Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Left Atrial Strain Helps Identifying the Cardioembolic Risk in Transient Ischemic Attacks Patients with Silent Paroxysmal Atrial Fibrillation
Authors Arnăutu SF , Morariu VI, Arnăutu DA , Tomescu MC , Dan TF, Jianu CD
Received 22 January 2022
Accepted for publication 3 March 2022
Published 10 March 2022 Volume 2022:18 Pages 213—222
DOI https://doi.org/10.2147/TCRM.S359490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Sergiu Florin Arnăutu,1,2,* Vlad Ioan Morariu,3,4,* Diana Aurora Arnăutu,3,4 Mirela Cleopatra Tomescu,3,4 Traian Flavius Dan,1,2 Cătălin Dragos Jianu1,2
1Neurology Department, Victor Babeș University of Medicine and Pharmacy, Timisoara, Romania; 2Neurology Clinic, Pius Brînzeu County Clinical Emergency Hospital, Timisoara, Romania; 3Multidisciplinary Heart Research Center, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania; 4Cardiology Clinic, Timisoara Municipal Clinical Emergency Hospital, Timisoara, Romania
*These authors contributed equally to this work
Correspondence: Diana Aurora Arnăutu; Mirela Cleopatra Tomescu, Victor Babes University of Medicine and Pharmacy, 2nd Eftimie Murgu Square, Timisoara, 300041, Romania, Tel +40 734600550 ; +40722979516, Fax +40 256220636, Email [email protected]; [email protected]
Purpose: Patients with transient ischemic attacks often present asymptomatic and paroxysmal atrial fibrillation. Since atrial fibrillation initiates in the atria, we aimed to identify whether the abnormalities in left atrial structure and function could identify the cardioembolic etiology of the transient ischemic attacks in patients at sinus rhythm.
Patients and Methods: A total of 190 patients over 50 years old with sinus rhythm discharged after a transient ischemic attack were included in the study and divided into two groups according to the presence (group I) or absence (group II) of documented paroxysmal atrial fibrillation. The documentation of paroxysmal atrial fibrillation was based on the examination of medical registers. Cardiac ultrasound assessment was performed at a minimum of 14 days after the onset of the transient ischemic attack, to avoid assessment of atrial stunning.
Results: The group I patients were older, more frequent women, with a history of stroke or transient ischemic attack and a higher CHA2DS2-VASc score. They also presented larger left atrial volumes, lower left atrial emptying fraction, and significantly impaired left atrial deformation patterns. Multivariate logistic regression identified three variables that were independently associated with paroxysmal atrial fibrillation: age, left atrial reservoir strain, and left atrial emptying fraction (P < 0.0001). The cut-off levels for the variables were age > 55 years, reservoir strain < − 17%, and emptying fraction < 51%.
Conclusion: The present study demonstrates that the LA strain is independently associated with paroxysmal atrial fibrillation in transient ischemic attack patients and might be of great help in identifying their cardioembolic etiology and preventing subsequent strokes by the initiation of anticoagulant therapy.
