Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Lenvatinib Plus PD-1 Inhibitors versus Regorafenib in Patients with Advanced Hepatocellular Carcinoma After the Failure of Sorafenib: A Retrospective Study
Authors Xu Y , Fu S, Liu K, Mao Y, Wu J
Received 26 June 2023
Accepted for publication 3 October 2023
Published 24 October 2023 Volume 2023:19 Pages 853—863
DOI https://doi.org/10.2147/TCRM.S420371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yongkang Xu, Shumin Fu, Kan Liu, Ye Mao, Jianbing Wu
Department of Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China
Correspondence: Jianbing Wu, Department of Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China, Email [email protected]
Purpose: To evaluate the clinical outcomes of lenvatinib plus PD-1 inhibitors (LP) and regorafenib (R) in patients with advanced hepatocellular carcinoma (HCC) after sorafenib failure.
Methods: From June 2018 to September 2021, 68 patients from a single center who received lenvatinib combined with PD-1 inhibitors or regorafenib after sorafenib treatment failure were analyzed. The tumor response and survival outcomes were compared between the LP group and R group. Prognostic factors for OS and PFS were determined using Cox proportional hazard regression models.
Results: The ORR increased in the LP group (19.5% vs 7.4%, p =0.294), and the DCR was better in the R group (73.2% vs 44.4%, p =0.017). Additionally, median PFS and OS were not significantly different between the LP group and R two groups in survival analysis (PFS: 5.3 months vs 3.0 months, p =0.633; OS: 11.8 months vs 8.0 months, p =0.699). The common adverse events (≥grade 3) were hand-foot skin reactions (13.1%). In multivariate analyses, AFP≥ 400 ng/mL and ECOG PS 2 were independent risk factors for poor prognosis.
Conclusion: The LP group appeared to have a trend of greater tumor response and a higher disease control rate than the R group among patients with sorafenib-resistant HCC, although PFS and OS did not differ significantly between the two groups.
Keywords: hepatocellular carcinoma, lenvatinib, PD-1 inhibitors, regorafenib, sorafenib, overall survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers in the world, and its incidence and mortality are on the rise.1 It is estimated that by 2040, there will be 1.4 million newly diagnosed cases (an increase of 55.0% from 2020), and 1.3 million people will die of liver cancer (an increase of 56.4% from 2020).2 Due to the insidious onset of HCC, early diagnosis is difficult, and many patients are diagnosed at a late stage.3,4 Treatment methods for advanced HCC include transarterial chemoembolization (TACE), radiofrequency ablation (RFA), hepatic artery infusion chemotherapy (HAIC), molecular-targeted drug therapy and immunotherapy.5–10 According to the results of the SHARP and Asia-Pacific HCC trials, sorafenib is the preferred therapy for those with late-stage HCC.11,12 However, owing to its rapid progression and intolerable side effects, many tumors are resistant to sorafenib, and the therapeutic efficacy remains poor.
The approval of new regimens, including targeted therapy and immunotherapy, has changed the second-line therapeutic landscape for patients with HCC. In the RESORCE trial,13 regorafenib effectively prolonged the survival of patients with sorafenib resistance. From the perspective of cost-effectiveness analysis, regorafenib is the most standard second-line treatment option in the era of molecular targeted therapy.14,15 Moreover, immune checkpoint inhibitors (ICIs) have also shown efficacy for sorafenib-resistant HCC. The advent of the CheckMate 040 study and KEYNOTE-224 study profoundly changed the systemic treatment pattern of advanced HCC.16,17 However, the ORR is still low, and the survival time is limited in these second-line therapy patients.
