Back to Journals » Vascular Health and Risk Management » Volume 16
Laser Doppler Flowmetry and Visible Light Spectroscopy of the Gastric Tube During Minimally Invasive Esophagectomy
Authors Safi N , Johannessen HO, Medhus AW , Mala T, Kazmi SSH
Received 1 July 2020
Accepted for publication 30 September 2020
Published 27 November 2020 Volume 2020:16 Pages 497—505
DOI https://doi.org/10.2147/VHRM.S269138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Nathkai Safi,1,2 Hans-Olaf Johannessen,3 Asle Wilhelm Medhus,4 Tom Mala,2,3 Syed SH Kazmi1,2
1Department of Vascular Surgery, Heart, Lung and Vascular Clinic, Oslo University Hospital, Oslo, Norway; 2Faculty of Medicine, Oslo University, Oslo, Norway; 3Department of Gastrointestinal Surgery, Oslo University Hospital, Oslo, Norway; 4Department of Gastroenterology, Oslo University Hospital, Oslo, Norway
Correspondence: Nathkai Safi
Department of Vascular Surgery, Heart, Lung and Vascular Clinic, Oslo University Hospital, Oslo University, Oslo 0586, Norway
Tel +47 97407460
Email [email protected]
Introduction: Ischemia is considered as the main reason for thoracic gastroesophageal anastomotic leaks after esophagectomy. Microcirculatory monitoring with laser Doppler flowmetry and visible light spectroscopy may provide valuable intraoperative real-time information about the gastric tube’s tissue perfusion and circulation.
Patients and Methods: Ten patients with esophageal cancer operated with minimally invasive esophagectomy participated in this single-center, prospective, observational pilot study. A single probe with laser Doppler flowmetry and visible light spectroscopy was used to perform transserosal microcirculation assessment of the gastric tube at predefined anatomical sites during different operation phases. Group comparison and changes were evaluated using the paired sample t-test.
Results: A reduction in StO2 was found at all measuring sites after the gastric tube formation compared with the baseline measurements. The mean StO2 reduction from baseline to gastric tube formation and after anastomosis was 16% (range 4%– 28%) and 42% (range, 35%– 52%), respectively. A statistically significant increase in the rHb concentration, representing venous congestion, was detected at the most cranial part of the gastric tube (P = 0.04). Three patients developed anastomotic leaks.
Conclusion: Intraoperative real-time laser Doppler flowmetry and visible light spectroscopy are feasible and may provide insight to microcirculatory changes in the gastric tube and at the anastomotic site. Patients with anastomotic leaks seem to have critical local tissue StO2 reduction and venous congestion that should be further evaluated in studies with larger sample sizes.
Keywords: esophagectomy, gastric tube circulation, gastroesophageal anastomosis complications
Introduction
Anastomotic leak is a severe complication after thoracic gastroesophageal anastomosis (TGEA) and is typically reported at incidences of 10–20%.1–3 Although multifactorial, ischemia at the anastomotic site plays a central role in TGEA leaks.4 The cranial 20% of the gastric tube is vascularized by an intramural vascular network and may be prone to the development of ischemia by manipulation, tension, and strangulation during gastric pull-up and anastomosis construction.3,5 The ischemic changes may not be visually apparent during the surgical procedure, and constructing the anastomosis at a hypoperfused site may result in an anastomotic leak. Intraoperative information about the gastric tube’s microcirculatory status may guide the surgeon to define the anastomotic site with the best possible microcirculation. Despite the introduction of numerous intraoperative bowel viability assessment techniques, only a few techniques are feasible for intraoperative assessment of the gastric tube during esophagectomy.6,7 In addition to laser Doppler flowmetry (LDF), which is one of the methods most extensively investigated, visible light spectroscopy (VLS) has emerged as a promising method for the detection of the microcirculatory changes in the gastrointestinal tract.4,8–10
The main aim of our pilot study was to evaluate the feasibility of combined use of LDF and VLS for transserosal microcirculation assessment of the stomach and the gastric tube during different phases of minimally invasive esophagectomy.
