.02.022
16. Tuli HS, Aggarwal V, Kaur J, et al. Baicalein: a metabolite with promising antineoplastic activity. Life Sci. 2020;259:118183.
17. Kashyap D, Kumar G, Sharma A, Sak K, Tuli HS, Mukherjee TK. Mechanistic insight into carnosol-mediated pharmacological effects: recent trends and advancements. Life Sci. 2017;169:27–36. doi:10.1016/j.lfs.2016.11.013
18. Wahab S, Annadurai S, Abullais SS, et al. Glycyrrhiza glabra (Licorice): a comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants. 2021;10(12):2751.
19. Zhang Z, Yang L, Hou J, Tian S, Liu Y. Molecular Mechanisms Underlying the Anticancer Activities of Licorice Flavonoids. Elsevier B.V; 2021:113635.
20. Jain R, Hussein MA, Pierce S, Martens C, Shahagadkar P, Munirathinam G. Oncopreventive and oncotherapeutic potential of licorice triterpenoid compound glycyrrhizin and its derivatives: molecular insights. Pharmacol Res. 2022;178:106138. doi:10.1016/j.phrs.2022.106138
21. Hussain H, Ali I, Wang D, et al. Glycyrrhetinic acid: a promising scaffold for the discovery of anticancer agents. Expert Opin Drug Discov. 2021;16(12):1497–1516. doi:10.1080/17460441.2021.1956901
22. Su X, Wu L, Hu M, Dong W, Xu M, Zhang P. Glycyrrhizic acid: a promising carrier material for anticancer therapy. Biomed Pharmacother. 2017;95:670–678. doi:10.1016/j.biopha.2017.08.123
23. Alagawany M, Elnesr SS, Farag MR, et al. Use of Licorice (Glycyrrhiza glabra) herb as a feed additive in poultry: current knowledge and prospects. Animals. 2019;9(8):536. doi:10.3390/ani9080536
24. Alagawany M, Elnesr SS, Farag MR. Use of liquorice (Glycyrrhiza glabra) in poultry nutrition: global impacts on performance, carcass and meat quality. Worlds Poult Sci J. 2019;75(2):293–303. doi:10.1017/S0043933919000059
25. Kitagawa I. Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl Chem. 2002;74(7):1189–1198. doi:10.1351/pac200274071189
26. Murphy SC, Agger S, Rainey PM. Too much of a good thing: a woman with hypertension and hypokalemia. Clin Chem. 2009;55(12):2093–2096. doi:10.1373/clinchem.2009.127506
27. Fallows S. Scientific committee on food. Nutr Food Sci. 2000;30(6):72–75.
28. Omar HR, Komarova I, Abdelmalak HD, et al. Licorice abuse: time to send a warning message. Ther Adv Endocrinol Metab. 2012;3(4):125–138. doi:10.1177/2042018812454322
29. Walker BR, Edwards CRW. Licorice-induced hypertension and syndromes of apparent mineralocorticoid excess. Endocrinol Metab Clin North Am. 1994;23(2):359–377. doi:10.1016/S0889-8529(18)30102-6
30. Kao TC, Wu CH, Yen GC. Bioactivity and potential health benefits of licorice. J Agric Food Chem. 2014;62(3):542–553. doi:10.1021/jf404939f
31. Chauhan P, Sharma H, Kumar U, Mayachari A, Sangli G, Singh S. Protective effects of Glycyrrhiza glabra supplementation against methotrexate-induced hepato-renal damage in rats: an experimental approach. J Ethnopharmacol. 2020;263:113209. doi:10.1016/j.jep.2020.113209
32. Fiore C, Eisenhut M, Ragazzi E, Zanchin G, Armanini D. A history of the therapeutic use of liquorice in Europe. J Ethnopharmacol. 2005;99(3):317–324. doi:10.1016/j.jep.2005.04.015
33. Asan-Ozusaglam M, Karakoca K. Evaluation of biological activity and antioxidant capacity of Turkish licorice root extracts. Rom Biotechnol Lett. 2014;19(1):8994–9005.
34. Karahan F, Avsar C, Ozyigit II, Berber I. Antimicrobial and antioxidant activities of medicinal plant Glycyrrhiza glabra var. glandulifera from different habitats. Biotechnol Biotechnol Equip. 2016;30(4):797–804. doi:10.1080/13102818.2016.1179590
35. Statti GA, Tundis R, Sacchetti G, Muzzoli M, Bianchi A, Menichini F. Variability in the content of active constituents and biological activity of Glycyrrhiza glabra. Fitoterapia. 2004;75(3–4):371–374. doi:10.1016/j.fitote.2003.12.022
36. Li YH, Li YN, Li HT, Qi YR, Wu ZF, Yang M. Comparative study of microwave-vacuum and vacuum drying on the physicochemical properties and antioxidant capacity of licorice extract powder. Powder Technol. 2017;320:540–545. doi:10.1016/j.powtec.2017.07.076
37. Cheel J, Tůmová L, Areche C, et al. Variations in the chemical profile and biological activities of licorice (Glycyrrhiza glabra L.), as influenced by harvest times. Acta Physiol Plant. 2013;35(4):1337–1349. doi:10.1007/s11738-012-1174-9
38. Cirillo G, Curcio M, Parisi OI, et al. Molecularly imprinted polymers for the selective extraction of glycyrrhizic acid from liquorice roots. Food Chem. 2011;125(3):1058–1063. doi:10.1016/j.foodchem.2010.09.077
39. Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phyther Res. 2018;32(12):2323–2339. doi:10.1002/ptr.6178
40. Shen S, Chang Z, Liu J, Sun X, Hu X, Liu H. Separation of glycyrrhizic acid and liquiritin from Glycyrrhiza uralensis Fisch extract by three-liquid-phase extraction systems. Sep Purif Technol. 2007;53(3):216–223. doi:10.1016/j.seppur.2006.07.003
41. Chen HR, Sheu SJ. Determination of glycyrrhizin and glycyrrhetinic acid in traditional Chinese medicinal preparations by capillary electrophoresis. J Chromatogr A. 1993;653(1):184–188. doi:10.1016/0021-9673(93)80411-Z
42. Baltina LA, Flekhter OB, Putieva ZM, Kondratenko RM, Krasnova LV, Tolstikov GA. Hydrolysis of β-glycyrrhizic acid. Pharm Chem J. 1996;30(4):263–266. doi:10.1007/BF02218774
43. Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, Tamir S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J Agric Food Chem. 2003;51(5):1201–1207. doi:10.1021/jf020935u
44. Dixon RA, Pasinetti GM. Flavonoids and isoflavonoids: from plant biology to agriculture and neuroscience. Plant Physiol. 2010;154(2):453–457. doi:10.1104/pp.110.161430
45. Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57(3):820–825. doi:10.1021/jf802601j
46. Song NR, Lee E, Byun S, et al. Isoangustone A, a novel licorice compound, inhibits cell proliferation by targeting PI3K, MKK4, and MKK7 in human melanoma. Cancer Prev Res. 2013;6(12):1293–1303. doi:10.1158/1940-6207.CAPR-13-0134
47. Chen M, Christensen SB, Blom J, et al. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob Agents Chemother. 1993;37(12):2550–2556. doi:10.1128/AAC.37.12.2550
48. Simmler C, Jones T, Anderson JR, et al. Species-specific standardisation of licorice by metabolomic profiling of flavanones and chalcones. Phytochem Anal. 2014;25(4):378–388. doi:10.1002/pca.2472
49. Li X, Sun R, Liu R. Natural products in licorice for the therapy of liver diseases: progress and future opportunities. Pharmacol Res. 2019;144:210–226. doi:10.1016/j.phrs.2019.04.025
50. Yoon G, Do JY, Cheon SH. Cytotoxic allyl retrochalcone from the roots of Glycyrrhiza inflata. Chem Pharm Bull. 2005;53(6):694–695. doi:10.1248/cpb.53.694
51. Manu KA, Shanmugam MK, Li F, et al. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J Mol Med. 2014;92(3):267–276. doi:10.1007/s00109-013-1095-0
52. Nasery MM, Abadi B, Poormoghadam D, et al. Curcumin delivery mediated by bio-based nanoparticles: a review. Molecules. 2020;25(3):689. doi:10.3390/molecules25030689
53. Zhang Z, Yang L, Hou J, Tian S, Liu Y. Molecular mechanisms underlying the anticancer activities of licorice flavonoids. J Ethnopharmacol. 2021;267:113635.
