Back to Journals » Journal of Pain Research » Volume 13
Less Pain, Better Sleep? The Effect of a Multidisciplinary Back Pain App on Sleep Quality in Individuals Suffering from Back Pain – a Secondary Analysis of App User Data
Authors Priebe JA, Utpadel-Fischler D , Toelle TR
Received 28 September 2019
Accepted for publication 27 April 2020
Published 20 May 2020 Volume 2020:13 Pages 1121—1128
DOI https://doi.org/10.2147/JPR.S232792
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Janosch A Priebe, Daniel Utpadel-Fischler, Thomas R Toelle
Center of Interdisciplinary Pain Medicine, Department of Neurology, Klinikum Rechts der Isar, Technical University of Munich, Munich, Germany
Correspondence: Janosch A Priebe
Center of Interdisciplinary Pain Medicine, Department of Neurology, Klinikum Rechts der Isar, Technical University of Munich, Munich, Germany
Tel +49 89 4140 2563
Email [email protected]
Purpose: Mobile health solutions are finding their way into health systems. The Kaia app has been shown to be able to reduce back pain in two studies. Since pain often comes along with disturbed sleep and both symptoms are strongly related we investigated whether the Kaia app training is associated with improved sleep quality.
Methods: User data of individuals with back pain were collected in two app versions (cohort 1: N = 180; cohort 2: N = 159). We analyzed the ratings of sleep quality and pain intensity on a 11-point numeric ratings scale (NRS; 0– 10) both at the beginning of usage (baseline: BL) and on the individual last day of usage (follow-up: LU) within a 3-month training program.
Results: In both cohorts, we found a significant reduction in pain intensity from BL to LU (cohort 1: MBL = 4.80; SD = 1.59 to MLU = 3.75; SD = 1.76, Δpain = – 1.04; SD = 2.12; t(158) = 6.207; p<.001/cohort 2: MBL = 4.20; SD = 1.98 to MLU = 3.65; SD = 1.78; Δpain = – 0.50; SD = 2.04; t(147) = 3.001; p = 0.003) and a significant improvement of sleep quality (cohort 1: MBL = 5.76; SD = 2.12 to MLU = 6.56; SD = 1.72; Δsleep = t(158) = 4.310; p < 0.001/cohort 2: MBL = 6.08; SD = 2.08 to MLU = 6.76; SD = 1.55; Δsleep = 0.67; SD = 2.13; sleep: t(147) = 3.825; p < 0.001). Interestingly, improvement of sleep quality was not fully mediated by pain reduction.
Conclusion: Our analysis underlines the relationship between pain and sleep in the clinical context. Improvement of sleep quality came along with pain reduction and vice versa. Further study should explain the exact mechanisms of action which are associated with the improvement of both symptom parameters.
Keywords: back pain, sleep, mHealth, multidisciplinary pain treatment, app, self-management
Introduction
Sleep and pain are closely – bidirectionally – related to each other. First, pain disturbs sleep quality. It is not surprising that pain patients suffer from a reduction in sleep quality and sleep disturbances.1–4 Yet, sleep also affects pain. It is well known, that disturbed sleep can worsen pain symptoms in pain patients.4–8 Basic research provides evidence that low sleep quality affects nociception with decreased pain thresholds, increased subjective pain ratings and also cortical processing.9–16 It is assumed that especially the functionality of the pain inhibiting circuits is negatively affected by poor sleep quality. Curbed activity of the endogenous pain inhibition leads to an imbalance of excitatory and inhibitory activation which in turn does not only increase pain sensitivity in healthy individuals but also worsens pain symptoms in patients with acute and chronic pain.6,17-21 In order to cover such symptom interactions in the multidimensional perspective on pain, it is not surprising that multidisciplinary therapy which comprises physical exercise, educative elements and psychological therapy is seen as the gold standard in the treatment of chronic pain.22
In recent times, mobile health applications have been seen as an innovative tool to provide effective treatment for pain patients at low costs.23–29 We investigated the effects of a 12-week program with the Kaia app which provides multidisciplinary elements (physical exercise, mindfulness and educational content, see method section for a description of the Kaia app and reference) for patients with unspecific low back pain (ie, back pain without medical signs of a specific underlying condition like cancer or fracture). In a secondary analysis of data of app users, we showed a substantial pain reduction by usage of a 12-week training program provided by the Kaia app.23 In a recent randomized controlled trial conducted by our group patients who received Kaia app treatment, less symptoms after 12 weeks compared to a control group who received physiotherapy and online education were reported.25
So far, to our best knowledge, sleep quality and its relationship to changes in pain symptoms by using a multidisciplinary pain app, has not been investigated yet. In the present paper, we performed a secondary analysis of user data of the Kaia app. Data regarding pain intensity and sleep quality were investigated for changes over a period of 3 months. In order to validate findings, two versions of the app (one version contained updates with user feedback27) were analyzed. Our hypothesis derived from previous studies23–27 was that both pain symptoms and sleep quality improve over time. Furthermore, the relationship between changes in pain intensity and sleep quality was investigated.
