Back to Journals » OncoTargets and Therapy » Volume 15
Leptomeningeal Metastatic L858R EGFR-mutant Lung Cancer: Prompt Response to Osimertinib in the Absence of T790M-mutation and Effective Subsequent Pulsed Erlotinib
Authors Kanbour A, Salih F, Abualainin W , Abdelrazek M, Szabados L, Al-Bozom I , Omar NE
Received 25 August 2021
Accepted for publication 30 December 2021
Published 14 June 2022 Volume 2022:15 Pages 659—667
DOI https://doi.org/10.2147/OTT.S336012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Video abstract of "Leptomeningeal Metastatic EGFR-Mutant Lung Cancer" [ID 336012].
Views: 953
Aladdin Kanbour,1,* Faroug Salih,1,* Wafa Abualainin,2 Mohamed Abdelrazek,3 Lajos Szabados,3 Issam Al-Bozom,4 Nabil E Omar5,*
1Medical Oncology Department, National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar; 2Solid Tumor Section, Molecular Genetics Laboratory, Diagnostic Genomic Division, Department of Laboratory Medicine and Pathology, Hamad Medical Corporation, Doha, Qatar; 3Clinical Imaging Department, National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar; 4Precision Medicine Section, Anatomical Pathology Department, Department of Laboratory Medicine and Pathology, Hamad Medical Corporation, Doha, Qatar; 5Pharmacy Department, National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar
*These authors contributed equally to this work
Correspondence: Nabil E Omar, Pharmacy Department, National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, 3050, Qatar, Email [email protected]
Abstract: Leptomeningeal carcinomatosis (LMC) is a known sequel of metastatic lung cancer and its treatment is challenging. Nevertheless, treatment options for LMC due to metastatic epidermal growth factor receptor-mutant (EGFR-mutant) lung adenocarcinoma are expanding. We present a 52-year-old male patient with metastatic non-small-cell lung cancer (NSCLC). The patient was found to have L858R mutation in exon 21 of the EGFR gene. He was initially treated with erlotinib, followed by afatinib/cetuximab, followed by chemotherapy. Thereafter, his disease progressed to LMC. Although tissue biopsy did not show T790M-mutation, osimertinib (160 mg once daily) promptly induced clinical and radiological response that continued for five months. High dose pulsed erlotinib (1500 mg weekly) improved his quality of life and extended his survival for a further four months.
Keywords: NSCLC, non-small-cell lung cancer, LMC, leptomeningeal carcinomatosis, EGFR, epidermal growth factor receptor, EGFR-TKIs, EGFR tyrosine kinase inhibitors, osimertinib, pulsed erlotinib
Introduction
The incidence of LMC in NSCLC has increased due to improved survival of patients from advances in systemic therapy;1 LMC is estimated to be present in about nine percent of EGFR-mutant NSCLC patients.2,3 The most common mutations in NSCLC patients that have been identified in the EGFR gene are in-frame deletion in exon 19 and L858R in exon 21. L858R mutation is a single nucleotide variant in exon 21 in the tyrosine kinase domain of the EGFR gene. This missense mutation located at the coding position 2573, causing nucleotide change from thymine (T) to guanine (G) causing amino acid substitution from leucine (L) to arginine (R) at codon 858. EGFR mutations are more frequent in adenocarcinoma than other NSCLCs, in females, and in never smokers.4,5
As demonstrated in previous studies, EGFR tyrosine kinase inhibitors (EGFR-TKIs) can improve prognosis of these patients.6,7 However, most lung adenocarcinoma patients with EGFR-mutations who are treated with EGFR tyrosine kinase inhibitors will relapse because of resistance development against the used drug.8 The most common resistance mechanism is acquisition of the EGFR exon 20 T790M mutation.