Patients and Methods
During seven months from July 2018 to January 2019, patients with esophageal cancer, scheduled for minimally invasive esophagectomy at Oslo University Hospital, Ullevål, were considered for this pilot study enrollment, at the discretion of the attending surgeon and from the availability of the principal investigator at the time of surgery. The institution is a regional center for esophageal cancer’s surgical treatment, with an annual volume of about 50 patients operated. Inclusion criteria for study enrollment included patients with the capacity to give informed consent and a potentially curable distal esophageal cancer. All patients received neoadjuvant radio-chemotherapy with 41.1 Gy and Carboplatin and paclitaxel according to the CROSS radio-chemotherapy regime.11
During surgery, a 2.6 mm microprobe with combined LDF and VLS modalities (O2C; LEA Medizintechnik, Germany), was channeled through a laparoscopic trocar to quantify transserosal, blood flow, velocity, mixed arterial and venous saturation of hemoglobin (StO2), and the amount of hemoglobin per tissue volume (rHb).
The O2C transmits continuous wave laser light (500–630 nm) and white light (830 nm) through an optical fiber to the tissue, where it is scattered and collected with fibers of the probe placed on the tissue surface.12 The white light tends to penetrate deeper into the tissue than the laser light due to its shorter wavelength.13 In VLS, the principle of absorbance and scattering of white light in biological tissues, gives a marked difference in absorption spectra of oxygenated and deoxygenated hemoglobin thereby, directly measures hemoglobin saturation and concentration.8,13,14
For LDF measurements, reflected laser light from the moving red blood cells in the tissue generates a Doppler shift. The frequency of this reflected light is dependent upon the velocity of the cells (erythrocytes) and is detected by a photodetector within the instrument and transformed into an electrical signal. The LDF produces a value referred to as a flow (red blood cell flux) expressed as mL/min/100-gram tissue.
Measurements were repeated in case of unstable or fluctuating recordings. The graphical picture provided by the LCD monitor of the O2C unit, help to keep the absorption spectra of oxyhemoglobin well above 50% of the arbitrary unit (AU) scale during examinations (Figure 1A and B).15 This step allowed us to perform the repeated transserosal measurements in all subjects without applying unnecessary pressure on the serosa. Before each recording, an ambient light correction was performed automatically, which allowed keeping the examined area illuminated and maintaining the O2C microprobe’s visual control throughout the examination. A standard measurement protocol of 5 seconds of continuous measurements at each anatomical position resulted in approximately 200 measurements at each anatomical site. The system provided a real-time quantitative measurement and stored the raw data for later analysis.
After establishing pneumoperitoneum with CO2, and before any intraperitoneal dissection, baseline measurements were recorded from predefined anatomical sites on the greater curvature’s anterior surface. Measurements were repeated at the same anatomical sites after gastric tube formation and subsequently, after the construction of the TGEA (Figure 2A–C). The distance between each measuring site was approximately 3–4 cm apart. In all patients, the gastroesophageal anastomosis was constructed at the site M7. A marking suture was placed at the gastric incisura towards the level of site M3, to identify the site after the gastric pull-up and anastomosis. All measurements were performed under stable hemodynamic conditions. No vasopressor medications were administered during the measurements, and the systemic oxygen saturation was kept > 97%.
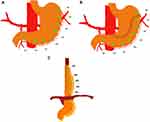 |
Figure 2 (A–C). Measuring points M1 to M8 (green dots) at baseline, after gastric tube construction, and gastroesophageal anastomosis. |
Operative Technique
A standard thoracolaparoscopic, “Ivor-Lewis” type minimal invasive esophagectomy was performed in all patients.16 This approach included complete mobilization of the stomach, dissection of the short gastric arteries and the left gastric artery, and gastric tube preparation after regional lymphadenectomy. A multi-step thoracolaparoscopic subtotal resection of the esophagus with two-field lymphadenectomy was performed. The gastric conduit was anastomosed to the proximal residual esophagus at the carina level by a circular staple device. The introduction site for the circular stapler was closed using a linear stapler and oversewn.
All surgical procedures were performed under general anesthesia with the same team of surgeons who were blinded to the results of the perioperative microcirculation measurements. Thoracic drains were placed close to the anastomosis and the diaphragmatic hiatus. A decompressing nasogastric tube was positioned, and the patients were extubated in the operating theatre. Postoperatively, a mean systemic arterial pressure of 65 mmHg or higher was targeted, and vasopressors were administered if needed. All patients stayed at the postoperative surveillance department for three days and were routinely examined on the third postoperative day with upper endoscopy and computed tomography (CT) with oral contrast of the esophagus. Complications are reported on and graded according to Clavien-Dindo classification of surgical complications as recommended by the Esophagectomy Complications Consensus Group.17
The study protocol was approved by the Regional Committees for Medical and Health Research Ethics in the South-Eastern region of Norway (approval number 2018/500/REK sør-øst A) and registered in Clinicaltrials.gov (ClinicalTrials.gov ID NCT03724162). The study conforms to the provisions of the Declaration of Helsinki. Informed written consent was obtained from all patients included in the study.
