Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Lichen Sclerosus of the Labial Mucosa: A Case Report and Literature Review
Authors Phuwaraks K, Rutnin S , Suchonwanit P
Received 6 November 2023
Accepted for publication 12 January 2024
Published 1 February 2024 Volume 2024:17 Pages 253—258
DOI https://doi.org/10.2147/CCID.S448367
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Koramon Phuwaraks, Suthinee Rutnin, Poonkiat Suchonwanit
Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Poonkiat Suchonwanit, Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok, 10400, Thailand, Tel +66-2-2011141, Fax +66-2-201-1211 Ext 4, Email [email protected]
Abstract: Lichen sclerosus (LS) is an uncommon, chronic, inflammatory mucocutaneous disorder found predominantly in females with unknown etiology. It presents as a white sclerotic plaque commonly located on the anogenital area. Extragenital LS is less prevalent, and LS affecting the oral mucosa is extremely rare, with only 39 biopsy-confirmed cases reported in the literature. Due to its several mimicking conditions, histological examination is usually required for a definitive diagnosis, particularly in patients with oral LS. Current evidence-based treatment recommendations for oral LS are unavailable; however, most cases tend to improve after treatment with topical or intralesional corticosteroids. We report a case of a 58-year-old female referred from the otolaryngology department for evaluating an asymptomatic whitish sclerotic plaque on the lower lip mucosa that had existed for 1 year. Following a punch biopsy, the patient was diagnosed with LS of labial mucosa. The condition improved after 2 months of treatment with topical and intralesional corticosteroids. The present case report raises awareness in recognizing oral LS and contributes to knowledge of this rare disorder.
Keywords: extragenital lichen sclerosus, lichen sclerosus et atrophicus, lichenoid dermatitis, lip, oral mucosa, sclerosis
Introduction
Lichen sclerosus (LS) is a relatively rare chronic inflammatory dermatosis with a prevalence of 0.1–0.3%.1 The condition was first mentioned by Hallopeau in 1887 in a patient with coalescent papules on the trunk and forearms and lichenification of the vulva; since then, it was coined various terms such as lichen sclerosus et atrophicus, kraurosis vulvae, balanitis xerotica obliterans, and white spot disease.2 In 1976, the disease was formally named LS by the International Society for the Study of Vulvovaginal Disease since it does not histologically exhibit atrophy in all cases.3 LS is predominantly found in females without racial predilection. It can occur at any age with a bimodal peak incidence in prepuberty and postmenopausal women.4
LS lesions most commonly occur on the anogenital area, with approximately 85% prevalence; however, extragenital LS develops in other body areas, particularly on the trunk and proximal extremities, with less frequency.5,6 Oral LS is considered an extremely rare subtype of extragenital LS, being infrequently reported in the literature.5 Due to its rarity, oral LS is frequently delayed in diagnosis and is often misdiagnosed with other imitative dermatological disorders. Here, we report an oral LS case in a 58-year-old female with a 1-year progressively developed asymptomatic whitish sclerotic plaque on the labial mucosa of the lower lip.
Case Presentation
A 58-year-old female visited the otolaryngology department with a 1-year history of an asymptomatic white spot on her lower lip. The lesion had no prior erythema or noticeable rash. The patient initially denied a biopsy and was prescribed a topical 0.1% triamcinolone acetonide gel for 1 month; nevertheless, the lesion showed no improvement and gradually became more prominent over time. She reported no history of chemical applications, injections, infections, or trauma on the affected site. She had allergic rhinitis, which was well controlled with cetirizine 10 mg daily. Owing to cosmetic concerns, the patient was referred to the dermatology department to evaluate and properly manage the lesion.
Physical examination revealed a whitish, slightly firm plaque on the left side of the lower labial mucosa. The lesion was well-defined and approximately 1.5 cm in diameter without involvement in the rest of the oral cavity. A subtle white striation was also seen on the right side of the lower labial mucosa (Figure 1). No significant skin lesions were observed on other body parts, and a review of other systems was unremarkable. By that time, LS was a provisional diagnosis. Despite an unusual location, the differential diagnosis included morphea, vitiligo, and contact leukoderma.
 |
Figure 1 Clinical manifestation: well-defined whitish, slightly firm plaque on the left side of the lower labial mucosa and subtle white striation on the right side of the lower labial mucosa. |
A punch biopsy was done at the sclerotic lesion with hematoxylin and eosin staining. The histological section showed focal atrophic epithelium associated with hydropic degeneration of the basal cell layer. The superficial lamina propria revealed edematous and homogenized collagen fiber with patchy, band-like lymphoplasmacytic inflammatory infiltration below the hyalinized area (Figures 2A and B). Regarding the histopathological findings, the final diagnosis in this patient was LS of the labial mucosa. The patient received treatment with intralesional triamcinolone injection (5 mg/mL) and topical 0.05% clobetasol propionate cream applied twice daily. After 2 months of treatment, the lesion was improved, becoming softened and decreased in size (Figure 3).