Methods
User data were collected in two different app versions. We included data of two app versions separately to increase the validity of our results (see below for a description of the differences in both versions). The data analyzed in the present study referred to individuals who downloaded the app and used it on their own initiative. The app is free for 1 week (demo version). After 1 week, the app was available for 9.99 Euro per months (meanwhile price has increased). All users agreed with the collection of data presented in this publication by signing the terms and conditions of Kaia. All data used for the study were anonymized before submission to the Klinikum rechts der Isar (MRI), Technical University of Munich for statistical analysis.
Inclusion criteria for app usage were age ≥18 years, declaration of medical treatment of back pain, no indicators for specific causes of back pain (red flags) that demand treatment and sufficient level of physical fitness (self-report). The Institutional Ethics Committee of the Medical Faculty of the Technische Universität Munich approved the study design (Reference: 273/17 S).
Data Collection
All data analyzed in this study were entered by app users as part of their self-test or in-app diaries and stored on company servers in Frankfurt, Germany. Only anonymized data were extracted from the user database and no personal data were submitted for scientific evaluation. The data protection officer of the University Hospital (Klinikum rechts der Isar) of the Technical Unversity of Munich approved the concept for protection of personal data.
The Kaia Back Pain App
Kaia treatment app has been described in detail previously.23,25,27 In short, the Kaia app (Kaia Health Software GmbH, Munich, Germany) is a multiplatform app for iOS, Android, and native Web solutions. Kaia came to market in September 2016 and is classified as a medical product class I. It is available via the App Store (iOS), the Google Play Store, or as a native website. The Kaia app involves the following pillars: (1) back pain-specific education, (2) physiotherapy/physical exercise, and (3) mindfulness/relaxation techniques (Figure 1). Daily content consists of all 3 pillars. The content for an individual patient is compiled and updated from day to day (or upon each login) from a large background of exercises and skills archived in the app. Depending on the patient´s status of knowledge, practice, and progress this is adapted continually. Thus, exercise regimen and content are tailored to the individual patient. Each section is comprehensive as a stand-alone—there is no obligation to perform all 3 sections in a single session. Content in the educational section covers a broad spectrum of general pain-related and back pain-specific education. There are over 30 different educational units in the app. Content is based on current German30 or international guidelines and standard textbooks in the field. Educational content was authored by board-certified physicians with relevant expertise in the field of back pain (ie, neurology, orthopedic surgery, and pain medicine) or clinical psychologists with experience in pain psychotherapy. The single exercises and the individual composition of exercises for every user per day (up to 5 exercises) were designed by physiotherapists of the Center for Interdisciplinary Pain Medicine at the Klinikum rechts der Isar, Technical University of Munich according to guidelines and curricula of the German Pain Society.30 Exercises within each class are ranked depending on exercise difficulty and strain. Mindfulness and relaxation techniques are an integral part of multidisciplinary inpatient and outpatient LBP rehabilitation. The Kaia app contains units of breathing techniques, body scan, visualization, and progressive muscle relaxation. The value of the various techniques is explained in the education part of the app. Mindfulness content is generally broadcasted as audio content only. There is also a chat function in the app that connects users to a coach (physiotherapist or sport scientist) for motivational and exercise-related questions.
 |
Figure 1 Illustration of the elements of the Kaia back pain app. |
Individuals who download the app have to provide information about region and intensity of back pain. If indications of red flags occurred, users were strongly recommended to see a physician before starting training with the app. Then, users had to confirm that a physician was consulted and excluded red flags by setting a checkmark.