Tumors that develop resistance to EGFR-TKIs, mostly due to EGFR-T790M mutation, represent a challenge for durable response.9
Osimertinib, a third-generation EGFR-TKI, is an approved agent for previously treated EGFR-TKIs mutant NSCLC patients who develop EGFR-T790M resistance mutation,10–13 and untreated EGFR-mutant advanced NSCLC patients.14
The updated results from BLOOM study indicate that osimertinib is effective for LMC, regardless of T790M-mutation status.15,16 Furthermore, intermittent high, rather than standard, dose of erlotinib reaches therapeutic concentrations within the cerebrospinal fluid (CSF) and is well tolerated in patients with LMC.17 Herein, we present a patient with T790M-negative metastatic NSCLC adenocarcinoma who developed LMC. The patient’s symptoms responded promptly well to osimertinib followed by pulsed erlotinib and showed radiological improvement that lasted for a total of nine months.
Case Report
A 52-year-old, nonsmoker, Asian male was first diagnosed with lung cancer in February 2017. His initial presentation was persistent coughing. Imaging showed a 4 cm sized left lung lesion lateral to the left lung hilum. Staging workup showed mediastinal (N2) lymph node, left adrenal involvement and multiple bone metastases with minimal left pleural fluid. A tissue biopsy of his lung mass revealed NSCLC adenocarcinoma; EGFR-mutant with L858R in exon 21, PD-L1 negative and ALK rearrangement negative.
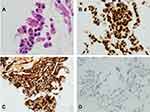
Pathological sections showed fragments of skeletal muscle fibers and fibroadipose tissue infiltrated by a tumor characterized by ill-defined glandular structures as well as showing solid sheets of pleomorphic polygonal cells some with cytoplasmic mucin (Figure 1A). These tumor cells are strongly positive for CK 7, TTF-1 (Figure 1B) and napsin-A (Figure 1C) while negative with CK 20, CD X2, PAPH and PSA. PD-L1 (TPS) is negative (Figure 1D). ALK 5 A4 by immunohistochemistry is negative.
The EGFR mutation was confirmed by molecular technique. The tumor area was marked by consultant pathologist on a formalin-fixed paraffin-embedded (FFPE) slide. Genomic DNA was extracted and analyzed by using EGFR-RT52 kit (EntroGen, USA). The kit is based on a real time-PCR qualitative genotyping assay that uses fluorescently labeled probes for detection of 30 recurrent mutations G719X in exons 18, deletions in exon 19, T790M, S768I and insertions in exon 20 (resistance mutations) and L858R and L861Q in exon 21 of EGFR gene. This test will not identify rare mutations, such as E709K and D770-N771ins.
The patient initially received erlotinib 150 mg then reduced to 100 mg because intolerance in the form of acne and mucositis; he had clinical and metabolic response until May 2018: a progression-free survival of 18 months.
In August 2018, positron emission tomography (PET) scan showed disease progression in the bones with stationary lung lesions. Liquid biopsy was tested and did not demonstrate T790M-mutation. At that point of his treatment, afatinib and cetuximab were introduced but then discontinued two weeks later, as he developed grade 3–4 oral/nasal mucositis. Carboplatin and pemetrexed were given as third line, and CT scan showed stationary course of the disease for another 12 months.