Keywords: transient ischemic attack, speckle tracking echocardiography, paroxysmal atrial fibrillation
Introduction
Atrial fibrillation (AF) causes significant problems to patients, medical doctors, and healthcare systems, demanding a multidisciplinary approach and management. It is the most common sustained cardiac arrhythmia in adults, with an estimated prevalence of 2–4%.1 Hypertension, diabetes, coronary heart disease, abnormalities of lipid metabolism are classic risk factors for both AF development and for ischemic stroke.2 Age is another important risk factor. When compared to those over 50, subjects under 50 have a substantially lower risk of developing AF.3 Men are more likely than women to develop atrial fibrillation (AF) at any age, but women with AF are at greater risk of having a stroke.4 Hematological disorders seem to be an often overlooked cause of acute stroke.5 Being in the coronavirus pandemic, we have to mention that AF is the most frequent arrhythmia related to COVID infection and that, when present, it is strongly related to mortality.6
Frequently, stroke is preceded by transient ischemic attacks (TIA´s). Ischemic strokes associated with AF are frequently severe, recurrent, and leading to death or permanent disability. About 20 to 30% of ischemic strokes have an unidentified etiology and are categorized as being cryptogenic.7 Using modern diagnostic procedures, up to 30% of cryptogenic strokes have been detected as having a cardioembolic etiology, due to silent atrial fibrillation (AF).1,7
Detecting individuals at increased risk of developing AF could improve a prompt AF detection. As soon as AF is detected, anticoagulation treatment is recommended, independent of the arrhythmia duration.1
The risk of stroke is important in patients with asymptomatic and paroxysmal atrial fibrillation (PAF), as these patients lack the protection offered by an adequate anticoagulant treatment.7,8 Silent PAF has been identified as the causing mechanism in about 15% of TIA´s.9,10 Holter-monitoring is commonly used for identifying PAF. Its efficiency is limited, as this arrhythmia is sporadic and frequently not recognized by the patients. Long term monitoring by insertable loop recorders represents a more efficient method, but is invasive and expensive and can´t be achieved in all stroke patients.11
Since AF initiates in the atria, we aimed to identify whether the abnormalities in LA structure and function could identify the cardioembolic etiology of the TIA´s in patients at sinus rhythm, using echocardiography. Echocardiography plays a significant role in the evaluation of patients who suffered an ischemic stroke, as it is non-invasive, efficient and repeatable.12
It is known that LA size is a valuable predictor of PAF in the general population, as well in patients with several disorders.13,14 Atrial enlargement is not specific for valvular and non-valvular AF, as it also occurs in left ventricular diastolic dysfunction and arterial hypertension. Additionally, it was reported that PAF may occur in patients with normal LA dimensions.15–17 Recent researches have proven the superiority of LA function versus LA size as a predictor of PAF,18 especially when studying deformation abnormalities by means of two-dimensional speckle-tracking echocardiography (2D-STE).19,20
In the present study, we aimed to assess whether LA two dimensional-speckle tracking echocardiography (2D-STE) could offer useful parameters to identify the cardioembolic etiology of TIA`s in patients with silent PAF.
Patients and Methods
Subject Selection
This is a retrospective cohort study that included all patients with transient ischemic attack aged ≥ 50 years old discharged consecutively from November 2017 to November 2021 from the Department of Neurology of the Timisoara County Hospital with a final diagnosis of TIA, and submitted for LA 2D-STE.
Exclusion criteria were: previous history of AF, the presence of AF within 24 hours after the TIA or at the time of cardiologic examination, moderate or severe valvular stenosis and/or regurgitation, mechanical heart valves, congenital heart disease, severe systolic dysfunction (left ventricular ejection fraction <40%), ventricular akinesia, permanent cardiac pacemaker, as well as inadequate echocardiography imaging.
The diagnosis of TIA was established in the presence of briskly evolving clinical signs of cerebral disorder (documented by neurological examination), lasting up to 24 h, and with no sign of infarction on brain-imaging.21
PAF was defined as an AF detected by a 12 leads standard resting electrocardiogram (ECG) or by a 24- hours Holter ECG monitoring, with a minimum duration of 30 seconds, and a spontaneously or interventional termination within 7 days of onset.1
Data Extraction
PAF was documented by review of the medical records when at least one episode was reported at any time in the patient’s history, during hospitalization for the TIA or during the post-hospital cardiologic examination. The clinical baseline data and the medical history of the TIA patients were obtained by review of the medical records.
The CHA2DS2-VASc risk score was assessed for every patient by giving 1 point for each of the following parameters: age between 65 and 74 years, a history of hypertension, diabetes mellitus, heart failure or atherosclerotic vascular disease, and female gender, and 2 points for a history of ischemic stroke/TIA or an age≥ 75 years.22
Echocardiographic Evaluation
All patients were evaluated by a 2-D transthoracic echocardiography during hospitalization for the TIA, using a Vivid 5S General Electric echocardiography device. LA 2D-STE was performed after a minimum of 14 days following the onset of the TIA, to avoid atrial stunning after a possible PAF.23,24 A single investigator with substantial echocardiography experience that was blinded to all other information performed all the echocardiographic evaluations, according to the common approach for the patients with TIA in the echocardiography laboratory.