Statistical Analysis
A descriptive data analysis was performed. Data are presented as median (range) or mean (standard deviation) dependent on data distribution. Group comparison and mean changes were evaluated using the paired sample t-test. A P -value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25 (IBM SPSS Statistics).
Results
During a period of seven months, ten patients with esophageal cancer were included. Patient characteristics are given in Table 1. There was no 30-day and 90-day mortality. During a median follow-up period of 16 months (range, 12–18 months), one patient died. Three patients (30%) had an anastomotic leak type I and Grade II surgical complication (Table 1). These three leakages were identified by standard upper endoscopy on the third postoperative day and confirmed with a CT with oral contrast on postoperative day six. In one patient, the leakage was treated solely with antibiotics. In the other two patients, antibiotics were administered, and the Jackson-Prat drain kept for an extended period of 5–7 postoperative days.
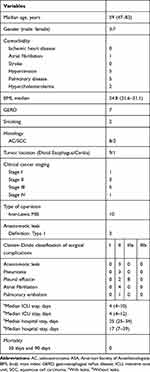 |
Table 1 Baseline Characteristics of Patients (n=10) |
Intraoperative transserosal microcirculation assessment was successfully performed in all patients. The median recording time required for the measurements was 6 minutes (range, 3–8 minutes).
When analyzing the whole study group, a reduction in StO2 was found at all measuring sites in the gastric tube as compared with the baseline measurements. The reduction was statistically significant at the sites M4 (P = 0.04), M5, and M7 (P = 0.03). The reduction in StO2 became amplified (P < 0.001) after the construction of TGEA at all measuring sites as compared with the baseline measurements (Figure 3A). The mean StO2 reduction from baseline to gastric tube formation and after anastomosis was 16% (range 4% - 28%) and 42% (range, 35% - 52%), respectively. A statistically significant increase in the rHb concentration was detected at the most cranial part of the gastric tube, site M7 (P = 0.04) (Figure 3B).
The mean LDF measurements of the whole study group showed a statistically significant increase in the local blood flow at the site M4 (P = 0.009) after TGEA (Figure 3C). A similar change in velocity measurements was observed in the gastric tube after TGEA (P = 0.004) (Figure 3D).
In the three patients with leaks, the mean StO2 reduction after anastomosis, as compared with the baseline StO2, was 49% (range, 25% - 69%), while in patients without leaks, it was 39% (range, 32% - 46%). After anastomosis, rHb increased from baseline to 61% (range, 33% - 147%), and 17% (range, 0–38%), respectively in the patients with and without leaks. Although, the mean change in velocity was similar in patients with or without leaks, respectively 12% (range, −13% - 41%) and 11% (range, −14% - 44%), the mean tissue blood flow after anastomosis was increased by 36% (range, −5% - 57%) and 26% (range, −4% - 51%) respectively. This increase was statistically significant only at M4 in patients without leaks (P = 0.02). Table 2 gives a detailed account of microcirculation assessment results in patients with and without anastomotic leaks.
Discussion
This is the first study to present data on the simultaneous use of LDF and VLS for assessment of the gastric tube microcirculation in patients with esophageal cancer undergoing minimally invasive surgery. The O2C technology utilized, provides a real-time information to the surgeons during surgery. We found a significant reduction in the tissue StO2 in the gastric tube. The tissue StO2 deteriorated further, and a statistically significant reduction in the transversal StO2 after anastomosis was observed in all patients, compared to baseline values. In this pilot study, the patients with leaks had a lower mean StO2 at baseline compared with the patients without leaks, and they also had a more reduction in the mean StO2 after anastomosis.
The mean proportional increase in rHb in the patients with leaks was higher (61%) than those without leaks (17%). This increase in rHb was most evident in the most cranial part of the patients with leaks. This is an important observation as the increase in rHb represents venous congestion that may impact tissue StO2. Gerau et al also found that in addition to significantly reduced StO2, there was an increase in the rHb in the gastric tube of patients with leaks.18 Furthermore, Buise et al found that the patients developed venous congestion after esophagectomy.19 Murakami et al performed microvascular anastomosis on the neck and showed that both the arterial circulation and venous congestion were relieved after such vascular anastomosis.20,21 However, in contradiction to Buise et al, they did not find reduced StO2. In the present study, we also found an increase in the mean tissue blood flow from baseline to the anastomosis in patients with leaks (39%). This increase in tissue blood flow is of smaller magnitude in the patients without leaks (26%). Thus, the combined use of LDF and VLS enables substantiated information regarding blood circulation at the anastomotic site for the surgeon during surgery and may also further enlighten pathophysiological changes induced in the gastric tube.