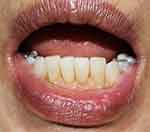 |
Figure 3 Improvement of the lesion after two months of treatment. |
Discussion
Oral LS is an extremely rare subtype of extragenital LS with unknown prevalence. It was first described by Miller in 1957 in a female patient presenting co-existing oral, genital, and skin lesions.7 According to the literature, 39 histologically confirmed OLS cases have been reported since 1955, with 20 patients with LS of labial mucosa.5 Typical clinical features of LS, including papules or plaques associated with white coloration, sclerosis, erosion, or atrophy, are also found in oral LS. However, genital LS is considered a more serious condition as this form usually manifests with pain, pruritus, or dryness and sometimes causes anogenital sequelae such as disfiguration, phimosis, recurrent balanitis, painful erection, and constipation.8 Moreover, evidence showed an increased rate of squamous cell carcinoma in genital LS with 0.4–6% prevalence.9 In contrast, extragenital LS is usually asymptomatic and exhibits less association with malignant transformation,10 with only two reported cases of squamous cell carcinoma arising from extragenital LS lesions on the leg and buttock.11,12 To our knowledge, malignant change has never been documented among previously reported oral LS cases.5
Oral LS commonly presents as whitish homogenous sclerotic plaque/plaques, some with telangiectasia and erosion.8 Approximately half of the occurrences were located on the labial mucosa, followed by lip, buccal mucosa, tongue, and palate with 37%, 34%, 29%, and 17%, respectively.5 Oral LS concomitant with extraoral LS is frequently seen, 31% in extragenital LS and 18% in genital LS; therefore, a complete body examination is recommended.5 Most oral LS patients present asymptomatic, and around 30% manifest with pruritus, pain, or tightness when opening the mouth.5 Gingival recession, periodontal detachment, and tooth mobility were reported in some cases with gingival involvement.13
Diagnosis of oral LS is challenging since the disease is rare, with an unusual distribution.8 The differential diagnosis includes other mimicking conditions such as morphea, vitiligo, leukoplakia, oral mucous fibrosis, and lichen planus.13 Histological examination is recommended for a definitive diagnosis, especially in cases of clinical uncertainty or uncommon location. Tissue biopsy should be performed at active sclerotic areas and edges of ulcers.4 Histopathological features gradually change upon disease progression. In the early stage, the disease is difficult to distinguish from other lichenoid disorders, showing superficial dermal edema with a band-like lymphocytic infiltration and vacuolization of the basal cell layer.2,14 Later, fibrosis occurs with hyalinized collagen in the dermis, causing sclerotic and/or atrophic lesions. Sometimes, subepidermal cleft and blister formation from interface dermatitis and dermal edema are visible.5
Morphea is a primary condition that must be distinguished from oral LS since it reveals similar clinical manifestations, including sclerosis and atrophy. However, no focal basal cell degeneration or loss of elastic fibers in the hyalinized tissue was observed in its histological sections.5 Vitiligo shows areas of well-defined depigmented skin without sclerosis, while leukoplakia predominantly presents whitish lesions on the buccal mucosa or tongue with a wrinkled surface.13–16 Oral submucous fibrosis shows diffuse lesions and progressive stiffness of the oral mucosa with a history of areca nut exposure.13 Lichen planus, a more prevalent disorder than oral LS, usually manifests painful, symmetrically distributed lesions with white stria, erythema, or erosion. It can affect several areas of the oral cavity, including buccal mucosa, gingiva, tongue, and labial mucosa.17,18 Although a potential malignant change in oral lichen planus has been noted, a recent meta-analysis revealed its low malignant transformation rate.19
Pathogenesis of LS has yet to be elucidated. Multifactorial etiology, including autoimmune process, genetic predisposition, and local inflammation, is hypothesized to play its pathogenetic role.20–22 IgG autoantibodies against extracellular matrix protein 1 were demonstrated in 74% of genital LS cases.23,24 A genetic predisposition, eg, HLA-DQ7 or HLA-DR12,25,26 and a history of local trauma exacerbating inflammation and oxidative stress with lipid and DNA peroxidation were also reported to be associated with the disease.8,27–29 In addition, medications such as imatinib30 and immune checkpoint inhibitors were documented to cause LS at 3–6 months after administration.31,32
Standard recommendations for the management of oral LS are still being determined due to its rarity and limited data on reported cases.10 Treatment purposes include reduction of symptoms, functional maintenance of affected areas, and prevention of malignant transformation.8 For oral LS, most patients are asymptomatic or report minor symptoms; therefore, cosmetic improvement is its main treatment objective.13 Treatment modalities reported for oral LS include potent topical corticosteroids, intralesional corticosteroids, topical calcineurin inhibitors, systemic corticosteroids, phototherapy, photodynamic therapy, and surgical excision.5,33,34 Retinoids show limited response to the treatment of oral LS.10,35 Our patient’s lesion was resolved with a combination of topical and intralesional corticosteroids.