At each day, the users logged into the app, they are asked to log their current pain level and the sleep quality in the previous night into the pain diary. Pain levels are recorded using a 11-point numeric ratings scale (NRS; 0 = no pain, 10 = unbearable pain). Sleep quality was rated on another 11-point numeric ratings scale (0 = worst imaginable, 10 = best imaginable). The in-app measures are meant to be visualized to the users in order to illustrate the development of their symptoms and were used as outcomes in the present analyses. From time to time users received motivating messages to continue or feedback (smilies and congratulation messages) for achieving improvement.
The differences in the two versions of the Kaia app used by the cohorts have been described elsewhere in detail.27 In short, in the updated Kaia app version (cohort 2) individual user feedback collected in the course of app usage was increasingly used to tailor the individual training program to the individual patient. Furthermore, push up reminders which however could be declined by users were used in the updated Kaia version (cohort 2). This in turn may have contributed to higher adherence in cohort 2 (see below).27
Statistical Analysis
For both samples, the same statistical analyses were performed separately. First, to test whether pain intensity and sleep quality improved from baseline (BL) to the last day of usage (LU), both pain and sleep ratings were subjected to paired sample t-tests (BL vs LU). Second, in order to test the relationship between pain reduction and improvement of sleep quality bivariate correlations were computed between the symptom improvement regarding pain and the symptom improvement regarding sleep. In order to generate a measure for symptom improvement, delta scores for pain and sleep (Δpain/Δsleep) were calculated by subtracting the baseline scores from the follow-up scores. In consequence, negative Δs for pain and positive Δs for sleep should result, if patients’ symptom load improves. Next, for testing, whether the changes in sleep disturbances are solely associated with a reduction of pain intensity sleep ratings were subjected to a unifactorial within-subjects ANCOVA with the factor time (BL vs FU) and the covariate Δpain. Analogically, a unifactorial within-subjects ANCOVA with the factor time (BL vs FU) and the covariate Δsleep was computed for the pain ratings. Last, as an exploratory analysis, two multiple regressions with the predictors sex, age and pain duration both for the criteria Δpain and Δsleep were computed in order to test potential relationships.
Results
Sample Characteristics
Data of 180 individuals (105 females) using app version 1 were analyzed. Mean age was M = 33.94 years (SD = 10.86). Individuals reported a mean pain duration of M = 8.5 weeks (SD = 5.0). Mean number of active days was M = 22.11 days (SD = 10.56) in this cohort.
In the second cohort using app version 2 data of 153 users (67 females) with a mean age of M = 46.96 years (SD = 13.10) and a mean pain duration of M = 19.20 weeks (SD = 27.32) were available. Mean number of active days of these users was M = 30.92 weeks (SD = 32.27). A day was classified as an active day when the user has logged into the app and completed at least on the module.
There was a substantial dropout over time. In cohort 1 only 18% of the users completed 12 weeks of the program. In cohort 2 we found a smaller drop-out with 38% of users being active for 12 weeks. We expected this large dropout since data were collected in users using the app on their own initiative in a non-controlled field-setting without (study) physicians being involved. Figure 2 illustrates the drop-out rate over time for both cohorts/app versions.
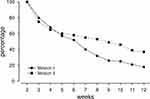 |
Figure 2 Adherence of users in the course of the 12-week program for both app versions: The figure illustrates the percentage of users who is active in the particular week. |
Symptom Load Over Time
Figure 3 illustrates the changes in symptom load regarding pain intensity and sleep quality.
According to our hypothesis, pain symptoms and sleep quality significantly improved for both app versions (Version 1: pain: t(158) = 6.207; p<.001; sleep: t(158) = 4.310; p < 0.001; Version 2: pain: t(147) = 3.001; p = 0.003; sleep: t(147) = 3.825; p < 0.001). Furthermore, there were substantial correlations between pain intensity at BL and pain intensity at LU as well as for sleep quality at BL and sleep quality at LU (Cohort 1: pain: r = 0.350; sleep: r = 0.321; Cohort 2: pain: r = 0.442; sleep: r = 0.341 with all p’s <.05).
Additionally, bivariate correlations between Δpain and Δsleep revealed a relationship between pain reduction and improvement of sleep quality in both versions (cohort 1: r = -0.369; cohort 2: r = -0.316; p’s <0.001). In order to test, if improvement of sleep quality is merely associated with pain reduction (and vice versa), sleep (pain) ratings were subjected to repeated-measurement one-way ANCOVAs with the factor time (BL vs LU) and the covariate Δpain (Δsleep).