54. Tuli HS, Kashyap D, Bedi SK, Kumar P, Kumar G, Sandhu SS. Molecular aspects of metal oxide nanoparticle (MO-NPs) mediated pharmacological effects. Life Sci. 2015;143:71–79. doi:10.1016/j.lfs.2015.10.021
55. Tuli HS, Joshi R, Kaur G, et al. Metal nanoparticles in cancer: from synthesis and metabolism to cellular interactions. J Nanostructure Chem. 2022;2022;1–28:64–66. doi:10.2105/ajph.66.1.64
56. Lu X, Shi H, Que Q, Qiu S. Research progress in immunotherapy of advanced non-small cell lung cancer. Trends Immunother. 2021;5(2.1):58–64. doi:10.24294/ti.v5.i2.1.1367
57. Shrihari TG. Dual role of inflammatory mediators in cancer. Ecancermedicalscience. 2017;11. doi:10.3332/ecancer.2017.721
58. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
59. Morgan D, Garg M, Tergaonkar V, Tan SY, Sethi G. Pharmacological significance of the non-canonical NF-κB pathway in tumorigenesis. Biochim Biophys Acta - Rev Cancer. 2020;1874(2):188449. doi:10.1016/j.bbcan.2020.188449
60. Garg M, Shanmugam MK, Bhardwaj V, et al. The pleiotropic role of transcription factor STAT3 in oncogenesis and its targeting through natural products for cancer prevention and therapy. Med Res Rev. 2020;41(3):1291–1336. doi:10.1002/med.21761
61. Dai X, Ahn KS, Kim C, et al. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol Oncol. 2015;9(4):818–833. doi:10.1016/j.molonc.2014.12.008
62. Raghunath A, Sundarraj K, Arfuso F, Sethi G, Perumal E. Dysregulation of Nrf2 in hepatocellular carcinoma: role in cancer progression and chemoresistance. Cancers. 2018;10(12):481. doi:10.3390/cancers10120481
63. Yang R, Yuan BC, Ma YS, Zhou S, Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol. 2017;55(1):5–18. doi:10.1080/13880209.2016.1225775
64. Yang R, Wang LQ, Yuan BC, Liu Y. The pharmacological activities of licorice. Planta Med. 2015;81(18):1654–1669. doi:10.1055/s-0035-1557893
65. Kageyama Y, Suzuki H, Saruta T. Role of glucocorticoid in the development of glycyrrhizin-induced hypertension. Clin Exp Hypertens. 1994;16(6):761–778. doi:10.3109/10641969409078024
66. Sun X, Zeng H, Wang Q, et al. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway. Exp Cell Res. 2018;369(1):112–119. doi:10.1016/j.yexcr.2018.05.012
67. Wang CY, Kao TC, Lo WH, Yen GC. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J Agric Food Chem. 2011;59:7726–7733. doi:10.1021/jf2013265
68. Luo L, Jin Y, Kim I-D, Lee J-K. Glycyrrhizin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Exp Neurobiol. 2013;22(2):107–115. doi:10.5607/en.2013.22.2.107
69. Fu Y, Zhou E, Wei Z, et al. Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 2014;281(11):2543–2557. doi:10.1111/febs.12801
70. Ni YF, Kuai JK, Lu ZF, et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J Surg Res. 2011;165(1):e29–e35. doi:10.1016/j.jss.2010.10.004
71. Bhattacharjee S, Bhattacharjee A, Majumder S, Majumdar SB, Majumdar S. Glycyrrhizic acid suppresses cox-2-mediated anti-inflammatory responses during Leishmania donovani infection. J Antimicrob Chemother. 2012;67(8):1905–1914. doi:10.1093/jac/dks159
72. Ishida T, Miki I, Tanahashi T, et al. Effect of 18β-glycyrrhetinic acid and hydroxypropyl γcyclodextrin complex on indomethacin-induced small intestinal injury in mice. Eur J Pharmacol. 2013;714(1–3):125–131. doi:10.1016/j.ejphar.2013.06.007
73. Kim J, Kim J, Shim J, et al. Licorice-derived dehydroglyasperin C increases MKP-1 expression and suppresses inflammation-mediated neurodegeneration. Neurochem Int. 2013;63(8):732–740. doi:10.1016/j.neuint.2013.09.013
74. Kim HJ, Lim SS, Park IS, Lim JS, Seo JY, Kim JS. Neuroprotective effects of dehydroglyasperin C through activation of heme oxygenase-1 in mouse hippocampal cells. J Agric Food Chem. 2012;60(22):5583–5589. doi:10.1021/jf300548b
75. Lee JH, Kim JE, Jang YJ, et al. Dehydroglyasperin C suppresses TPA-induced cell transformation through direct inhibition of MKK4 and PI3K. Mol Carcinog. 2016;55(5):552–562. doi:10.1002/mc.22302
76. Fu Y, Chen J, Li YJ, Zheng YF, Li P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013;141(2):1063–1071. doi:10.1016/j.foodchem.2013.03.089
77. Furusawa JI, Funakoshi-Tago M, Mashino T, et al. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-κB p65 in LPS signaling pathway. Int Immunopharmacol. 2009;9(4):499–507. doi:10.1016/j.intimp.2009.01.031
78. Kim SJ, Kim CG, Yun SR, Kim JK, Jun JG. Synthesis of licochalcone analogues with increased anti-inflammatory activity. Bioorganic Med Chem Lett. 2014;24(1):181–185. doi:10.1016/j.bmcl.2013.11.044
79. Chu X, Jiang L, Wei M, et al. Attenuation of allergic airway inflammation in a murine model of asthma by Licochalcone A. Immunopharmacol Immunotoxicol. 2013;35(6):653–661. doi:10.3109/08923973.2013.834929
80. Franceschelli S, Pesce M, Ferrone A, et al. Biological effect of licochalcone C on the regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO signaling pathways in H9c2 cells in response to LPS stimulation. Int J Mol Sci. 2017;18(4):690.