Conclusion
Oral LS is a rare condition mainly distributed on the labial mucosa. Due to its rarity and various imitative disorders, oral LS can be under-recognized and easily misdiagnosed. This case report reminds and raises awareness of physicians regarding this uncommon condition. However, the lack of dermoscopic features of oral LS in our case is the main limitation. Our case also highlights the importance of histopathological examination, which is essential for a definitive diagnosis and preventing delays in treatment. Moreover, tissue biopsy should be performed in patients with treatment recalcitrance or suspected malignant transformation despite no report of squamous cell carcinoma in oral LS. Early recognition and management of LS and its consequences help limit the disease burden and improve treatment outcomes.
Ethics Approval and Consent to Participate
This article was performed in accordance with the principles of Declaration of Helsinki. Ethical review and approval was not required to publish the case details in accordance with local legislation and institutional requirements. Written informed consent was obtained from the patient for publication of this case report and any accompanying images as per our standard institutional rules.
Funding
No sources of funding were used to prepare this manuscript.
Disclosure
The authors declare that this manuscript was prepared in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57(1):9–30.
2. Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32(3):393–416. doi:10.1016/0190-9622(95)90060-8
3. Friedrich EG, DiPaola GR, Hewitt J, Woodruff JD. New nomenclature for vulvar disease. J Cutan Pathol. 1976;3(3):159–161. doi:10.1111/j.1600-0560.1976.tb01105.x
4. Singh N, Etiology GP, Features C. Diagnosis of Vulvar Lichen Sclerosus: a Scoping Review. Obstet Gynecol Int. 2020;2020:7480754. doi:10.1155/2020/7480754
5. Kakko T, Salo T, Siponen MK. Oral lichen sclerosus: a systematic review of reported cases and two new cases. Int J Dermatol. 2018;57(5):521–528. doi:10.1111/ijd.13870
6. Vachiramon V, Suchonwanit P, Thadanipon K. Bilateral linear lichen planus pigmentosus associated with hepatitis C virus infection. Case Rep Dermatol. 2010;2(3):169–172. doi:10.1159/000320775
7. Miller RF. Lichen sclerosus et atrophicus with oral involvement: histopathologic study and dermabrasive treatment. AMA Arch Dermatol. 1957;76(1):43–55. doi:10.1001/archderm.1957.01550190047010
8. De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med Lausanne. 2023;10:1106318. doi:10.3389/fmed.2023.1106318
9. Meani R, Howard A, Veysey E. Incidence of vulval squamous cell carcinoma in women with vulval lichen sclerosus in an Australian tertiary referral centre. Australas J Dermatol. 2019;60(1):76–77. doi:10.1111/ajd.12873
10. Burshtein A, Burshtein J, Rekhtman S. Extragenital lichen sclerosus: a comprehensive review of clinical features and treatment. Arch Dermatol Res. 2023;315(3):339–346. doi:10.1007/s00403-022-02397-1
11. Sergeant A, Vernall N, Mackintosh LJ, McHenry P, Leman JA. Squamous cell carcinoma arising in extragenital lichen sclerosus. Clin Exp Dermatol. 2009;34(7):e278–279. doi:10.1111/j.1365-2230.2008.03195.x
12. Sotillo Gago I, Martínez Sahuquillo A, Matilla A, García Pérez A. [Spinocellular epithelioma following scleroatrophic autoaneous licher]. Actas Dermosifiliogr. 1977;68(3–4):219–220. Spanish.