Conventional Echocardiography
All patients were examined in a left lateral decubitus posture, using parasternal long and short axis, apical 4-, 3-, and 2-chambers visualizations. ECG trace was registered throughout the assessment. All recordings were performed at end-expiration, with a 3-cardiac cycle registration. The systolic left ventricular function was assessed by measuring left ventricular ejection fraction (LVEF) using Simpson’s biplane method. The diastolic LV function was assessed by means of transmitral pulsed Doppler and tissue Doppler imaging. The ratio between the peak early diastolic inflow and the peak late diastolic inflow (E/A), respectively the septal E/E´ ratio were calculated.24 Left atrial indexed volume (LAVI) to body surface area was calculated in the apical view, using the biplane area length method. The total left atrial emptying fraction (LAEF) was calculated using the formula: (LAEDV−LAESV)/LAEDV × 100.
2D-Speckle Tracking Echocardiography
Global peak longitudinal strain (GLS) of the LV was calculated from the three peak longitudinal strain curves of the apical 4-, 2-, and 3-chamber views using appropriate frame rates.
Left atrial reservoir (RV), conduit (CD), and booster pump (BP) strains (S) were assessed using 2D-STE on images obtained in the apical 4-chamber view. The images were analyzed offline using the General Electric Echo PAC software version 201. ECG was referenced to the peak of the R wave, and the region of interest was adjusted to the thickness of the LA wall. LARVS was calculated as the peak positive longitudinal strain during ventricular systole, while the negative strains during early and late diastole assessed the CD and BP atrial functions (Figure 1).
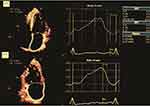 |
Figure 1 Strain curves from left atrial speckle-tracking, in apical four-chamber and two-chamber. |
To define the intra- and inter-observer variability, the 2D- STE measurements were repeated for 10 randomly selected patients at one month distance by the same observer on the same echocardiographic images. The intra-class correlation coefficient and the mean absolute difference between the repeated measures were calculated.24
Statistical Analysis
Normally distributed continuous variables are shown mean ± SD, while categorical variables are presented as number (percentage). Continuous variables were compared using an unpaired 2-tailed t-test, and categorical variables were compared using the Χ2 or Fisher exact test, as suitable Logistic regression analysis was performed to evaluate the risk prediction of PAF in TIA patients after adjusting for baseline characteristics and echocardiographic parameters. Receiver–operating characteristic (ROC) curves were utilized to determine the optimal sensitivity and specificity of the analyzed parameters. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models. The threshold for statistical significance was established at a P-value of <0.05 using the MedCalc Statistical Software version 20.15 (MedCalc Software Ltd, Ostend, Belgium) for Windows.
Ethics
The Ethics Committee of the Victor Babes University of Medicine and Pharmacy in Timisoara gave its approval for the research to be conducted (Nr. 47/2017). In accordance with the Helsinki Declaration’s principles of informed consent, all patients provided written informed consent prior to participating in the study.
Results
A total of 232 patients were registered in the present analysis. Seven patients were eliminated because of unacceptable quality of conventional echocardiographic images and 25 because AF was documented during the out-of-hospital cardiologic examination. Furthermore 10 patients had inadequate image quality for 2D-STE.
At last, 190 TIA patients were enrolled in the study. They were divided into two groups, according to presence (group I) or absence (group II) of documented episodes of PAF. Demographics, clinical characteristics and echocardiographic data are shown in Tables 1 and 2.
 |
Table 1 Baseline Demographics of the Patients with Transient Ischemic Attacks |
 |
Table 2 Echocardiography |
PAF was detected in 33% of patients that had suffered a TIA. The patients with detected PAF were older (mean age 67.5 vs 60 years, P<0.0001), more frequent women (48% vs 32%, P=0.04) and had more often a history of TIA or stroke (65% vs 23%, P<0.0001). Significant differences were noted among the two groups regarding the LA functional parameters. The patients with documented episodes of PAF had larger LA indexed volume (P<0.001), poorer LA total emptying fraction (P<00001), and impaired LA deformation patterns (Table 2, Figure 2). In contrast, other echocardiographic measures of LV systolic and diastolic function, as well as LV GLS, were not notably different between the groups (Table 2).