81. Li CX, Li TH, Zhu M, Lai J, Wu ZP. Pharmacological properties of glabridin (a flavonoid extracted from licorice): a comprehensive review. J Funct Foods. 2021;85:104638. doi:10.1016/j.jff.2021.104638
82. Wang D, Liang J, Zhang J, Wang Y, Chai X. Natural chalcones in Chinese materia medica: licorice. Evidence Based Complement Altern Med. 2020;16. doi:10.1155/2020/3821248
83. Zhu L, Wei H, Wu Y, et al. Licorice isoliquiritigenin suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Int J Biochem Cell Biol. 2012;44(7):1139–1152. doi:10.1016/j.biocel.2012.04.003
84. Honda H, Nagai Y, Matsunaga T, et al. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor Toll-like receptor 4/MD-2 complex signaling in a different manner. J Leukoc Biol. 2012;91(6):967–976. doi:10.1189/jlb.0112038
85. Honda H, Nagai Y, Matsunaga T, et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet‐induced adipose tissue inflammation. J Leukoc Biol. 2014;96(6):1087–1100. doi:10.1189/jlb.3A0114-005RR
86. Nakamura S, Watanabe T, Tanigawa T, et al. Isoliquiritigenin ameliorates indomethacin-induced small intestinal damage by inhibiting NOD-like receptor family, pyrin domain-containing 3 inflammasome activation. Pharmacology. 2018;101(5–6):236–245. doi:10.1159/000486599
87. Wu Y, Chen X, Ge X, et al. Isoliquiritigenin prevents the progression of psoriasis-like symptoms by inhibiting NF-κB and proinflammatory cytokines. J Mol Med. 2016;94(2):195–206. doi:10.1007/s00109-015-1338-3
88. Wang R, Zhang CY, Bai LP, et al. Flavonoids derived from liquorice suppress murine macrophage activation by up-regulating heme oxygenase-1 independent of Nrf2 activation. Int Immunopharmacol. 2015;28(2):917–924. doi:10.1016/j.intimp.2015.03.040
89. Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. 2020;77(22):4459–4483. doi:10.1007/s00018-020-03536-5
90. Jasim HA, Nahar L, Jasim MA, Moore SA, Ritchie KJ, Sarker SD. Chalcones: synthetic chemistry follows where nature leads. Biomolecules. 2021;11(8). doi:10.3390/biom11081203
91. Torres de Pinedo A, Peñalver P, Morales JC. Synthesis and evaluation of new phenolic-based antioxidants: structure-activity relationship. Food Chem. 2007;103(1):55–61. doi:10.1016/j.foodchem.2006.07.026
92. Li W, Asada Y, Yoshikawa T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry. 2000;55(5):447–456. doi:10.1016/S0031-9422(00)00337-X
93. Haraguchi H, Ishikawa H, Mizutani K, Tamura Y, Kinoshita T. Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorganic Med Chem. 1998;6(3):339–347. doi:10.1016/S0968-0896(97)10034-7
94. Chen X, Liu Z, Meng R, Shi C, Guo N. Antioxidative and anticancer properties of Licochalcone A from licorice. J Ethnopharmacol. 2017;198:331–337.
95. Franceschelli S, Pesce M, Vinciguerra I, et al. Licocalchone-C extracted from glycyrrhiza glabra inhibits lipopolysaccharide-interferon-γ inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression. Molecules. 2011;16(7):5720–5734. doi:10.3390/molecules16075720
96. Liu H, Wang J, Zhou W, Wang Y, Yang L. Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J Ethnopharmacol. 2013;146(3):773–793. doi:10.1016/j.jep.2013.02.004
97. Asano T, Ishihara K, Morota T, Takeda S, Aburada M. Permeability of the flavonoids liquiritigenin and its glycosides in licorice roots and davidigenin, a hydrogenated metabolite of liquiritigenin, using human intestinal cell line Caco-2. J Ethnopharmacol. 2003;89(2–3):285–289. doi:10.1016/j.jep.2003.09.009
98. Zang Y. Pharmacological activities of coumarin compounds in licorice: a review. Nat Prod Commun. 2020;15(9):1–17.
99. Fylaktakidou K, Hadjipavlou-Litina D, Litinas K, Nicolaides D. Natural and synthetic coumarin derivatives with anti-inflammatory / antioxidant activities. Curr Pharm Des. 2005;10(30):3813–3833. doi:10.2174/1381612043382710
100. Nizioł-łukaszewska Z, Bujak T. Saponins as natural raw materials for increasing the safety of bodywash cosmetic use. J Surfactants Deterg. 2018;21(6):767–776. doi:10.1002/jsde.12168
101. Simayi Z, Rozi P, Yang X, et al. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: systematic review. Int J Biol Macromol. 2021;183:387–398.
102. Cerulli A, Masullo M, Montoro P, Piacente S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and Their Constituents as Active Cosmeceutical Ingredients. Cosmet. 2022;9(1):7. doi:10.3390/cosmetics9010007
103. Hani U, Yasmin Begum M, Wahab S, et al. Review of current perspectives on novel drug delivery systems and approaches for lung cancer management. J Pharm Innov. 2021;24:1–24.
104. Ahmad MF. Ganoderma lucidum: a rational pharmacological approach to surmount cancer. J Ethnopharmacol. 2020;260. doi:10.1016/j.jep.2020.113047
105. Tuli HS, Kumar G, Sandhu SS, Sharma AK, Kashyap D. Apoptotic effect of cordycepin on A549 human lung cancer cell line. Turkish J Biol. 2015;39(2):306–311. doi:10.3906/biy-1408-14
106. Kashyap D, Sharma A, Tuli HS, et al. Apigenin: a natural bioactive flavone-type molecule with promising therapeutic function. J Funct Foods. 2018;484:57–471.
107. Wu C-P, Ohnuma S, Ambudkar V. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12(4):609–620. doi:10.2174/138920111795163887
108. Liu C, Ho PCL, Wong FC, Sethi G, Wang LZ, Goh BC. Garcinol: current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015;362(1):8–14. doi:10.1016/j.canlet.2015.03.019
109. Prasannan R, Kalesh KA, Shanmugam MK, et al. Key cell signaling pathways modulated by zerumbone: role in the prevention and treatment of cancer. Biochem Pharmacol. 2012;84(10):1268–1276. doi:10.1016/j.bcp.2012.07.015
110. Tuli HS, Mistry H, Kaur G, et al. Gallic acid: a dietary polyphenol that exhibits anti-neoplastic activities by modulating multiple oncogenic targets. Anticancer Agents Med Chem. 2021;22(3):499–514. doi:10.2174/1871520621666211119085834
111. Guo X, Hu H, Jin Q, Li H, Cheng Q. Studies on the proliferation inhibition effects of tua from actinidia chinensis radix on lung cancer xenografts in nude mice and its preliminary mechanism. Trends Immunother. 2021;5(21):14–23. doi:10.24294/ti.v5.i2.1.1371
112. Al-Radadi NS. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: antimicrobial and anticancer study against HepG2 cell line. Arab J Chem. 2021;14(2):102956. doi:10.1016/j.arabjc.2020.102956
113. Vlaisavljević S, Šibul F, Sinka I, Zupko I, Ocsovszki I, Jovanović-šanta S. Chemical composition, antioxidant and anticancer activity of licorice from Fruska Gora locality. Ind Crops Prod. 2018;112:217–224. doi:10.1016/j.indcrop.2017.11.050
114. Hosseinzadeh H, Nassiri-Asl M. Pharmacological effects of glycyrrhiza spp. and its bioactive constituents: update and review. Phytother Res. 2015;29(12):1868–1886. doi:10.1002/ptr.5487
115. Li X, Guo R, Zhang X, Li X. Extraction of glabridin using imidazolium-based ionic liquids. Sep Purif Technol. 2012;88:146–150.
116. Çevik D, Kan Y, Akhan Güzelcan E, Durmaz I, Çetin-atalay R, Kırmızıbekmez H. Bioactivity-guided isolation of cytotoxic secondary metabolites from the roots of Glycyrrhiza glabra and elucidation of their mechanisms of action. Ind Crops Prod. 2018;124:389–396. doi:10.1016/j.indcrop.2018.08.014
117. Zhu K, Li K, Wang H, Kang L, Dang C, Zhang Y. Discovery of glabridin as potent inhibitor of epidermal growth factor receptor in SK-BR-3 cell. Pharmacology. 2019;104(3–4):113–125. doi:10.1159/000496798
118. Huang HL, Hsieh MJ, Chien MH, Chen HY, Yang SF, Hsiao PC. Glabridin mediate caspases activation and induces apoptosis through JNK1/2 and p38 MAPK pathway in human promyelocytic leukemia cells. PLoS One. 2014;9(6).