Lenvatinib can target a variety of molecular targets, such as VEGFR1-3, FGFR1-4, PDGFR, KIT, and RET, which have driving effects on cancers. Thyroid cancer, kidney cancer, liver cancer, and endometrial cancer have all been treated with lenvatinib on a large scale.18,19 Although the overall survival (OS) of lenvatinib was not inferior to that of sorafenib (13.6 months vs 12.3 months) according to REFLECT, the progression-free survival (PFS) was significantly better (7.4 months vs 3.7 months, p<0.0001).20 The NCCN guidelines also recommend that lenvatinib be selected as a second-line treatment for sorafenib-resistant HCC.21 The Keynote-524 study showed that the combination of lenvatinib and PD-1 inhibitors can significantly improve treatment efficacy and progression-free survival.22 According to preclinical research, lenvatinib plus PD-1 inhibitors produce a synergistic impact, improving the immune microenvironment and fostering anticancer immunity.23,24 Combination therapy offers a potentially effective treatment protocol, but few reports have been published on its therapeutic effects in patients with sorafenib-resistant HCC. Therefore, we performed a comparative study to evaluate the clinical outcomes of lenvatinib plus PD-1 inhibitors (LP) and regorafenib (R) in patients with sorafenib-resistant HCC.
Materials and Methods
Patients
Between June 2018 and September 2021, 85 patients with sorafenib-refractory HCC who received lenvatinib plus PD-1 inhibitors or regorafenib were recruited. After screening based on the inclusion and exclusion criteria, 68 cases were ultimately enrolled in the study, including 41 cases in the LP group and 27 cases in the R group. The study protocol was approved by The Ethics Committee of the Second Affiliated Hospital of Nanchang University. All patients provided informed consent prior to treatment. The study was conducted according to the Declaration of Helsinki.
The inclusion criteria were as follows: (1) HCC was diagnosed according to American Association for the Study of Liver Disease (AASLD) guidelines;10 (2) Barcelona Clinic Liver Cancer (BCLC) stage B/C; (3) sorafenib-resistant HCC patients had received LP/R; (4) performance score of Eastern Cooperative Oncology Group (ECOG PS) 0–2 score; (5) Child‒Pugh (CP) A/B; and (6) received at least 2 cycles of LP/R.
The exclusion criteria were as follows: (1) malignant tumor other than HCC; (2) severe organ dysfunction; (3) missing clinical data; and (4) loss to follow-up.
Treatment Procedures
Tislelizumab, sintilimab, camrelizumab, and pembrolizumab are PD-1 inhibitors that are administered intravenously at 200 mg every 3 weeks. The initial oral dose of lenvatinib is 8 mg/day for individuals whose body weight is below 60 kg and 12 mg/day for those whose weight is over 60 kg. The patients received regorafenib orally at a dose of 160 mg once daily for 3 weeks and 1 week off. All treatment drug dosage reductions and local treatment combinations (TACE/HAIC) followed the recommendations of the multidisciplinary tumor board.
Efficacy and Safety Assessments
The status of tumor growth and metastasis was evaluated according to the RESIST1.1 and mRECIST standards based on contrast-enhanced CT/MRI, which was performed every 6–8 weeks. Laboratory tests, including biochemical tests, alpha-fetoprotein (AFP) levels and thyroid function tests, were performed at each follow-up visit. Objective response rate (ORR)= [complete response (CR)+partial response (PR)]/total number of cases. Disease control rate (DCR) = [CR+PR+ disease stability (SD)]/total number of cases. Progression-free survival (PFS) was defined as the time interval from the initial treatment to PD or death. Overall survival (OS) was defined as the time interval from the initial treatment to death or last day of follow-up. The grade of adverse events (AEs) was recorded using the National Cancer Institute General Toxicity Criteria (NCI-CTC 5.0). All patients were followed up after treatment until reaching the OS endpoint or the last follow-up date (January 1, 2023).
Statistical Analysis
The statistical tools SPSS (version 26.0; IBM Corporation) and R (version 4.2.1) were used for all statistical analyses. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. Survival curves were constructed using the Kaplan–Meier method and compared using the log rank test. To identify the variables that were independently associated with PFS and OS, variables with p <0.05 in the univariate analysis were further adopted into the Cox proportional hazards regression model. P<0.05 was considered statistically significant.