Based on the present findings, patients with leaks may have more ischemic changes, aggravated by the venous congestion as represented by rHb increase. The increase in local blood flow is probably a compensatory physiological response to ischemia that may contribute to venous congestion due to reduced venous drainage caused by surgical trauma to the veins during esophagectomy and gastric tube formation. Extrinsic compression at the esophageal hiatus may also obstruct venous drainage and could have caused venous congestion in the patients with leaks.21 Manipulation and axial tension in the tube may also play a part in venous drainage.4 The physiological counter-current mechanism of shunting blood flow from the mucosa to the serosal layer during an ischemic insult may also have contributed in the increase in local blood flow measured with transserosal LDF in our study cohort.22 To avoid vascular injury, a fair distance from the left gastroepiploic artery was maintained during free dissection of the greater omentum. Although the diaphragmatic hiatus’s opening was wide, this could still have been a potential source of external compression on the venous drainage, secondary to expected postoperative edema.
The advantages of LDF and VLS are the quickness of measurements, low invasiveness, and high reproducibility.6 VLS is validated for the investigation of chronic mesenteric ischemia.14 Many studies have utilized LDF and VLS to assess the microcirculation in gastric tube. However, most of these studies of the microcirculatory assessment of gastric tube have been conducted on with the patients with anastomosis constructed on the neck. Most of the studies of the microcirculatory assessment of the gastric tubes are initial experiences and feasibility studies. Furthermore, either LDF or light spectrophotometry has been utilized. Only two studies incorporated both LDF and VLS.15,23 The former was the investigation of transmucosal microcirculation in patients with non-specific abdominal pain, and the latter included patients with chronic mesenteric ischemia and a control group with non-specific abdominal pain. Other studies such as Pham et al had no real-time measurements, while Wang et al excluded patients with anastomotic leaks.24,25
Although the assessment of ischemia can be identified as a common aim in these studies, the studies differ in patient demographics and the different measurement units. It is, therefore, hard to draw conclusions and to standardize these methods for routine clinical use. Interestingly, many of the available studies could not provide intraoperative real-time information about microcirculation.26,27 In contrast, the present study confirms the feasibility of the combined use of LDF and VLS intraoperatively, as made possible by the O2C technology.
Although studies have shown adverse effects of both radio- and chemotherapy on the tissue, the neoadjuvant radio-chemotherapy is the current standard of treatment of esophageal cancer before radical surgical resection for most patients.11 Furthermore, the clinical results of major studies have so far not demonstrated more anastomotic leakages.28 A major limitation of our pilot study is the small sample size. The study was not designed and powered to investigate anastomosis leaks in general. Therefore, statistical evaluations should be interpreted cautiously. Anastomotic leaks were identified early due to an aggressive diagnostic approach, including routine gastroscopy at day three and liberal use of early CT with oral contrast. The standardized surgical technique applied represents a strength of the study. The study results hold promise for future appropriately powered studies to provide intraoperative useful cut-off values for StO2, rHb, local tissue blood flow, and velocity. The results of more extensive studies may be used to standardize microcirculation assessment modalities for regular clinical use.
Conclusion
Concomitant intraoperative transserosal LDF and VLS may help identify local ischemia in the gastric tube during esophagectomy. Patients with anastomotic leaks seems to have a profound local tissue StO2 reduction, which is further aggravated by the development of venous congestion.
Data Sharing Statement
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures), will be made available and shared with investigators whose proposed use of the data has been approved, but an independent review committee identified for this purpose. Proposals should be directed to associate professor Syed Sajid Hussain Kazmi MD Ph.D. [email protected], project leader. To gain access, data requesters will need to sign a data access agreement.
Acknowledgment
We are thankful for the kind assistance of Mrs. Hilde Iren Flaatten, University medical library, Oslo University Hospital and Mrs. Manuela Zucknick, Associate professor, Department of Biostatistics, Institute of Basic Medical Sciences, University of Oslo.
Disclosure
The authors have nothing to disclose.