119. Lin KW, Huang AM, Hour TC, Yang SC, Pu YS, Lin CN. 18β-Glycyrrhetinic acid derivatives induced mitochondrial-mediated apoptosis through reactive oxygen species-mediated p53 activation in NTUB1 cells. Bioorganic Med Chem. 2011;19(14):4274–4285. doi:10.1016/j.bmc.2011.05.054
120. Sharma G, Kar S, Palit S, Das PK. 18β-glycyrrhetinic acid (concur) induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J Cell Physiol. 2012;227(5):1923–1931. doi:10.1002/jcp.22920
121. Satomi Y, Nishino H, Shibata S. Glycyrrhetinic acid and related compounds induce G1 arrest and apoptosis in human hepatocellular carcinoma HepG2. Anticancer Res. 2005;25(6B):4043–4047.
122. Thiugnanam S, Xu L, Ramaswamy K, Gnanasekar M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol Rep. 2008;20(6):1387–1392.
123. Lin SC, Chu PY, Liao WT, et al. Glycyrrhizic acid induces human MDA-MB-231 breast cancer cell death and autophagy via the ROS-mitochondrial pathway. Oncol Rep. 2018;39(2):703–710. doi:10.3892/or.2017.6123
124. Wang H, Ge X, Qu H, et al. Glycyrrhizic acid inhibits proliferation of gastric cancer cells by inducing cell cycle arrest and apoptosis. Cancer Manag Res. 2020;12:2853–2861. doi:10.2147/CMAR.S244481
125. Katsori AM, Hadjipavlou-Litina D. Recent progress in therapeutic applications of chalcones. Expert Opin Ther Pat. 2011;21(10):1575–1596. doi:10.1517/13543776.2011.596529
126. Tang ZH, Li T, Tong YG, et al. A systematic review of the anticancer properties of compounds isolated from licorice (gancao). Planta Med. 2015;81(18):1670–1687. doi:10.1055/s-0035-1558227
127. Cho JJ, Chae JI, Yoon G, et al. a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int J Oncol. 2014;45(2):667–674. doi:10.3892/ijo.2014.2461
128. Xiao XY, Hao M, Yang XY, et al. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011;302(1):69–75. doi:10.1016/j.canlet.2010.12.016
129. Fu Y, Hsieh TC, Guo J, et al. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun. 2004;322(1):263–270. doi:10.1016/j.bbrc.2004.07.094
130. Lu WJ, Wu GJ, Chen RJ, et al. Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest. Food Funct. 2018;9(8):4500–4507. doi:10.1039/C8FO00728D
131. Yuan X, Li T, Xiao E, et al. LicochalconeB inhibits growth of bladder cancer cells by arresting cell cycle progression and inducing apoptosis. Food Chem Toxicol. 2014;65:242–251. doi:10.1016/j.fct.2013.12.030
132. Kang TH, Yoon G, Kang IA, Oh HN, Chae JI, Shim JH. Natural compound licochalcone B Induced extrinsic and intrinsic apoptosis in human skin melanoma (A375) and squamous cell carcinoma (A431) cells. Phyther Res. 2017;31(12):1858–1867. doi:10.1002/ptr.5928
133. Ngameni B, Touaibia M, Patnam R, et al. Inhibition of MMP-2 secretion from brain tumor cells suggests chemopreventive properties of a furanocoumarin glycoside and of chalcones isolated from the twigs of Dorstenia turbinata. Phytochemistry. 2006;67(23):2573–2579. doi:10.1016/j.phytochem.2006.09.017
134. Si L, Yan X, Hao W, et al. Licochalcone D induces apoptosis and inhibits migration and invasion in human melanoma A375 cells. Oncol Rep. 2018;39(5):2160–2170. doi:10.3892/or.2018.6329
135. Wang P, Yuan X, Wang Y, Zhao H, Sun X, Zheng Q. Licochalcone C induces apoptosis via B-cell lymphoma 2 family proteins in T24 cells. Mol Med Rep. 2015;12(5):7623–7628. doi:10.3892/mmr.2015.4346
136. Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: novel approach for breast cancer therapy. Semin Cancer Biol. 2016;40:192–208. doi:10.1016/j.semcancer.2016.09.001
137. Zhou Y, Ho WS. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol Rep. 2014;31(1):298–304. doi:10.3892/or.2013.2849
138. Kim DH, Park JE, Chae IG, Park G, Lee SY, Chun KS. Isoliquiritigenin inhibits the proliferation of human renal carcinoma Caki cells through the ROS-mediated regulation of the Jak2/STAT3 pathway. Oncol Rep. 2017;38(1):575–583. doi:10.3892/or.2017.5677
139. Hsia SM, Yu CC, Shih YH, et al. Isoliquiritigenin as a cause of DNA damage and inhibitor of ataxia-telangiectasia mutated expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinoma. Head Neck. 2016;38:E360–E371. doi:10.1002/hed.24001
140. Kwak AW, Choi JS, Lee MH, et al. Retrochalcone echinatin triggers apoptosis of esophageal squamous cell carcinoma via rosand ER stress-mediated signaling pathways. Molecules. 2019;24(22). doi:10.3390/molecules24224055
141. Pauty J, Usuba R, Cheng IG, et al. A vascular endothelial growth factor-dependent sprouting angiogenesis assay based on an in vitro human blood vessel model for the study of anti-angiogenic drugs. EBioMedicine. 2018;27:225–236. doi:10.1016/j.ebiom.2017.12.014
142. Kim C, Lee SG, Yang WM, et al. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018;431:123–141. doi:10.1016/j.canlet.2018.05.038
143. Siveen KS, Ahn KS, Ong TH, et al. γ-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget. 2014;5(7):1897–1911. doi:10.18632/oncotarget.1876
144. Yang Y, Ren L, Yang H, et al. Research progress on anti-angiogenesis drugs in hepatocellular carcinoma. Cancer Plus. 2021;3(2).
145. Kashyap D, Mondal R, Tuli HS, Kumar G, Sharma AK. Molecular targets of gambogic acid in cancer: recent trends and advancements. Tumor Biol. 2016;37(10):12915–12925. doi:10.1007/s13277-016-5194-8
146. Aggarwal V, Kashyap D, Sak K, et al. Molecular mechanisms of action of tocotrienols in cancer: recent trends and advancements. Int J Mol Sci. 2019;20(3). doi:10.3390/ijms20030656
147. Sharma A, Kashyap D, Sak K, Tuli HS, Sharma AK. Therapeutic charm of quercetin and its derivatives: a review of research and patents. Pharm Pat Anal. 2018;7(1):15–32. doi:10.4155/ppa-2017-0030
148. Yadav P, Jaswal V, Sharma A, et al. Celastrol as a pentacyclic triterpenoid with chemopreventive properties. Pharm Pat Anal. 2018;7(4):155–167. doi:10.4155/ppa-2017-0035
149. Hoseinkhani Z, Norooznezhad F, Rastegari-Pouyani M, Mansouri K. Medicinal plants extracts with antiangiogenic activity: where is the link? Adv Pharm Bull. 2020;10(3):370–378. doi:10.34172/apb.2020.045
150. Tuli HS, Sak K, Iqubal A, et al. STAT signaling as a target for intervention: from cancer inflammation and angiogenesis to non-coding RNAs modulation. Mol Biol Rep. 2022;49:8987–8999. doi:10.1007/s11033-022-07399-w
151. Ma Z, Wang LZ, Cheng JT, et al. Targeting hypoxia-inducible factor-1-mediated metastasis for cancer therapy. Antioxid Redox Signal. 2021;34(18):1484–1497. doi:10.1089/ars.2019.7935
152. Wang XF, Zhou QM, Lu YY, Zhang H, Huang S, Su SB. Glycyrrhetinic acid potently suppresses breast cancer invasion and metastasis by impairing the p38 MAPK-AP1 signaling axis. Expert Opin Ther Targets. 2015;19(5):577–587. doi:10.1517/14728222.2015.1012156
153. Sheela ML, Ramakrishna MK, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol. 2006;6(3):494–498. doi:10.1016/j.intimp.2005.07.002
154. Kim YH, Shin EK, Kim DH, Lee HH, Park JHY, Kim JK. Antiangiogenic effect of licochalcone A. Biochem Pharmacol. 2010;80(8):1152–1159. doi:10.1016/j.bcp.2010.07.006
155. Kwon SJ, Park SY, Kwon GT, et al. Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. Cancer Prev Res. 2013;6(6):603–613. doi:10.1158/1940-6207.CAPR-13-0012
156. Jiang F, Mu J, Wang X, et al. The repressive effect of miR-148a on TGF beta-SMADs signal pathway is involved in the glabridin-induced inhibition of the cancer stem cells-like properties in hepatocellular carcinoma cells. PLoS One. 2014;9(5).