Results
Patients
The study selection process is illustrated in Figure 1. From June 2018 to September 2021, 85 HCC patients who had not responded to sorafenib were enrolled and given LP or R therapy. After filtering based on the inclusion criteria, 68 patients participated in the study. Seventeen patients were excluded. Of these, 10 were excluded because of incomplete data, and 7 were excluded due to a lack of follow-up data.
 |
Figure 1 Flow diagram of study participants. |
There were no significant differences in the clinical characteristics of the patients, including age, sex, ECOG PS, CP A/B, BCLC B/C, hepatitis B virus (HBV) infection, liver cirrhosis, AFP, portal vein tumor thrombus and extrahepatic metastasis, between the LP group and the R group, as shown in Table 1 (p>0.05). After developing sorafenib resistance, 41 and 27 patients received LP and R treatments, respectively. Of all the patients, 62 (91.2%) were male, and 6 (8.8%) were female. Forty-seven patients (60.1%) had advanced-stage HCC, and 21 had BCLC stage B HCC (39.9%). The CP scores were A (n = 54), with 85.4% in the LP group and 70.4% in the R group. Among the patients, 44 (64.7%) presented with extrahepatic metastases, and 19 (27.9%) presented with portal vein thrombosis. Twenty-four patients (58.5%) in the LP group and 21 patients (77.8%) in the R group received TACE/HAIC therapy.
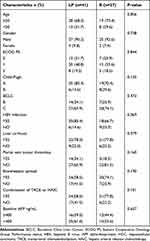 |
Table 1 Characteristics of Patients in This Study |
Subsequent Treatment
Following the progression of the disease, 24 patients had additional therapies, as shown in Table 2. In the LP group, 14 (34.1%) patients received new treatment after progression, including HAIC+ PD-1 inhibitors + apatinib (2), atezolizumab + bevacizumab (1), PD-1 inhibitors + regorafenib (4), apatinib (1), PD-1 inhibitors + apatinib (2), radiotherapy (1), and regorafenib (3). After termination of the study treatment, 10 patients (37.4%) in the R group received third-line therapy, including PD-1 inhibitors + regorafenib (1), PD-1 inhibitors + apatinib (1), PD-1 inhibitors +lenvatinib (4), TACE +PD-1 inhibitors + lenvatinib (2), and lenvatinib (2).
 |
Table 2 Subsequent Treatment |
Treatment Outcomes and Survival Outcomes
Tumor responses are shown in Table 3. One patient (2.4%) in the LP group but 0 patients in the R group achieved CR, whereas 7 (17.1%) and 2 (7.4%) patients achieved PR, as determined by RECIST1.1. Between the LP/R groups, there was no appreciable change in ORR (19.5% vs 7.4%; p = 0.294). One patient (3.7%) in the R group and 3 (7.3%) patients in the LP group experienced CR, whereas 9 (22.0%) and 3 (11.1%) patients achieved PR, as determined by mRECIST. The ORR was higher in the LP group (29.3% vs 14.8%, p =0.755), and the DCR was better in the R group (73.2% vs 44.4%, p= 0.017). The median PFS in the LP group was 5.3 months (95% CI, 4.0–7.2), and it was 3.0 months (95% CI, 2.3–6.3) in the R group [p = 0.633; Figure 2A]. The median OS was 11.8 months (95% CI, 9.2–16.2) in the LP group and 8.0 months (95% CI, 6.5–22.0) in the R group [p =0.699; Figure 2B].
 |
Table 3 Response to Treatment |
 |
Figure 2 OS and PFS with sorafenib resistance HCC who were treated with LP or R, respectively. (A) Kaplan–Meier analysis of PFS. (B) Kaplan–Meier analysis of OS. |
Treatment Toxicities
AEs that occurred throughout treatment with LP/R are shown in Table 4. During the time of the trial, there were no treatment-related fatalities. The most common symptoms in the LP group were hypertension (41.5%), hand-foot skin reactions (HFSR, 36.6%), and proteinuria (22.0%). The most typical AE grade (≥3) was hypertension (9.8%). The most frequent AEs in the R group were HFSR (44.0%), hyperbilirubinemia (25.9%), and hypertension (18.5%). The most frequent severe AEs during regorafenib treatment were HFSR (n=4, 14.8%) and elevated AST levels (n =2, 7.4%).