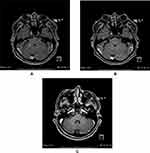
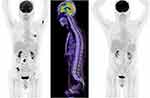
In October 2019, the patient presented to the Emergency Department complaining of new onset severe generalized headache, dizziness, blurred vision, and repeated vomiting evolving over two weeks. Examination revealed neck rigidity without papilledema, intact cranial nerves, power 5/5 in both upper and lower limbs, reflexes +1, and downgoing plantar response. Magnetic resonance imaging (MRI) of the brain showed abnormal sulcal and cisternal enhancement representing extensive leptomeningeal metastases, with small cerebellar and cerebral cortical metastases (Figure 2A). Whole body PET scan showed disease progression (Figure 3). Patient refused a lumbar puncture. He received dexamethasone 8 mg twice daily for three days along with paracetamol and ondansetron without symptom improvement. The patient was given osimertinib as a replacement of his treatment option, 160 mg once daily, osimertinib was started on day four, all his symptoms have improved dramatically within the following 24 h. On day six, a tissue biopsy of a right pelvic bone lesion showed metastatic adenocarcinoma of the lung. The T790M-mutation was not detected in this biopsy (Figure 4). Fourteen days post osimertinib initiation, MRI of the brain showed complete clearance of some tumor lesions and decrease in size of other lesions in the brain. Furthermore, a decrease in the leptomeningeal regions (Figure 2B). A month later, November 2019, a follow-up PET scan showed favorable response (Figure 3), and in January 2020 a follow-up MRI for brain and spine showed regression of the tiny cerebral enhancing metastatic lesions (Figure 2C).
In February 2020, headache and vomiting recurred but generally the patient complained of less severe symptoms than the symptoms presented in October 2019. CSF examination showed atypical cells highly suspicious for metastatic malignancy. MRI spine showed diffuse meningeal thickening, and subtle nodularity along the lumbar spinal cord and thecal sac representing metastases.
PET scan showed practically stable presentation (Figure 3). Osimertinib was stopped at this point. The patient was followed with a trial of intrathecal methotrexate, twice weekly for two weeks, which induced severe post lumbar puncture headache without relieving his symptoms. Palliative whole brain radiotherapy and meningeal radiation were discussed with the patient several times as an additional treatment option, but he refused.
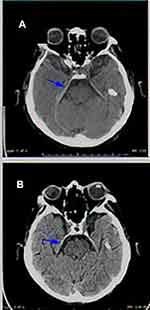
By the end of March 2020, the patient was given pulsed erlotinib 1050 mg once weekly increased to 1500 mg one week later. In April, the patient showed up following a syncope and fall, CT scanning was performed for the head and showed changes related to the leptomeningeal metastasis with thickening of the tentorium cerebelli and along the right greater wing of sphenoid (Figure 5A). In July, while presenting with seizures, CT scanning of the head showed reduction in the leptomeningeal and tentorium cerebelli thickening (Figure 5B). He continued pulsed erlotinib for almost four months, as his overall symptoms had improved without significant medication side effect. Unfortunately, his performance status kept deteriorating and, unfortunately; he died by the end of July 2020. Overall, he survived almost nine months post LMC diagnosis: five months on osimertinib and four months on pulsed erlotinib.
Discussion
Metastatic NSCLC and LMC have been reported with poor prognosis.1 Our patient had stage IV NSCLC adenocarcinoma with EGFR-mutation L858R in exon-21. His first line was conventional dose erlotinib with good partial metabolic response for 18 months. Following disease progression second line was tried as afatinib/cetuximab but discontinued after two weeks only due to poor tolerance and grade 3–4 oral/nasal mucositis. Furthermore, third-line chemotherapy with carboplatin/pemetrexed was used and was effective for further 12 months. Unfortunately, he developed LMC and new systemic lesions were detected without showing T790M-mutation from a metastatic bone site. Since his tumor carried an EGFR-mutation, the decision to start osimertinib was made, to overcome the relapse and progression as several studies confirmed that the osimertinib has possible effective outcome even if the tumor was T790M-negative.