The univariate analysis of the clinical and echocardiographic features presented in Tables 1 and 2 found that age, female gender, LAVI, LAEF and LA strains were significantly associated with the risk of PAF (Table 3). The CHA2D2S-VASc risk score, although significantly higher in group I (P<0.001), was not significantly associated with PAF in the TIA patients at logistic regression analysis.
 |
Table 3 Factors Associated with Paroxysmal Atrial Fibrillation in Patients with Transient Ischemic Attacks |
Multivariate logistic regression (forward method) included the variables associated with PAF identified by univariate analysis and found that three of them were independently associated with PAF in patients with TIA´s: age, LAEF and LARVS (Table 3).
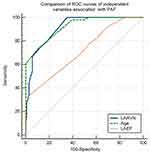
The ROC curves analyses of these independent variables associated with PAF indicated appropriate sensitivities and specificities: age (AUC=0.922, sensitivity: 72.92, specificity 90.1, P<0.0001), LARVS (AUC=0.915, sensitivity: 100.0, specificity 64.8, P<0.0001), and LAEF (AUC=0.717; sensitivity: 72.9; specificity 90.1; P<0.0001). The comparison of the ROC curves showed significant differences between the areas under the ROC curves (AUC) for age and LARVS versus LAEF (0.198, P<0.0001), as shown in Figure 3. The cut-off levels of the variables associated with PAF were as follows: LARVS < −17%, LAEF <51%, and age >55 years.
Discussion
The findings from our research prove the value of echocardiography for detection of atrial disorder in the background of TIA`s. We performed a combined evaluation of LA size and function by conventional and 2D-STE. Moreover, echocardiographic cut-off points were calculated for the variables independently associated with PAF in TIA patients.
LA enlargement implies adverse LA structural remodelling, which is associated with electrical instability and thrombus formation.25,26 But AF was also diagnosed in patients with normal LA size,17 and explained by LA functional remodelling. This is demonstrated by a decreased myocardial deformation and is more sensitive in detecting subclinical atrial dysfunction.27,28 Atrial remodelling implies morphologic changes in the atrial tissue with the development of interstitial fibrosis.29,30 The degree of atrial fibrosis can be rated using cardiac imaging methods,31 and is inversely correlated with the value of the strain calculated by echocardiography.32 In this respect, retrospective studies achieved in patients with cryptogenic strokes have shown that STE improved the detection accuracy of AF.33
These findings have been confirmed in the present study, that increased the challenge by enrolling TIA patients with and without documented episodes of PAF. Logistic multivariable regression analysis demonstrated that age, LARVS and LAEF were independently associated with PAF. We found the following cut-off values predictive for PAF in TIA´s patients: LARVS < −17%, LAEF <51%, and age >55 years. These values are confirmed by another study that analysed LA strain predictive power of AF in cryptogenic ischemic strokes.34
The strong association between reduced LA strains and AF has also been reported in other settings, such as predicting recurrence of AF after catheter ablation.35 Pagola reported the presence of silent AF in 86% of cryptogenic stroke patients with normal LA size and decreased LA strain.18 Bayes de Luna described a frequently under-recognized clinical condition characterized by advanced interatrial block, that is strongly associated with atrial fibrillation and that was identified as a risk factor for non-lacunar cardioembolic ischemic stroke and vascular dementia.36
Vera et al37 elaborated an interesting decryptoring score to predict AF in cryptogenic strokes that includes clinical, laboratory, and left atrial strain parameters (age > 75 years, hypertension, troponin T > 40 ng/L, NT pro-BNP > 200 pg/mL, LA reservoir strain < 25.3%, and LA conduct strain<10.4%). The rate of AF detection was 0% among patients with a score of 10 and 80% among patients with a score of >35. This score, however, requires more validation before being utilized in clinical practice.