 |
Table 4 Summary of Adverse Events |
Prognostic Factor Analysis
According to the univariate analysis, ECOG PS (HR 2.745, 95% CI 1.449–5.200, p =0.002), HBV infection (HR 0.479, 95% CI 0.263–0.870, p =0.016), and AFP (HR 3.000, 95% CI 1.798–5.004, p<0.001) remained independent predictors of PFS (Table 5). Predictors of worse PFS according to the multivariate analysis were ECOG PS 2 score (HR 2.721, 95% CI 1.372–5.395, p =0.004) and AFP≥400 ng/mL (HR 3.000, 95% CI 1.798–5.004, p < 0.001). In the two groups, the univariate analysis showed that ECOG PS (HR 3.474, 95% CI 1.812–6.660, p<0.001), Child‒Pugh (HR 2.90, 95% CI 1.29–6.54, p=0.010) and AFP (HR 2.661, 95% CI 1.572–4.505, p < 0.001) were significantly correlated with OS (Table 6). Predictors of worse OS were ECOG PS 2 score (HR 4.530, 95% CI 2.291–8.960, p < 0.001) and AFP≥400 ng/mL (HR 3.111, 95% CI 1.790–5.407, p < 0.001).
 |
Table 5 | Univariate and Multivariate Analysis of Risk Factors for Progression-Free Survival |
 |
Table 6 Univariate and Multivariate Analysis of Risk Factors for Overall Survival |
After stratification by ECOG PS, the median PFS in the ECOG PS 0–1 score group was significantly higher than that in the ECOG PS 2 score group [5.3 months (95% CI, 4.0–7.2) vs 3.0 months (95% CI, 1.9–4.1), p=0.001, Figure 3A], and the differences in OS outcomes were also statistically significant [12.8 months (95% CI, 10.1–19.0) vs 4.0 (95% CI, 3.0–6.8) months, p <0.001, Figure 3B]. After stratification by AFP, the median PFS in the AFP<400 ng/mL group was significantly longer than that in the AFP≥400 ng/mL group [6.5 months (95% CI, 5.3–8.7) vs 2.7 months (95% CI, 2.1–3.7), p <0.001, Figure 3C]. Similarly, the differences in OS outcomes were also statistically significant [14.1 months (95% CI, 11.7–22.2) vs 6.6 months (95% CI, 5.1–11.1), p <0.001, Figure 3D].
Discussion
Numerous current guidelines for treatment options after sorafenib resistance in hepatocellular carcinoma include lenvatinib, regorafenib, ramucirumab, and PD-1/PD-L1 inhibitors.25,26 With the great success of the IMbrave15027 and KEYNOTE-524 study,22 immunotherapy combined with targeted therapy has become the optimal choice for targeted therapy in advanced HCC.Although the immunosuppressive microenvironment of HCC promotes immune tolerance and evasion through multiple mechanisms, molecularly targeted drugs can improve the immune microenvironment and synergize the effects.28,29 Therefore, it is important to explore the efficacy of immunotherapy combined with targeted therapy in the second-line treatment of advanced hepatocellular carcinoma. Lenvatinib combined with PD-1 inhibitors for sorafenib-resistant HCC has seldom been reported; therefore, we conducted exploratory analyses to determine whether any clinical benefit factors were associated with survival time. This retrospective single-center study compared the clinical outcomes of using LP/R for sorafenib-resistant HCC patients, including tumor response, prognostic outcomes, and adverse events. Although this study did not show a statistically significant prolongation of survival time, the benefit of tumor response seemed to increase, and there was a clear difference in the DCR. The multivariate analyses found that the prognosis of sorafenib-resistant patients with AFP ≥ 400 ng/mL and ECOG PS 2 was poor.