First generation TKIs erlotinib and gefitinib have shown some response in the treatment of LMC in small studies and case series.18–21 Osimertinib, a third-generation TKI, is superior over standard platinum-based chemotherapy for the treatment of patients with T790M-positive disease who had progressed after first-line EGFR-TKI.12,13
It is also superior over first generation TKIs in untreated patients regardless of T790M status.14 The update from the BLOOM study which included 21 unselected patients and 11 T790M-positive patients concluded that osimertinib penetrates the blood–brain barrier.16 Ballard et al added that osimertinib penetration to the blood–brain barrier is better than gefitinib, afatinib or rociletinib.22 Osimertinib presents encouraging activity and manageable tolerability in patients with leptomeningeal metastases from EGFR-mutant NSCLC observed at 160 mg a day.16 The findings from BLOOM study was observed in several case reports (Table 1).15,23–25
 |
Table 1 Summary of Previous Literature of Osimertinib 160 mg for Treatment of Patients with Leptomeningeal Metastases from EGFR-Mutant NSCLC |
Our patient demonstrated a prompt dramatic clinical response (within 24 h of commencing osimertinib) with significant response shown in brain imaging. Even though the T790M was negative based on bone biopsy, which might be a false negative,26,27 but in our case the chance of having a false negative was low as the bone biopsy showed acceptable DNA quality. In addition, the L858R mutation was detected at this metastatic site. The prompt, relatively sustained improvement in the patient condition following treatment with osimertinib should not be ignored. Our case report has shown great benefit of the sequential treatment strategy that we applied to manage our patient, including erlotinib (18 months), afatinib/cetuximab, chemotherapy with carboplatin/pemetrexed (12 months), osimertinib radiological response that continued for (5 months) and finally high-dose pulsed erlotinib (additional 4 months). Osimertinib has shown dramatic quick effective and well-tolerated therapeutic option for LMC in EGFR-mutant NSCLC, even in T790M-negative disease, our case showed response within 24 h post-osimertinib initiation.
The overall treatment time since the patient was first diagnosed is approximately four years. Moreover, he responded to pulsed erlotinib for additional four months. Median survival of EGFR-mutated NSCLC patients with LMC was 3.1 months in previous studies,2,3 while our patient had survived almost nine months post LMC diagnosis as he was under selective sequential treatment.
We have managed this patient with different treatment options first to reduce the disease progression according to the molecular results and second to overcome any developed resistance, taking into consideration the patient status and performance. Patients with LMC are known to have poor performance status; a treatment that is tolerable with fewer side effects should always be taken into consideration.
Conclusion
Osimertinib can result in clinical and radiological response, with extended survival when used for EGFR-mutant T790M-negative lung adenocarcinoma progressed to LMC. Pulsed erlotinib can be one of the options to help in controlling the symptoms of LMC and extension of the patient’s survival.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The case report was approved by the Medical Research Centre at Hamad Medical Corporation and the Hamad Institutional Review Board (IRB) under number MRC-04-21-249.
Consent for Publication
This case report does not contain any personal identifier of the patient. It only includes radiological and pathological imaging, which does not contain any identifications. A written informed consent of patient information, images and publication was signed by the patient.
Acknowledgments
Open Access funding provided by the Qatar National Library.
Author Contributions
AK and FS contributed equally to this work; they conceived and designed the idea, literature review, data collection and wrote the initial manuscript draft. WA wrote the initial manuscript draft and contributed to the solid tumor section of the molecular genetic laboratory of the case report. IAB provided the histopathology figures, interpretation and helped in manuscript writing. MA and LS helped in the radiology figures and helped in manuscript writing. NEO contributed to the manuscript writing and organize the overall case report. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in his work.
References
1. Lee SJ, Il LJ, Nam DH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. 2013;8(2):185–191. doi:10.1097/JTO.0b013e3182773f21
2. Kuiper JL, Hendriks LE, van der Wekken AJ, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer. 2015;89(3):255–261. doi:10.1016/j.lungcan.2015.05.023
3. Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12(11):1193–1199. doi:10.1093/neuonc/noq076
4. Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346.
5. Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101(36):13306–13311. doi:10.1073/pnas.0405220101
6. Jorge SEDC, Kobayashi SS, Costa DB. Epidermal growth factor receptor (EGFR) mutations in lung cancer: preclinical and clinical data. Braz J Med Biol Res. 2014;47(11):929–939. doi:10.1590/1414-431X20144099
7. Yi HG, Kim HJ, Kim YJ, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009;65(1):80–84. doi:10.1016/j.lungcan.2008.10.016
8. Cheng L, Alexander RE, MacLennan GT, et al. Molecular pathology of lung cancer: key to personalized medicine. Mod Pathol. 2012;25(3):347–369. doi:10.1038/modpathol.2011.215
9. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi:10.1158/1078-0432.CCR-12-2246
10. Stinchcombe TE. AZD9291 in epidermal growth factor receptor inhibitor-Resistant non-small-cell lung cancer. Transl Lung Cancer Res. 2016;5(1):92–94. doi:10.3978/j.issn.2218-6751.2015.07.19
11. Uemura T, Oguri T, Okayama M, et al. Dramatic intracranial response to osimertinib in a poor performance status patient with lung adenocarcinoma harboring the epidermal growth factor receptor T790M mutation: a case report. Mol Clin Oncol. 2017;6(4):525–528. doi:10.3892/mco.2017.1181
12. Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
13. Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum–pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31(11):1536–1544. doi:10.1016/j.annonc.2020.08.2100
14. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR -mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
15. Yang JCH, Kim SW, Kim DW, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38(6):538–547. doi:10.1200/JCO.19.00457
16. Yang JC-H, Kim D-W, Kim S-W, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a Phase I study. J Clin Oncol. 2016;34(15_suppl):9002. doi:10.1200/JCO.2016.34.15_suppl.9002
17. How J, Mann J, Laczniak AN, Baggstrom MQ. Pulsatile Erlotinib in EGFR-positive non–small-cell lung cancer patients with leptomeningeal and brain metastases: review of the literature. Clin Lung Cancer. 2017;18(4):354–363. doi:10.1016/j.cllc.2017.01.013
18. Masuda T, Hattori N, Hamada A, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol. 2011;67(6):1465–1469. doi:10.1007/s00280-011-1555-6
19. Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4(11):1415–1419. doi:10.1097/JTO.0b013e3181b62572
20. Yang H, Yang X, Zhang Y, et al. Erlotinib in combination with pemetrexed/cisplatin for leptomeningeal metastases and cerebrospinal fluid drug concentrations in lung adenocarcinoma patients after gefitinib failure. Target Oncol. 2015;10(1):135–140. doi:10.1007/s11523-014-0326-9
21. Lee E, Keam B, Kim DW, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8(8):1069–1074. doi:10.1097/JTO.0b013e318294c8e8
22. Ballard P, Yates JWT, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140. doi:10.1158/1078-0432.CCR-16-0399
23. Li J, Liu X, Yuan C. Treatment response to osimertinib in a patient with leptomeningeal metastasis from lung adenocarcinoma following failure of gefitinib and erlotinib: a case report. Mol Clin Oncol. 2018;9(3):321–324. doi:10.3892/mco.2018.1666
24. Tsang MW. Osimertinib 160 mg daily for advanced non‐small cell lung cancer with leptomeningeal metastasis: a case report. Asia Pac J Clin Oncol. 2019;15(S6):5–7. doi:10.1111/ajco.13246
25. Li H, Yu T, Huang M, Guo A, Qian X, Yin Z. Treatment response to osimertinib in EGFR-Mutated leptomeningeal metastases from non-small cell lung cancer: a case series. Onco Targets Ther. 2019;12:7785–7790. doi:10.2147/OTT.S199452
26. Kobayashi K, Naoki K, Manabe T, et al. Comparison of detection methods of EGFR T790M mutations using plasma, serum, and tumor tissue in EGFR-TKI-resistant non-small cell lung cancer. Onco Targets Ther. 2018;11:3335–3343. doi:10.2147/OTT.S161745
27. Stockley T, Souza CA, Cheema PK, et al. Evidence-based best practices for EGFR T790M testing in lung cancer in Canada. Curr Oncol. 2018;25(2):163–169. doi:10.3747/co.25.4044
 © 2022 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2022 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.