13. Marangon Júnior H, Souza PEA, Soares RV, Gomez RS, Pereira GHM, Horta MCR. Oral lichen sclerosus: a rare case report and review of the literature. Head Neck Pathol. 2017;11(2):212–218. doi:10.1007/s12105-016-0766-x
14. Attili VR, Attili SK. Lichen sclerosus of lips: a clinical and histopathologic study of 27 cases. Int J Dermatol. 2010;49(5):520–525. doi:10.1111/j.1365-4632.2010.04288.x
15. Mahasaksiri T, Kositkuljorn C, Anuntrangsee T, Suchonwanit P. Application of topical immunotherapy in the treatment of alopecia areata: a review and update. Drug Des Devel Ther. 2021;15:1285–1298. doi:10.2147/dddt.S297858
16. Rattanakaemakorn P, Suchonwanit P. Scalp pruritus: review of the pathogenesis, diagnosis, and management. Biomed Res Int. 2019;2019:1268430. doi:10.1155/2019/1268430
17. Lehner J, Agbo-Godeau S, Bertolus C. A retrospective study of 23 cases: are lichenoid lesions of the labial mucosa induced? Cureus. 2022;14(5):e25012. doi:10.7759/cureus.25012
18. Chanprapaph K, Pomsoong C, Tankunakorn J, Eden C, Suchonwanit P, Rutnin S. Comparative analyses of clinical features, histopathology, and CD123 immunohistochemistry of oral lupus erythematosus, lichen planus, and other lichenoid lesions. Dermatology. 2022;238(3):464–475. doi:10.1159/000517971
19. Idrees M, Kujan O, Shearston K, Farah CS. Oral lichen planus has a very low malignant transformation rate: a systematic review and meta-analysis using strict diagnostic and inclusion criteria. J Oral Pathol Med. 2021;50(3):287–298. doi:10.1111/jop.12996
20. Suchonwanit P, Kositkuljorn C, Pomsoong C. Alopecia areata: an autoimmune disease of multiple players. Immunotargets Ther. 2021;10:299–312. doi:10.2147/itt.S266409
21. Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14(1):27–47. doi:10.1007/s40257-012-0006-4
22. Suchonwanit P, Kositkuljorn C, Mahasaksiri T, Leerunyakul K. A comparison of the efficacy and tolerability of three corticosteroid treatment regimens in patients with alopecia areata. J DermatolTreat. 2022;33(2):756–761. doi:10.1080/09546634.2020.1773384
23. Kreuter A, Kryvosheyeva Y, Terras S, et al. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm Venereol. 2013;93(2):238–241. doi:10.2340/00015555-1512
24. Bieber AK, Steuer AB, Melnick LE, Wong PW, Pomeranz MK. Autoimmune and dermatologic conditions associated with lichen sclerosus. J Am Acad Dermatol. 2021;85(1):228–229. doi:10.1016/j.jaad.2020.08.011
25. Azurdia RM, Luzzi GA, Byren I, et al. Lichen sclerosus in adult men: a study of HLA associations and susceptibility to autoimmune disease. Br J Dermatol. 1999;140(1):79–83. doi:10.1046/j.1365-2133.1999.02611.x
26. Marren P, Yell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol. 1995;132(2):197–203. doi:10.1111/j.1365-2133.1995.tb05013.x
27. Chanprapaph K, Sutharaphan T, Suchonwanit P. Scalp biophysical characteristics in males with androgenetic alopecia: a comparative study with healthy controls. Clin Interv Aging. 2021;16:781–787. doi:10.2147/cia.S310178
28. Paulis G, Berardesca E. Lichen sclerosus: the role of oxidative stress in the pathogenesis of the disease and its possible transformation into carcinoma. Res Rep Urol. 2019;11:223–232. doi:10.2147/rru.S205184
29. Suchonwanit P, Triyangkulsri K, Ploydaeng M, Leerunyakul K. Assessing biophysical and physiological profiles of scalp seborrheic dermatitis in the Thai population. Biomed Res Int. 2019;2019:5128376. doi:10.1155/2019/5128376
30. Skupsky H, Abuav R, High W, Pass C, Goldenberg G. Development of lichen sclerosus et atrophicus while receiving a therapeutic dose of imatinib mesylate for chronic myelogenous leukemia. J Cutan Pathol. 2009;37:877–880. doi:10.1111/j.1600-0560.2009.01398.x
31. Conteduca V, Medri M, Mazzoni L, De Giorgi U, Stanganelli I. Anogenital lichen sclerosus et atrophicus lesions in a case series of cancer patients on immunotherapy. Cancer Immunol Immunother. 2022;71(6):1545–1548. doi:10.1007/s00262-021-03094-0
32. Miraglia E, Soda G, Giustini S. Genital lichen sclerosus after nivolumab. Dermatol Online J. 2020;26:11.
33. Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) Guideline on (anogenital) Lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29(10):e1–43. doi:10.1111/jdv.13136
34. Robledo-Sierra J, Bäckman K, Öhman J, Jontell M. Oral lichen sclerosus: an overview and report of three cases. Int J Oral Maxillofac Surg. 2018;47(12):1550–1556. doi:10.1016/j.ijom.2018.04.006
35. Ravits HG, Welsh AL. Lichen sclerosus et atrophicus of the mouth. AMA Arch Dermatol. 1957;76(1):56–58. doi:10.1001/archderm.1957.01550190060011
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