Our findings demonstrated that the median PFS and OS of the patients treated with LP were 5.3 and 11.8 months, respectively. According to RECIST1.1, the ORR and DCR of patients treated with LP were 19.5% and 73.2%, respectively. In contrast to earlier research findings, which showed safe and acceptable outcomes, our results are different. The RESCUE study demonstrated that camrelizumab plus apatinib is a prospective regimen for second-line treatment of advanced HCC, with an ORR of 22.5% and a median OS of 21.8 months (17.3–26.8).30 Only patients with CP class A, ECOG PS 0–1, and intrahepatic vessel invasion (24.2%) were included in the RESCUE. Moreover, compared with previous single molecular targeting agent trials involving patients with sorafenib-resistant HCC, the PFS in the LP group had certain benefits.13,31–33 Another small study also showed that LP is a hopeful new strategy for second-line or later treatment of patients.34
In patients treated with regorafenib, the ORR, DCR, median PFS, and median OS were 7.4%, 44.4%, 3.0 months, and 8.0 months, respectively. These results are worse than the ORR, DCR, median PFS, and median OS of 11.0%, 65.2%, 3.2 months, and 10.6 months, respectively, in the Phase III RESORCE trial.13 These disparities likely resulted from several factors, including differences in patient characteristics, such as a larger proportion of patients with HBV infection (66.7%), ECOG PS 2 score (18.5%), and CP class B (29.6%). However, they have been similarly described in several recent studies on real-world experiences.35–37Although these results suggested that LP group appeared to have a trend of greater tumor response and a higher disease control rate than the R group among patients with sorafenib-resistant HCC (ORR, 19.5% vs 7.4%, p = 0.294; DCR, 73.2% vs 44.4%, p= 0.017), there were different conclusions regarding the ORR. Interestingly, when compared with the LP group and R group, the combination regimen did not show an obvious advantage in improving the prognosis of patients. The median PFS and OS were not significantly different between the LP group and R group in the prognosis analysis (PFS 5.3 months vs 3.0 months, p =0.633; OS 11.8 months vs 8.0 months, p =0.699). The author’s team believed that the sample size was insufficient to assess the effect of treatment on survival. Importantly, combination immunotherapy is recommended, as it may effectively slow tumor growth.
Multivariate analysis failed to demonstrate that treatment option (LP vs R) may be an independent risk factor for PFS/OS. However, ECOG PS and AFP were also independent risk factors, as revealed by the multivariate analysis of the whole population. Further analysis indicated that patients with better physical condition (ECOG PS 0–1 score) had longer PFS (5.3 months vs 3.0 months, p=0.001) and OS (12.8 months vs 4.0 months, p <0.001). Kaplan–Meier analysis also indicated that patients with lower levels of AFP (<400 ng/mL) had longer PFS (6.5 months vs 2.7 months, p <0.001) and OS (14.1 months vs 6.6 months, p <0.001). Considerable research has suggested that tumor burden and physical status are important factors that may affect the efficacy of systematic treatment of HCC.38,39
From a clinical perspective, our findings support the adverse reaction incidence of previous studies.16,34–36 The study found that the frequency of severe AEs was 13.1% for hand-foot skin reactions and 4.9% for elevated AST levels. All AEs had noticeably higher percentages in the LP group, with the leading five AEs being hypertension (41.5), hand-foot skin reaction (36.6%), proteinuria (22.0%), diarrhea (19.5%), and hypothyroidism (17.1%). Immune-related adverse effects (irAEs) related to immunotherapy were observed during the study period. Despite the lack of experience in the treatment of irAEs, no serious side reactions were recognized in the immunotherapy group, and all were resolved with symptomatic treatment.
This study had several strengths. The therapeutic effects of lenvatinib plus PD-1 inhibitors or regorafenib alone in patients with sorafenib-resistant were investigated. Using real-world data, our study describes the basic characteristics of sorafenib-resistant HCC patients. We also identified that prognostic factors (ECOG PS and AFP) were key indicators affecting survival time in our study based on the length of follow-up.
There are a number of underlying restrictions in this study. First, this was a single-center retrospective study and may have been affected by specific treatment practices. Second, the number of patients included in the study was too small to detect relevant differences.As no randomized controlled studies have been conducted, caution should be exercised when interpreting these findings. Finally, because the enrolled patients used various combination therapeutic regimens, there was a certain bias in the process of selecting patients and evaluating treatment efficacy.
In summary, our study suggests that the LP group had a trend of higher tumor response and better disease control rate than the R group; nevertheless, their PFS and OS did not demonstrate the possibility of benefits. Future research on second-line treatments for HCC patients is needed and should be conducted with a larger sample, particularly in those who have previously received treatment with ICIs. Let us look forward to more and better drugs in the future and better guidance for clinical diagnosis and treatment so that more patients can benefit.