157. Kim M, Park SC, Lee DY. Glycyrrhizin as a nitric oxide regulator in cancer chemotherapy. Cancers. 2021;13(22):5762.
158. Shanmugam MK, Rajendran P, Li F, et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J Mol Med. 2011;89(7):713–727. doi:10.1007/s00109-011-0746-2
159. Manu KA, Shanmugam MK, Ramachandran L, et al. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin Cancer Res. 2012;18(8):2220–2229. doi:10.1158/1078-0432.CCR-11-2470
160. Kashyap D, Garg VK, Tuli HS, et al. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules. 2019;9(5):174. doi:10.3390/biom9050174
161. Kashyap D, Tuli HS, Sharma AK. Ursolic acid (UA): a metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi:10.1016/j.lfs.2016.01.017
162. Kashyap D, Pal D, Sharma R, et al. Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res Int. 2022;2022:1–16. doi:10.1155/2022/9605439
163. Kashyap D, Tuli HS, Sak K, Garg VK, Goel N, Punia S. Role of reactive oxygen species in cancer progression. Curr Pharmacol Rep. 2019;5:79–86. doi:10.1007/s40495-019-00171-y
164. Zhang J, Ahn KS, Kim C, et al. Nimbolide-induced oxidative stress abrogates STAT3 signaling cascade and inhibits tumor growth in transgenic adenocarcinoma of mouse prostate model. Antioxidants Redox Signal. 2016;24(11):575–589. doi:10.1089/ars.2015.6418
165. Kim SM, Lee JH, Sethi G, et al. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014;354(1):153–163. doi:10.1016/j.canlet.2014.08.002
166. Luna J, Sotoca A, Fernández P, Miralles C, Rodríguez A. Recent advances in early stage lung cancer. J Clin Transl Res. 2021;7(2):163.
167. Kang SY, Hwang D, Shin S, et al. Potential of bioactive food components against gastric cancer: insights into molecular mechanism and therapeutic targets. Cancers. 2021;13(18):4502.
168. Monisha J, Roy NK, Padmavathi G, et al. NGAL is downregulated in oral squamous cell carcinoma and leads to increased survival, proliferation, migration and chemoresistance. Cancers. 2018;10(7):228. doi:10.3390/cancers10070228
169. Kothapalli R, Siveen KS, Tan TZ, et al. Functional characterization of selective exosite-binding inhibitors of matrix metalloproteinase-13 (MMP-13) - Experimental validation in human breast and colon cancer. Biosci Biotechnol Biochem. 2016;80(11):2122–2131. doi:10.1080/09168451.2016.1200456
170. Tay K-C, Tan LT-H, Chan CK, et al. Formononetin: a review of its anticancer potentials and mechanisms. Front Pharmacol. 2019;10. doi:10.3389/fphar.2019.00820
171. Ko JH, Um JY, Lee SG, Yang WM, Sethi G, Ahn KS. Conditioned media from adipocytes promote proliferation, migration, and invasion in melanoma and colorectal cancer cells. J Cell Physiol. 2019;234(10):18249–18261. doi:10.1002/jcp.28456
172. Jung YY, Lee JH, Nam D, et al. Anti-myeloma effects of icariin are mediated through the attenuation of JAK/STAT3-dependent signaling cascade. Front Pharmacol. 2018;9:531.
173. Tsai JP, Hsiao PC, Yang SF, et al. Licochalcone a suppresses migration and invasion of human hepatocellular carcinoma cells through downregulation of MKK4/JNK via NF-κB mediated urokinase plasminogen activator expression. PLoS One. 2014;9(1). doi:10.1371/journal.pone.0086537
174. Dai X, Wang L, Deivasigamni A, et al. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget. 2017;8(8):12831–12842. doi:10.18632/oncotarget.14606
175. Ong PS, Wang LZ, Dai X, Tseng SH, Loo SJ, Sethi G. Judicious toggling of mTOR activity to combat insulin resistance and cancer: current evidence and perspectives. Front Pharmacol. 2016;73:95.
176. Ko JH, Lee JH, Jung SH, et al. 2,5-dihydroxyacetophenone induces apoptosis of multiple myeloma cells by regulating the MAPK activation pathway. Molecules. 2017;22(7):1157. doi:10.3390/molecules22071157
177. Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):1–17. doi:10.1038/s41392-019-0089-y
178. Siveen KS, Nguyen AH, Lee JH, et al. Negative regulation of signal transducer and activator of transcription-3 signalling cascade by lupeol inhibits growth and induces apoptosis in hepatocellular carcinoma cells. Br J Cancer. 2014;111(7):1327–1337. doi:10.1038/bjc.2014.422
179. Mirzaei S, Zarrabi A, Hashemi F, et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: inhibiting or promoting carcinogenesis? Cancer Lett. 2021;509:63–80. doi:10.1016/j.canlet.2021.03.025
180. Hsieh MJ, Lin CW, Yang SF, Chen MK, Chiou HL. Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-κB and AP-1 activity in human liver cancer cells. Br J Pharmacol. 2014;171(12):3037–3050. doi:10.1111/bph.12626
181. Bozorgi A, Khazaei S, Khademi A, Khazaei M. Natural and herbal compounds targeting breast cancer, a review based on cancer stem cells. Iran J Basic Med Sci. 2020;23(8):970–983. doi:10.22038/ijbms.2020.43745.10270
182. Chen HY, Chiang YF, Huang JS, et al. Isoliquiritigenin reverses epithelial-mesenchymal transition through modulation of the tgf-β/smad signaling pathway in endometrial cancer. Cancers. 2021;13(6):1–20.
183. Dehshahri A, Ashrafizadeh M, Ghasemipour Afshar E, et al. Topoisomerase inhibitors: pharmacology and emerging nanoscale delivery systems. Pharmacol Res. 2020;151:104551. doi:10.1016/j.phrs.2019.104551
184. Paskeh MDA, Asadi S, Zabolian A, et al. Targeting cancer stem cells by dietary agents: an important therapeutic strategy against human malignancies. Int J Mol Sci. 2021;22(21):11669. doi:10.3390/ijms222111669
185. Srivani G, Peela S, Alam A, Nagaraju GP. Gemcitabine for pancreatic cancer. Cancer Plus. 2021;69:24–42.
186. Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3(1):7. doi:10.1038/s41392-017-0004-3
187. Carrasco-Esteban E, Domínguez-Rullán JA, Barrionuevo-Castillo P, et al. Current role of nanoparticles in the treatment of lung cancer. J Clin Transl Res. 2021;7(2):140.
188. Mirzaei S, Gholami MH, Zabolian A, et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: new hope in the fight against cancer. Pharmacol Res. 2021;171:105759. doi:10.1016/j.phrs.2021.105759
189. Ashrafizadeh M, Ahmadi Z, Kotla NG, et al. Nanoparticles targeting STATs in cancer therapy. Cells. 2019;8(10):1158. doi:10.3390/cells8101158
190. Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25(1):55–71. doi:10.1007/s11095-007-9348-7
191. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85. doi:10.1186/s13045-021-01096-0
192. Enrico C. Nanotechnology-based drug delivery of natural compounds and phytochemicals for the treatment of cancer and other diseases. In: Studies in Natural Products Chemistry. Elsevier B.V; 2019:91–123.