Compared to other stroke studies,18,37,38 we selected younger patients, as the risk of non-valvular AF increases substantially in patients over 50 years. We found that TIA patients with detected PAF presented notable LA functional impairment, indicated by larger LAVI (P<0.001), poorer LAEF (P<0.0001), and reduced LA strains (P<0.001).
Our results support the importance of regular atrial function evaluation in patients with TIA`s of undetermined origin. Atrial reduced contractility determines an abnormal stasis of blood within the atrium, with thrombus formation and risk of cerebral embolization. The progression from paroxysmal to persistent atrial fibrillation supports the concept of atrial cardiomyopathy as a cause of stroke.39,40 Although many researches assess stroke risk in AF patients, TIA is rarely included. We believe that the inclusion of TIA is valuable, because of the common pathogenic mechanism, and the high risk of subsequent stroke.
An improvement of stroke risk assessment is demanded, and echocardiography provides additional high-risk features.
Our study has several limitations. It was done in a single neurology centre with a relatively small number of patients. It needs validation of the results by studies in a greater population. Because of the limited duration of PAF, and the lack of a long-term ECG monitoring for AF detection, many episodes might have been unrecognized. No investigations were done to assess an atherosclerotic origin of the TIA´s. Furthermore, longitudinal studies able to accurately predict the development of AF should provide additional information.
Conclusion
The current study shows that LA strain is an independently and strongly associated with PAF in TIA patients, implying a cardioembolic etiology that could be prevented by initiating anticoagulant medication. More information is expected from longitudinal studies that aim to predict the occurrence of AF.
Our findings have considerable clinical implications because, until now, LA 2D-STE was not included in the usual evaluation of TIA patients, but it may be the ideal next step in this regard.
Acknowledgments
We would like to offer our gratitude to everyone who assisted us in the preparation of this document. Grateful appreciation to those who participated in the peer review process and provided their valuable suggestions and opinions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hindricks G, Potpara T, Dagres G, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery. Eur Heart J. 2020;00:1–125.
2. Wańkowicz P, Nowacki P, Gołąb-Janowska M. Risk factors for ischemic stroke in patients with non-valvular atrial fibrillation and therapeutic international normalized ratio range. Arch Med Sci. 2019;15:1217–1222.
3. Wańkowicz P, Nowacki P, Gołąb-Janowska M. Atrial fibrillation risk factors in patients with ischemic stroke. Arch Med Sci. 2021;17(1):19–24.
4. Michelena HI, Powell BD, Brady PA, et al. Gender in atrial fibrillation: ten years later. Gend Med. 2010;7:175.
5. Arboix A, Jiménez C, Massons J, et al. Hematological disorders: a commonly unrecognized cause of acute stroke. Expert Rev Hematol. 2016;9(9):891–901.
6. Genovesi S, Rebora P, Occhino G, et al. Atrial fibrillation and clinical outcomes in a cohort of hospitalized patients with Sars-Cov-2 infection and chronic kidney disease. J Clin Med. 2021;10(18):4108–4122.
7. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477.
8. Saver JL. Cryptogenic stroke. N Engl J Med. 2016;374:2065–2074.
9. Jianu DC. Diagnosis of Symptomatic Intracranial Atherosclerotic Disease. Scholar´s Press; 2020. ISBN-13: 9783639762440.
10. Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486.
11. Liao J, Khalid Z, Scallan C, et al. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke J Cereb Circ. 2007;38:2935–2940.
12. Pepi M, Evangelista A, Nihoyannopoulos P, et al.; for the European Association of Echocardiography (EAE). Recommendation for echocardiography use in the diagnosis and management of cardiac sources of embolism. Eur J Echocardiogr. 2010;11:461–476.
13. Parvanescu T, Vitel A, Sporea I, et al. Significant association between left ventricular diastolic dysfunction, left atrial performance and liver stiffness in patients with metabolic syndrome and non-alcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2021;14:1535–1545.
14. Ogata T, Matsuo R, Kiyuna F, et al. Left atrial size and long-term risk of recurrent stroke after acute ischemic stroke in patients with nonvalvular atrial fibrillation. J Am Heart Assoc. 2017;6:e006402.