The ANCOVA for the sleep ratings in app version 1 revealed a marginally significant main effect of the factor time, F(1157) = 3.769; p = 0.054 and a significant interaction of time and the covariate Δpain, F(1157) = 24.751; p < 0.001. This confirms that there is a substantial relationship between pain reduction and improvement of sleep quality (interaction). Yet, the improvement of sleep quality cannot solely be explained by pain reduction (marginally significant main effect). The analysis of app version 2 confirmed this finding both with a significant main effect of time, F(1146) = 8.600; p = 0.004, and a significant interaction of time and Δpain, F(1146) = 16.234; p < 0.001.
For the sake of completeness, the same analysis was run for the pain ratings with sleep quality as covariate. For both app versions, there was a significant interaction of time and sleep quality (all p’s <0.001). The main effect of time (BL vs LU) was significant for version 1 (p<.001) while it reached only marginal significance in version 2, F(1146) = 2.219; p = 0.075. Hence, pain reduction is not only associated with improvement in sleep quality.
Last the multiple regression analysis with the predictors age, sex and pain duration revealed no significant model neither for the criterion Δpain nor Δsleep (R2s <0.10; p’s >0.10).
Discussion
The objective of the present study was to investigate if individuals with back pain report increased sleep quality after a 12-week program with the Kaia app. For this purpose, secondary data of app users collected in two app versions were analyzed. According to our hypothesis, the users showed a substantial increase in sleep quality which was related to pain reduction. Yet, improvement of sleep quality was not only explainable by improvement in pain symptoms (ANCOVA).
The efficacy of multidisciplinary mobile health applications in treating back pain has been demonstrated in a small number of clinical studies.23–27 To our best knowledge, this is the first mHealth study on (self-reported) sleep quality in individuals suffering from back pain. First of all, the increase in sleep quality is not surprising. By intuition, less pain will determine better sleep. This is also in line with existing literature.1–3,7
It is interesting, however, that improvement in sleep quality is (statistically) only partially mediated by improvement of pain. Since it is well known that mindfulness can improve sleep quality31,32 most likely by stress reduction and improvement of wellbeing,33 this indicates on a speculative level that the mindfulness exercises may be associated with improvement of sleep.
One may also speculate that also education about pain can directly or indirectly facilitate sleep quality. Assuming that pain symptoms are related to anxiety or even fear of potentially serious causes of the pain symptoms, these conditions may be reduced by education providing medical information about the cause of the pain and adequate management, as provided by the app. For example, patients may learn that the causes even of intense back pain usually are harmless. In consequence, both mindfulness exercise and education units may be related to stress reduction and anxiety which – besides pain reduction – is associated with better sleep quality.
In addition, we also confirmed our assumption that pain reduction is not merely associated improvement of sleep quality - an analysis only performed for the sake of completeness.
We can only speculate whether the improvement of sleep quality in turn contributes to the pain relief in our study. It is at least well shown that disturbed sleep leads to substantial changes in pain processing like enhanced pain perception or hyperalgesia.1–7,9,19 It is assumed that especially the functionality of the descending inhibitory pain circuits and their top-down activation may be disturbed in individuals suffering from sleep disturbances.18–20 This may lead to an increase in pain perception, which worsens sleep disturbances and may induce a vicious cycle.4,6-8
Taken the bidirectional relationship between sleep and pain, treatment options targeting both symptoms, ie, sleep and pain, may, in consequence, be more effective since they intervene with both mechanisms. The results of the present observation are interesting since they indicate that mobile health applications, such as the Kaia app, are able to effectively deliver multiple elements of the multidisciplinary pain therapy.
One may argue that treatment with mHealth is not able to replace face to face treatments by a physician or psychologist. Therefore, a health care professional should be involved in order to exclude red flags and to monitor symptom development. Yet, a mobile app can be integrated into regular treatment. Treatment via mobile solutions may be able to bridge waiting times for a day hospital treatment opportunity. Furthermore, mobile apps could be applied after the completion of regular treatment in order to maintain treatment effects.