193. Li Y-L, Zhu X-M, Liang H, Orvig C, Chen Z-F. Recent advances in asialoglycoprotein receptor and glycyrrhetinic acid receptor-mediated and/or pH-responsive hepatocellular carcinoma- targeted drug delivery. Curr Med Chem. 2020;28(8):1508–1534. doi:10.2174/0929867327666200505085756
194. Seon MR, Park SY, Kwon SJ, et al. Hexane/ethanol extract of Glycyrrhiza uralensis and its active compound isoangustone A induce G1 cycle arrest in DU145 human prostate and 4T1 murine mammary cancer cells. J Nutr Biochem. 2012;23(1):85–92. doi:10.1016/j.jnutbio.2010.11.010
195. Lee E, Son JE, Byun S, et al. CDK2 and mTOR are direct molecular targets of isoangustone A in the suppression of human prostate cancer cell growth. Toxicol Appl Pharmacol. 2013;272(1):12–20. doi:10.1016/j.taap.2013.04.030
196. Huang W, Tang S, Qiao X, et al. Isoangustone A induces apoptosis in SW480 human colorectal adenocarcinoma cells by disrupting mitochondrial functions. Fitoterapia. 2014;94:36–47. doi:10.1016/j.fitote.2014.01.016
197. Tang S, Cai S, Ji S, et al. A induces autophagic cell death in colorectal cancer cells by activating AMPK signaling. Fitoterapia. 2021;152:104935. doi:10.1016/j.fitote.2021.104935
198. Kim JS, Park MR, Lee SY, et al. Licochalcone a induces apoptosis in KB human oral cancer cells via a caspase-dependent FasL signaling pathway. Oncol Rep. 2014;31(2):755–762. doi:10.3892/or.2013.2929
199. Lin RC, Yang SF, Chiou HL, et al. Licochalcone A-induced apoptosis through the activation of p38MAPK pathway mediated mitochondrial pathways of apoptosis in human osteosarcoma cells in vitro and in vivo. Cells. 2019;8(11):1441. doi:10.3390/cells8111441
200. Wang J, Zhang YS, Thakur K, et al. Licochalcone A from licorice root, an inhibitor of human hepatoma cell growth via induction of cell apoptosis and cell cycle arrest. Food Chem Toxicol. 2018;120:407–417. doi:10.1016/j.fct.2018.07.044
201. Wu P, Yu T, Wu J, Chen J, Chen J. Licochalcone a induces ROS-mediated apoptosis through trxr1 inactivation in colorectal cancer cells. Biomed Res Int. 2020;2020:1–11.
202. Yu SJ, Cho IA, Kang KR, et al. Licochalcone-E induces caspase-dependent death of human pharyngeal squamous carcinoma cells through the extrinsic and intrinsic apoptotic signaling pathways. Oncol Lett. 2017;13(5):3662–3668. doi:10.3892/ol.2017.5865
203. Zhang Q, Zhang J, Liu B, Wei J. Licochalcone E inhibits trxR1 expression, alters Nrf2/STAT6 signal, and induces antitumor effects in vitro against human SH-SY5Y and SK-N-BE(2) neuroblastoma cells. Environ Toxicol. 2022;37(5):1173–1184. doi:10.1002/tox.23474
204. Farese RV, Biglieri EG, Shackleton CHL, Irony I, Gomez-Fontes R. Licorice-Induced Hypermineralocorticoidism. N Engl J Med. 1991;325(17):1223–1227. doi:10.1056/NEJM199110243251706
205. Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;46(3):167–192. doi:10.1016/j.yrtph.2006.06.002
206. Celik MM, Karakus A, Zeren C, et al. Licorice induced hypokalemia, edema, and thrombocytopenia. Hum Exp Toxicol. 2012;31(12):1295–1298. doi:10.1177/0960327112446843
207. Strandberg TE, Järvenpää AL, Vanhanen H, McKeigue PM. Birth outcome in relation to licorice consumption during pregnancy. Am J Epidemiol. 2001;153(11):1085–1088. doi:10.1093/aje/153.11.1085
208. Strandberg TE, Andersson S, Järvenpää AL, McKeigue PM. Preterm birth and licorice consumption during pregnancy. Am J Epidemiol. 2002;156(9):803–805. doi:10.1093/aje/kwf130
209. Kwon YJ, Son DH, Chung TH, Lee YJ. A review of the pharmacological efficacy and safety of licorice root from corroborative clinical trial findings. J Med Food. 2020;23(1):12–20. doi:10.1089/jmf.2019.4459
210. Yamamura Y, Santa T, Kotaki H, Uchino K, Sawada Y, Iga T. Administration-route dependency of absorption of glycyrrhizin in rats: intraperitoneal administration dramatically enhanced bioavailability. Biol Pharm Bull. 1995;18(2):337–341. doi:10.1248/bpb.18.337
211. Jiang B, Qu H, Kong H, et al. The effects of sweet foods on the pharmacokinetics of glycyrrhizic acid by icELISA. Molecules. 2017;22(3):498. doi:10.3390/molecules22030498
212. Yamamura Y, Kawakami J, Santa T, et al. Pharmacokinetic profile of glycyrrhizin in healthy volunteers by a new high‐performance liquid chromatographic method. J Pharm Sci. 1992;81(10):1042–1046. doi:10.1002/jps.2600811018
213. Ishida S, Ichikawa T, Sakiya Y. Binding of glycyrrhetinic acid to rat plasma, rat serum albumin, human serum, and human serum albumin. Chem Pharm Bull. 1988;36(1):440–443. doi:10.1248/cpb.36.440
214. Akao T, Hayashi T, Kobashi K, et al. Intestinal bacterial hydrolysis is indispensable to absorption of 18β‐glycyrrhetic acid after oral administration of glycyrrhizin in rats. J Pharm Pharmacol. 1994;46(2):135–137. doi:10.1111/j.2042-7158.1994.tb03756.x
215. Wang YG, Zhou JM, Ma ZC, et al. Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: a possible mechanism for its hepatoprotective property against lithocholic acid-induced injury. Chem Biol Interact. 2012;200(1):11–20. doi:10.1016/j.cbi.2012.08.023
216. Kočevar Glavač N, Kreft S. Excretion profile of glycyrrhizin metabolite in human urine. Food Chem. 2012;131(1):305–308. doi:10.1016/j.foodchem.2011.08.081
217. Nabekura T, Yamaki T, Ueno K, Kitagawa S. Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals. Cancer Chemother Pharmacol. 2008;62(5):867–873. doi:10.1007/s00280-007-0676-4
218. Cao S, Zu Y, Zhang L, Huang Y, Shang X. Effect of oral administration of glycyrrhetinic acid on six metal elements in rat serum. Zhongguo Zhongyao Zazhi. 2012;37(4):490–494.