15. Skaarup KG, Christensen H, Høst N, et al. Diagnosing paroxysmal atrial fibrillation in patients with ischemic strokes and transient ischemic attacks using echocardiographic measurements of left atrium function. Am J Cardiol. 2016;17(1):91–99.
16. Fatema K, Barnes ME, Bailey KR, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10:282–286.
17. Ble M, Benito B, Cuadrado-Godia E, et al. Left atrium assessment by speckle tracking echocardiography in cryptogenic stroke: seeking silent atrial fibrillation. J Clin Med. 2021;10(16):3501–3511.
18. Pagola J, Juega J, Francisco-Pascual J, et al. Predicting atrial fibrillation with high risk of embolization with atrial strain and NT-proBNP. Transl Stroke Res. 2021;12:735–741.
19. Olsen FJ, Jørgensen PG, Møgelvang R, et al. Predicting paroxysmal atrial fibrillation in cerebrovascular ischemia using tissue Doppler imaging and speckle tracking echocardiography. J Stroke Cerebrovasc Dis. 2016;25:350–359.
20. Kamel H, Bartz TM, Longstreth WT, et al. Cardiac mechanics and incident ischemic stroke: the Cardiovascular Health Study. Sci Rep. 2021;11:17358.
21. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276.
22. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272.
23. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography. J Am Soc Echocardiogr. 2005;18:1440–1463.
24. Leung M, van Rosendael PJ, Abou R, et al. Left atrial function to identify patients with atrial fibrillation at high risk of stroke: new insights from a large registry. Eur Heart J. 2018;39:1416–1425.
25. Jordan K, Yaghi S, Poppas A, et al. Left atrial volume index is associated with cardioembolic stroke and atrial fibrillation detection after embolic stroke of undetermined source. Stroke. 2019;50:1997–2001.
26. Gupta DK, Shah AM, Giugliano RP, et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35:1457–1465.
27. Morris DA, Takeuchi M, Krisper M, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. 2014;16:364–372.
28. Kojima T, Kawasaki M, Tanaka R, et al. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging. 2011;13:227–234.
29. The Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554.
30. Thomas L, Abhayaratna W. Left atrial reverse remodeling. JACC Cardiovasc Imaging. 2017;10:65–77.
31. De Sensi F, Penela D, Soto-Iglesias D, et al. Imaging techniques for the study of fibrosis in atrial fibrillation ablation: from molecular mechanisms to therapeutical perspectives. J Clin Med. 2021;10:2277.
32. Cameli M, Lisi M, Righini FM, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601.
33. Rasmussen SMA, Olsen FJ, Jørgensen PG, et al. Utility of left atrial strain for predicting atrial fibrillation following ischemic stroke. Int J Cardiovasc Imaging. 2019;35:1605–1613.
34. Kusunose K, Takahashi H, Nishio S, et al. Predictive value of left atrial function for latent paroxysmal atrial fibrillation as the cause of embolic stroke of undetermined source. J Cardiol. 2021;5:355–361.
35. Wen S, Indrabhinduwat M, Brady PA, et al. Post procedural peak left atrial contraction strain predicts recurrence of arrhythmia after catheter ablation of atrial fibrillation. Cardiovasc Ultrasound. 2021;19:22–34.
36. Arboix A, Martí L, Dorison S, Sánchez MJ. Bayés syndrome and acute cardioembolic ischemic stroke. World J Clin Cases. 2017;5(3):93–101.
37. Vera A, Cecconi A, Ximénez-Carrillo Á, et al.; Sudy Investigators. A comprehensive model to predict atrial fibrillation in cryptogenic stroke: the decryptoring score. J Stroke Cerebrovasc Dis. 2021;31(1):106–161.
38. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283–292.
39. Padfield GJ, Steinberg C, Swampillai J, et al. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm. 2017;14:801–807.
40. Guichard J-B, Nattel S. Atrial cardiomyopathy. J Am Coll Cardiol. 2017;70:756–765.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.