Funding
This work was supported by a funding from the Jiangxi Provincial Department of Science and Technology (No. 20202BAB206052).
Disclosure
The authors state that there are no potential conflicts of interest in this article.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi:10.1016/j.jhep.2022.08.021
3. Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
5. Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. doi:10.1159/000514174
6. Kudo M. Recent advances in systemic therapy for hepatocellular carcinoma in an aging society: 2020 update. Liver Cancer. 2020;9(6):640–662. doi:10.1159/000511001
7. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
8. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491. doi:10.1053/j.gastro.2018.08.065
9. Korean Liver Cancer Association. 2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019;20(7):1042–1113. doi:10.3348/kjr.2019.0140
10. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
11. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
12. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
13. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
14. Parikh ND, Singal AG, Hutton DW. Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer. 2017;123(19):3725–3731. doi:10.1002/cncr.30863
15. Solimando AG, Susca N, Argentiero A, et al. Second-line treatments for advanced hepatocellular carcinoma: a systematic review and bayesian network meta-analysis. Clin Exp Med. 2022;22(1):65–74. doi:10.1007/s10238-021-00727-7
16. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
17. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
18. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs. 2019;79(6):665–674. doi:10.1007/s40265-019-01116-x
19. Xu Y, Fu S, Shang K, et al. PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus regorafenib in patients with advanced hepatocellular carcinoma after failure of sorafenib. Front Oncol. 2022;12:958869. doi:10.3389/fonc.2022.958869
20. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
21. Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi:10.6004/jnccn.2021.0022
22. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
23. Yi C, Chen L, Lin Z, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544–2560. doi:10.1002/hep.31921
24. Adachi Y, Kamiyama H, Ichikawa K, et al. Inhibition of FGFR reactivates ifngamma signaling in tumor cells to enhance the combined antitumor activity of lenvatinib with Anti-PD-1 antibodies. Cancer Res. 2022;82(2):292–306. doi:10.1158/0008-5472.CAN-20-2426
25. Pinter M, Scheiner B, Peck-Radosavljevic M. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021;70(1):204–214. doi:10.1136/gutjnl-2020-321702
26. Fu Y, Liu S, Zeng S, et al. From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):396. doi:10.1186/s13046-019-1396-4
27. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
28. Chen S, Huang C, Liao G, et al. Distinct single-cell immune ecosystems distinguish true and de novo HBV-related hepatocellular carcinoma recurrences. Gut. 2023;72(6):1196–1210. doi:10.1136/gutjnl-2022-328428
29. Shigeta K, Matsui A, Kikuchi H, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e001435. doi:10.1136/jitc-2020-001435
30. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
31. Lin Y, Qin S, Li Z, et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: the REALITY randomized clinical trial. JAMA Oncol. 2022;8(2):242–250. doi:10.1001/jamaoncol.2021.6268
32. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
33. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
34. Huang X, Xu L, Ma T, et al. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: a retrospective study. Front Oncol. 2021;11:751159. doi:10.3389/fonc.2021.751159
35. Kuo YH, Yen YH, Chen YY, et al. Nivolumab versus regorafenib in patients with hepatocellular carcinoma after sorafenib failure. Front Oncol. 2021;11:683341. doi:10.3389/fonc.2021.683341
36. Huang J, Guo Y, Huang W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2022;9:157–170. doi:10.2147/JHC.S353956
37. Choi WM, Choi J, Lee D, et al. Regorafenib versus nivolumab after sorafenib failure: real-world data in patients with hepatocellular carcinoma. Hepatol Commun. 2020;4(7):1073–1086. doi:10.1002/hep4.1523
38. Moriguchi M, Aramaki T, Sato R, et al. Intrahepatic tumor burden as a novel factor influencing the introduction of second-line chemotherapy for hepatocellular carcinoma. Anticancer Res. 2020;40(7):3953–3960. doi:10.21873/anticanres.14387
39. Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008. doi:10.1016/j.jhep.2017.06.026
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