219. Zhou N, Zou C, Qin M, Li Y, Huang J. A simple method for evaluation pharmacokinetics of glycyrrhetinic acid and potential drug-drug interaction between herbal ingredients. Sci Rep. 2019;9(1):11308. doi:10.1038/s41598-019-47880-4
220. Lin D, Sun W, Wang Z, et al. The effect of glycyrrhetinic acid on pharmacokinetics of cortisone and its metabolite cortisol in rats. J Biomed Biotechnol. 2012;2012(0001447641):856324. doi:10.1155/2012/856324
221. Li HY, Xu W, Su J, Zhang X, Hu LW, Zhang WD. In vitro and in vivo inhibitory effects of glycyrrhetinic acid on cytochrome P450 3A activity. Pharmacology. 2010;86(5–6):287–292. doi:10.1159/000320956
222. Lu Y, Jing J, Ren W, et al. Biliary excretion of glycyrrhetinic acid: glucuronide-conjugate determination following a pharmacokinetic study of rat bile. Phyther Res. 2014;28(12):1887–1889. doi:10.1002/ptr.5170
223. Han B, Chen W, Zheng Q, et al. Determination of isoliquiritigenin and its distribution in mice by synchronous fluorescence spectrometry. Anal Sci. 2011;27(11):1115–1119. doi:10.2116/analsci.27.1115
224. Guo J, Liu D, Nikolic D, Zhu D, Pezzuto JM, Van Breemen RB. In vitro metabolism of isoliquiritigenin by human liver microsomes. Drug Metab Dispos. 2008;36(2):461–468. doi:10.1124/dmd.107.018721
225. Vaya J, Mahmood S, Goldblum A, et al. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry. 2003;62(1):89–99. doi:10.1016/S0031-9422(02)00445-4
226. Wang S, Dunlap TL, Huang L, et al. Evidence for chemopreventive and resilience activity of licorice: glycyrrhiza glabra and G. Inflata extracts modulate estrogen metabolism in ACI rats. Cancer Prev Res. 2018;11(12):819–830. doi:10.1158/1940-6207.CAPR-18-0178
227. Van De Schans MGM, Bovee TFH, Stoopen GM, Lorist M, Gruppen H, Vincken JP. Prenylation and backbone structure of flavonoids and isoflavonoids from licorice and hop influence their phase I and II metabolism. J Agric Food Chem. 2015;63(49):10628–10640. doi:10.1021/acs.jafc.5b04703
228. He W, Wu JJ, Ning J, et al. Inhibition of human cytochrome P450 enzymes by licochalcone A, a naturally occurring constituent of licorice. Toxicol Vitr. 2015;29(7):1569–1576. doi:10.1016/j.tiv.2015.06.014
229. Zhang F, Liu W, Huang J, et al. Inhibition of drug-metabolizing enzymes by Jingyin granules: implications of herb–drug interactions in antiviral therapy. Acta Pharmacol Sin. 2022;43(4):1072–1081. doi:10.1038/s41401-021-00697-2
230. Huang L, Nikolic D, van Breemen RB. Hepatic metabolism of licochalcone A, a potential chemopreventive chalcone from licorice (Glycyrrhiza inflata), determined using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2017;409(30):6937–6948. doi:10.1007/s00216-017-0642-x
231. Choi JS, Choi JS, Choi DH. Effects of licochalcone A on the bioavailability and pharmacokinetics of nifedipine in rats: possible role of intestinal CYP3A4 and P-gp inhibition by licochalcone A. Biopharm Drug Dispos. 2014;35(7):382–390. doi:10.1002/bdd.1905
232. Goel B, Sharma A, Tripathi N, et al. In-vitro antitumor activity of compounds from Glycyrrhiza glabra against C6 glioma cancer cells: identification of natural lead for further evaluation. Nat Prod Res. 2021;35(23):5489–5492. doi:10.1080/14786419.2020.1786830
233. Zhang B, Yan M, Zhang W, Ke ZY, Ma LG. Glycyrrhiza glabra suppresses nasopharyngeal carcinoma cell proliferation through inhibiting the expression of lncRNA, AK027294. Biosci Biotechnol Biochem. 2020;84(2):314–320. doi:10.1080/09168451.2019.1673695
234. Zheng C, Han L, Wu S. A metabolic investigation of anticancer effect of G. glabra root extract on nasopharyngeal carcinoma cell line, C666-1. Mol Biol Rep. 2019;46(4):3857–3864. doi:10.1007/s11033-019-04828-1
235. Chen G, Hu X, Zhang W, et al. Mammalian target of rapamycin regulates isoliquiritigenin induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis. 2012;17(1):90–101. doi:10.1007/s10495-011-0658-1
236. Nourazarian SM, Nourazarian A, Majidinia M, Roshaniasl E. Effect of root extracts of medicinal herb Glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pacific J Cancer Prev. 2016;16(18):8563–8566. doi:10.7314/APJCP.2015.16.18.8563
237. Zhu J, Huang R, Yang R, et al. Licorice extract inhibits growth of non-small cell lung cancer by down-regulating CDK4-Cyclin D1 complex and increasing CD8+ T cell infiltration. Cancer Cell Int. 2021;21(1):1–18. doi:10.1186/s12935-021-02223-0
238. Xiu-Rong Z, Shi-Yao W, Wen S, Chao W. Isoliquiritigenin inhibits proliferation and metastasis of MKN28 gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. Mol Med Rep. 2018;18(3):3429–3436. doi:10.3892/mmr.2018.9318
239. Song L, Luo Y, Li S, et al. Isl induces apoptosis and autophagy in hepatocellular carcinoma via downregulation of pi3k/akt/mtor pathway in vivo and in vitro. Drug Des Devel Ther. 2020;14:4363–4376. doi:10.2147/DDDT.S270124
240. Niu Q, Zhao W, Wang J, et al. LicA induces autophagy through ULK1/Atg13 and ROS pathway in human hepatocellular carcinoma cells. Int J Mol Med. 2018;41(5):2601–2608. doi:10.3892/ijmm.2018.3499
241. Lin PH, Chiang YF, Shieh TM, et al. Dietary compound isoliquiritigenin, an antioxidant from licorice, suppresses triple-negative breast tumor growth via apoptotic death program activation in cell and xenograft animal models. Antioxidants. 2020;9(3). doi:10.3390/antiox9030228
242. Huang W-C, Su -H-H, Fang L-W, Wu S-J, Liou C-J. Licochalcone A inhibits cellular motility by suppressing E-cadherin and MAPK signaling in breast cancer. Cells. 2019;8(3):218. doi:10.3390/cells8030218
243. Jiang YX, Dai YY, Pan YF, et al. Total flavonoids from radix Glycyrrhiza exert anti-inflammatory and antitumorigenic effects by inactivating iNOS signaling pathways. Evidence Based Complement Altern Med. 2018;2018:1–10. doi:10.1155/2018/6714282
244. Bortolotto LFB, Barbosa FR, Silva G, et al. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomed Pharmacother. 2017;85:425–433. doi:10.1016/j.biopha.2016.11.047
245. Dong S, Inoue A, Zhu Y, Tanji M, Kiyama R. Activation of rapid signaling pathways and the subsequent transcriptional regulation for the proliferation of breast cancer MCF-7 cells by the treatment with an extract of Glycyrrhiza glabra root. Food Chem Toxicol. 2007;45(12):2470–2478. doi:10.1016/j.fct.2007.05.031
246. Park SY, Kwon SJ, Lim SS, Kim JK, Lee KW, Park JHY. Licoricidin, an active compound in the hexane/ethanol extract of Glycyrrhiza uralensis, inhibits lung metastasis of 4T1 murine mammary carcinoma cells. Int J Mol Sci. 2016;17(6).
247. Si L, Yang X, Yan X, Wang Y, Zheng Q. Isoliquiritigenin induces apoptosis of human bladder cancer T24 cells via a cyclin-dependent kinase-independent mechanism. Oncol Lett. 2017;14(1):241–249. doi:10.3892/ol.2017.6159
248. Yuan X, Li D, Zhao H, et al. Licochalcone A-induced human bladder cancer T24 cells apoptosis triggered by mitochondria dysfunction and endoplasmic reticulum stress. Biomed Res Int. 2013;2013:1–9. doi:10.1155/2013/474272
249. Wu CH, Chen HY, Wang CW, et al. Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget. 2016;7(45):73432–73447. doi:10.18632/oncotarget.12369
250. Gioti K, Papachristodoulou A, Benaki D, et al. Glycyrrhiza glabra-enhanced extract and adriamycin antiproliferative effect on PC-3 prostate cancer cells. Nutr Cancer. 2020;72(2):320–332. doi:10.1080/01635581.2019.1632357
251. Chen HY, Huang TC, Shieh TM, Wu CH, Lin LC, Hsia SM. Isoliquiritigenin induces autophagy and inhibits ovarian cancer cell growth. Int J Mol Sci. 2017;18(10).