Limitations
Our study has some limitations. First, we report a secondary analysis which refers to data collected from users downloading and using the app on their own demands and initiative and not in a clinical setting. This leads to a hardly controlled setting which may also be the reason for the large drop in app adherence over time. Furthermore, causal conclusions are hard to draw. Second, the exact mechanisms of action cannot be investigated in the present analysis since we do not have access to information regarding the completion of the particular modules for data protection reasons. Third, also due to the non-systematic data collection the sample is heterogenous regarding symptom duration (see above) or exact location of pain (no information available for us due to data protection demands). Only information about age, sex and pain duration was available in addition to pain and sleep ratings which limits preciseness of conclusions. Fourth, interindividual differences in adherence and frequency of app usage make it difficult to draw systematic conclusions on “the minimum dose” that is needed to affect sleep and pain. Studies with larger sample sizes must be conducted in order to investigate usage behavior in a more detailed manner. Yet, in our previous RCT, we at least did not find relationships between frequency of app usage and symptom improvement.25
Despite these limitations, we consider our findings as important since it is the first study investigating the relationship between sleep and pain in the mHealth context. Furthermore, we extensively discussed the value of secondary analyses in the mHealth context in a recent commentary.34
Conclusions
The objective of the present analysis was to investigate whether a back pain app with a multidisciplinary pain management approach can improve the quality of sleep in back pain patients. We found a substantial improvement in sleep quality which was not solely associated with pain reduction. Despite the limitations, these results indicate that mobile health solutions are not only a promising tool to deliver pain treatment. They rather seem to be able to positively affect symptoms besides pain. Yet, further research has to be conducted in order to explore the specific effects of different elements of the multidisciplinary pain treatment provided by the mHealth tool.
Data Sharing Statement
All data are available upon request.
Disclosure
Interim findings of this paper were presented at the EFIC 2019 in Valencia, Spain, as a poster. The poster’s abstract was published in the abstract book of the conference: https://efic-congress.org/wp-content/uploads/2020/03/EFIC-2019-Abstractbook.pdf.
Janosch A. Priebe received a consultancy fee for data analysis of demographic data of Kaia subscribers via internet download and reports personal fees from Kaia Health during the conduct of the study. Daniel Utpadel-Fischler declares that he has no conflict of interest. Thomas R. Toelle declares consultancies, travel grants and speaking fees from AOP Orphan, Almiral Hermal, Bionest Partners, Benkitt Renkiser, Grünenthal, Hexal, Indivior, Kaia Health, Lilly, Medscape Mundipharma, MSD, Novartis, Pfizer, Recordati Pharma, Sanofi-Aventis, and TAD Pharma. In addition he reports grants from Innovationsfonds Germany and personal fees from AOP, Almiral Hermal, Bionest Partners, Benkitt Renkiser, Gruenenthal, Hexal, and Indivior during the conduct of the study.
The authors report no other possible conflicts of interest in this work.
References
1. Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14(4):311–314. doi:10.1097/00002508-199812000-00007
2. Wittig RM, Zorick FJ, Blumer D, Heilbronn M, Roth T. Disturbed sleep in patients complaining of chronic pain. J Nerv Ment Dis. 1982;170(7):429–431. doi:10.1097/00005053-198207000-00011
3. Atkinson JH, Ancoli-Israel S, Slater MA, Garfin SR, Gillin C. Subjective sleep disturbance in chronic back pain. Clin J Pain. 1988;4(4):225–232. doi:10.1097/00002508-198812000-00007
4. Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5(5):385–396. doi:10.1053/smrv.2001.0179
5. Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10(1):35–42. doi:10.1046/j.1365-2869.2001.00240.x
6. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi:10.1016/j.jpain.2013.08.007
7. Lautenbacher S. Pain, sleeping problems and their many relatives. Pain. 2012;153(6):1138. doi:10.1016/j.pain.2012.03.001
8. Lautenbacher S. Sleep and pain are definitely coupled—but how tight is this coupling? Pain. 2018;159(1):3–4. doi:10.1097/j.pain.0000000000001082
9. Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. doi:10.1016/j.smrv.2005.08.001