252. Tsai JP, Lee CH, Ying TH, et al. Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances Licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget. 2015;6(30):28851–28866. doi:10.18632/oncotarget.4767
253. Li C, Zhou X, Sun C, Liu X, Shi X, Wu S. Isoliquiritigenin inhibits the proliferation, apoptosis and migration of osteosarcoma cells. Oncol Rep. 2019;41(4):2502–2510. doi:10.3892/or.2019.6998
254. Chen J, Liu C, Yang QQ, et al. Isoliquiritigenin suppresses osteosarcoma U2OS cell proliferation and invasion by regulating the PI3K/Akt signalling pathway. Chemotherapy. 2018;63(3):155–161. doi:10.1159/000490151
255. Ayeka PA, Bian Y, Mwitari PG, et al. Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complement Altern Med. 2016;16(1). doi:10.1186/s12906-016-1171-4
256. Huang Y, Liu C, Zeng WC, et al. Isoliquiritigenin inhibits the proliferation, migration and metastasis of Hep3B cells via suppressing cyclin D1 and PI3K/AKT pathway. Biosci Rep. 2020;40(1).
257. Shi H, Wu Y, Wang Y, et al. Liquiritigenin potentiates the inhibitory effects of cisplatin on invasion and metastasis via downregulation MMP-2/9 and PI3 K/AKT signaling pathway in B16F10 melanoma cells and mice model. Nutr Cancer. 2015;67(5):761–770. doi:10.1080/01635581.2015.1037962
258. Wakamatsu T, Nakahashi Y, Hachimine D, Seki T, Okazaki K. The combination of glycyrrhizin and lamivudine can reverse the cisplatin resistance in hepatocellular carcinoma cells through inhibition of multidrug resistance-associated proteins. Int J Oncol. 2007;31(6):1465–1472.
259. Kim YJ, Jung EB, Myung SC, Kim W, Lee CS. Licochalcone a enhances geldanamycin-induced apoptosis through reactive oxygen species-mediated caspase activation. Pharmacology. 2013;92(1–2):49–59. doi:10.1159/000351846
260. Zhao H, Yuan X, Li D, et al. Isoliquiritigen enhances the antitumour activity and decreases the genotoxic effect of cyclophosphamide. Molecules. 2013;18(8):8786–8798. doi:10.3390/molecules18088786
261. Wang Y, Wang S, Liu J, Lu Y, Li D. Licoricidin enhances gemcitabine-induced cytotoxicity in osteosarcoma cells by suppressing the Akt and NF-κB signal pathways. Chem Biol Interact. 2018;290:44–51. doi:10.1016/j.cbi.2018.05.007
262. Lee CK, Son SH, Park KK, et al. Licochalcone a inhibits the growth of colon carcinoma and attenuates cisplatin-induced toxicity without a loss of chemotherapeutic efficacy in mice. Basic Clin Pharmacol Toxicol. 2008;103(1):48–54. doi:10.1111/j.1742-7843.2008.00238.x
263. Wang QS, Gao LN, Zhu XN, et al. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics. 2019;9(21):6239–6255. doi:10.7150/thno.35972
264. Ochi MM, Amoabediny G, Rezayat SM, Akbarzadeh A, Ebrahimi B. In vitro co-delivery evaluation of novel pegylated nano-liposomal herbal drugs of silibinin and glycyrrhizic acid (Nano-phytosome) to hepatocellular carcinoma cells. Cell J. 2016;18(2):135–148. doi:10.22074/cellj.2016.4308
265. Zhang Q, Feng Z, Wang H, et al. Preparation of camptothecin micelles self-assembled from disodium glycyrrhizin and tannic acid with enhanced antitumor activity. Eur J Pharm Biopharm. 2021;164:75–85. doi:10.1016/j.ejpb.2021.04.012
266. Chopdey PK, Tekade RK, Mehra NK, Mody N, Jain NK. Glycyrrhizin conjugated dendrimer and multi-walled carbon nanotubes for liver specific delivery of doxorubicin. J Nanosci Nanotechnol. 2015;15(2):1088–1100. doi:10.1166/jnn.2015.9039
267. Wu M, Lian B, Deng Y, et al. Resveratrol-loaded glycyrrhizic acid-conjugated human serum albumin nanoparticles wrapping resveratrol nanoparticles: preparation, characterization, and targeting effect on liver tumors. J Biomater Appl. 2017;32(2):191–205. doi:10.1177/0885328217713357
268. El-Marakby EM, Hathout RM, Taha I, Mansour S, Mortada ND. A novel serum-stable liver targeted cytotoxic system using valerate-conjugated chitosan nanoparticles surface decorated with glycyrrhizin. Int J Pharm. 2017;525(1):123–138. doi:10.1016/j.ijpharm.2017.03.081
269. Wu F, Xue H, Li X, et al. Enhanced targeted delivery of adenine to hepatocellular carcinoma using glycyrrhetinic acid-functionalized nanoparticles in vivo and in vitro. Biomed Pharmacother. 2020;131(1):110682. doi:10.1016/j.biopha.2020.110682
270. Xue H, Qin L, Zhang L, et al. Preparation of docetaxel‑loaded, glycyrrhetinic acid‑modified nanoparticles and their liver‑targeting and antitumor activity. Exp Ther Med. 2021;22(4):1144. doi:10.3892/etm.2021.10578
271. Mezghrani O, Tang Y, Ke X, et al. Hepatocellular carcinoma dually-targeted nanoparticles for reduction triggered intracellular delivery of doxorubicin. Int J Pharm. 2015;478(2):553–568. doi:10.1016/j.ijpharm.2014.10.041
272. Chen G, Li J, Cai Y, et al. A glycyrrhetinic acid-modified curcumin supramolecular hydrogel for liver tumor targeting therapy. Sci Rep. 2017;7(1):44210. doi:10.1038/srep44210
273. Zhang C, Liu Z, Zheng Y, et al. Glycyrrhetinic acid functionalized graphene oxide for mitochondria targeting and cancer treatment in vivo. Small. 2018;14(4):1703306. doi:10.1002/smll.201703306
274. Lv Y, Li J, Chen H, Bai Y, Zhang L. Glycyrrhetinic acid-functionalized mesoporous silica nanoparticles as hepatocellular carcinoma-targeted drug carrier. Int J Nanomedicine. 2017;12:4361–4370. doi:10.2147/IJN.S135626
275. Tian G, Sun X, Bai J, et al. Doxorubicin-loaded dual-functional hyaluronic acid nanoparticles: preparation, characterization and antitumor efficacy in vitro and in vivo. Mol Med Rep. 2019;19(1):133–142. doi:10.3892/mmr.2018.9687
276. Qiao F, Zhao Y, Mai Y, et al. Isoliquiritigenin nanosuspension enhances cytostatic effects in A549 lung cancer cells. Planta Med. 2020;86(8):538–547. doi:10.1055/a-1134-3378
277. Gao F, Zhang J, Fu C, et al. iRGD-modified lipid–polymer hybrid nanoparticles loaded with isoliquiritigenin to enhance anti-breast cancer effect and tumor-targeting ability. Int J Nanomedicine. 2017;12:4147–4162. doi:10.2147/IJN.S134148
278. Wang G, Yu Y, Wang YZ, Yin PH, Xu K, Zhang H. The effects and mechanisms of isoliquiritigenin loaded nanoliposomes regulated AMPK/mTOR mediated glycolysis in colorectal cancer. Artif Cells Nanomed Biotechnol. 2020;48(1):1231–1249. doi:10.1080/21691401.2020.1825092
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.