10. Simpson NS, Scott-Sutherland J, Gautam S, Sethna N, Haack M. Chronic exposure to insufficient sleep alters processes of pain habituation and sensitization. Pain. 2018;159(1):33–40. doi:10.1097/j.pain.0000000000001053
11. Tiede W, Magerl W, Baumgärtner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148(1):36–42. doi:10.1016/j.pain.2009.08.029
12. Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145–151. doi:10.1093/sleep/29.2.145
13. Azevedo E, Manzano GM, Silva A, Martins R, Andersen ML, Tufik S. The effects of total and REM sleep deprivation on laser-evoked potential threshold and pain perception. Pain. 2011;152(9):2052–2058. doi:10.1016/j.pain.2011.04.032
14. Kundermann B, Hemmeter-Spernal J, Huber MT, Krieg JC, Lautenbacher S. Effects of total sleep deprivation in major depression: overnight improvement of mood is accompanied by increased pain sensitivity and augmented pain complaints. Psychosom Med. 2008;70(1):92–101. doi:10.1097/PSY.0b013e31815c1b5d
15. Busch V, Haas J, Crönlein T, et al. Sleep deprivation in chronic somatoform pain—effects on mood and pain regulation. Psychiatry Res. 2012;195(3):134–143. doi:10.1016/j.psychres.2011.07.021
16. Irwin MR, Olmstead R, Carrillo C, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35(4):537–543. doi:10.5665/sleep.1742
17. Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1–2):295–302. doi:10.1016/j.pain.2004.12.032
18. Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009;13(10):1043–1047. doi:10.1016/j.ejpain.2008.12.007
19. Karmann AJ, Kundermann B, Lautenbacher S. Sleep deprivation and pain: a review of the newest literature. Schmerz (Berlin, Germany). 2014;28(2):141–146. doi:10.1007/s00482-014-1394-6
20. Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi:10.1093/sleep/30.4.494
21. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. doi:10.1097/ACO.0b013e32833c348b
22. Deckert S, Kaiser U, Kopkow C, Trautmann F, Sabatowski R, Schmitt J. A systematic review of the outcomes reported in multimodal pain therapy for chronic pain. Eur J Pain. 2016;20(1):51–63. doi:10.1002/ejp.721
23. Huber S, Priebe JA, Baumann KM, Plidschun A, Schiessl C, Tölle TR. Treatment of low back pain with a digital multidisciplinary pain treatment app: short-term results. JMIR Rehabil Assist Technol. 2017;4(2):e11. doi:10.2196/rehab.9032
24. Irvine AB, Russell H, Manocchia M, et al. Mobile-Web app to self-manage low back pain: randomized controlled trial. J Med Internet Res. 2015;17(1):e1. doi:10.2196/jmir.3130
25. Toelle TR, Utpadel-Fischler DA, Haas KK, Priebe JA. App-based multidisciplinary back pain treatment versus combined physiotherapy plus online education: a randomized controlled trial. NPJ Digit Med. 2019;2(1):34. doi:10.1038/s41746-019-0109-x
26. Shebib R, Bailey JF, Smittenaar P, Perez DA, Mecklenburg G, Hunter S. Randomized controlled trial of a 12-week digital care program in improving low back pain. NPJ Digit Med. 2019;2(1):1. doi:10.1038/s41746-018-0076-7
27. Clement I, Lorenz A, Ulm B, Plidschun A, Huber S. Implementing systematically collected user feedback to increase user retention in a mobile app for self-management of low back pain: retrospective Cohort Study. JMIR Mhealth Uhealth. 2018;6(6):e10422. doi:10.2196/10422
28. Garg S, Garg D, Turin TC, Chowdhury MFU. Web-based interventions for chronic back pain: a systematic review. J Med Internet Res. 2016;18:e139. doi:10.2196/jmir.4932
29. Nicholl BI, Sandal LF, Stochkendahl MJ, et al. Digital support interventions for the self-management of low back pain: a systematic review. J Med Internet Res. 2017;19:e179. doi:10.2196/jmir.7290
30. Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Nationale VersorgungsLeitlinie Nicht-spezifischer Kreuzschmerz – Langfassung, 2 Auflage [National Guidelines for the treatment of unspecific low back pain - long version, 2nd edition]. Version 1; 2017. Available from: https://www.kbv.de/media/sp/nvl_kreuzschmerz_lang.pdf. German.
31. Black DS, O’reilly GA, Olmstead R, Breen EC, Irwin MR. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med. 2015;175(4):494–501. doi:10.1001/jamainternmed.2014.8081
32. Winbush NY, Gross CR, Kreitzer MJ. The effects of mindfulness-based stress reduction on sleep disturbance: a systematic review. Explore. 2007;3(6):585–591. doi:10.1016/j.explore.2007.08.003
33. Cramer H, Haller H, Lauche R, Dobos G. Mindfulness-based stress reduction for low back pain. A systematic review. BMC Complement Altern Med. 2012;12(1):162. doi:10.1186/1472-6882-12-162
34. Priebe JA, Toelle TR. Is there a right control condition in mHealth trials? A critical view on pain medicine. NPJ Digit Med. 2019;2(1):1–3. doi:10.1038/s41746-019-0184-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