References
1. Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256(1):95–103. doi:10.1097/SLA.0b013e3182590603
2. Lagarde SM, Vrouenraets BC, Stassen LP, van Lanschot JJ. Evidence-based surgical treatment of esophageal cancer: overview of high-quality studies. Ann Thorac Surg. 2010;89(4):1319–1326. doi:10.1016/j.athoracsur.2009.09.062
3. Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: A review. Am J Surg. 1995;169(6):634–640.
4. Ikeda Y, Niimi M, Kan S, Shatari T, Takami H, Kodaira S. Clinical significance of tissue blood flow during esophagectomy by laser doppler flowmetry. J Thorac Cardiovasc Surg. 2001;122(6):1101–1106. doi:10.1067/mtc.2001.117835
5. Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54(6):1110–1115. doi:10.1016/0003-4975(92)90077-H
6. Urbanavicius L, Pattyn P, de Putte DV, Venskutonis D. How to assess intestinal viability during surgery: A review of techniques. World J Gastrointest Surg. 2011;3(5):59–69. doi:10.4240/wjgs.v3.i5.59
7. Linder G, Hedberg J, Bjorck M, Sundbom M. Perfusion of the gastric conduit during esophagectomy. Dis Esophagus. 2017;30(1):143–149.
8. Jansen SM, de Bruin DM, van Berge Henegouwen MI, et al. Optical techniques for perfusion monitoring of the gastric tube after esophagectomy: A review of technologies and thresholds. Dis Esophagus. 2018;31(6). doi:10.1093/dote/dox161
9. Schilling MK, Redaelli C, Maurer C, Friess H, Buchler MW. Gastric microcirculatory changes during gastric tube formation: assessment with laser doppler flowmetry. J Surg Res. 1996;62(1):125–129. doi:10.1006/jsre.1996.0184
10. Benaron D, Parachikov I, Cheong W-F, et al. Design of a visible-light spectroscopy clinical tissue oximeter. J Biomed Opt. 2005;10(4):044005. doi:10.1117/1.1979504
11. Wong I, Law S. The cross road in neoadjuvant therapy for esophageal cancer: long-term results of cross trial. Transl Cancer Res. 2016;5(S3):S415–S419. doi:10.21037/tcr.2016.08.32
12. Vongsavan N, Matthews B. Some aspects of the use of laser doppler flow meters for recording tissue blood flow. Exp Physiol. 1993;78(1):1–14. doi:10.1113/expphysiol.1993.sp003664
13. Frank K, Kessler M, Appelbaum K, Dümmler W. The Erlangen micro-lightguide spectrophotometer empho i. Phys Med Biol. 1990;34:1883–1900. doi:10.1088/0031-9155/34/12/011
14. Van Noord D, Sana A, Benaron DA, et al. Endoscopic visible light spectroscopy: A new, minimally invasive technique to diagnose chronic gi ischemia. Gastrointest Endosc. 2011;73(2):291–298. doi:10.1016/j.gie.2010.10.025
15. Berge ST, Safi N, Medhus AW, et al. Gastroscopy assisted laser doppler flowmetry and visible light spectroscopy in patients with chronic mesenteric ischemia. Scand J Clin Lab Invest. 2019;79(7):541–549. doi:10.1080/00365513.2019.1672084
16. Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H. Comparing perioperative mortality and morbidity of minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: A nationwide retrospective analysis. Ann Surg. 2019. doi:10.1097/SLA.0000000000003500
17. Low DE, Alderson D, Cecconello I, et al. International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg. 2015;262(2):286–294. doi:10.1097/SLA.0000000000001098
18. Gareau DS, Truffer F, Perry KA, et al. Optical fiber probe spectroscopy for laparoscopic monitoring of tissue oxygenation during esophagectomies. J Biomed Opt. 2010;15(6):061712. doi:10.1117/1.3512149
19. Buise MP, Ince C, Tilanus HW, Klein J, Gommers D, van Bommel J. The effect of nitroglycerin on microvascular perfusion and oxygenation during gastric tube reconstruction. Anesth Analg. 2005;100(4):1107–1111. doi:10.1213/01.ANE.0000147665.60613.CA
20. Murakami M, Sugiyama A, Ikegami T, et al. Additional microvascular anastomosis in reconstruction after total esophagectomy for cervical esophageal carcinoma. Am J Surg. 1999;178(3):263–266.
21. Murakami M, Sugiyama A, Ikegami T, et al. Revascularization using the short gastric vessels of the gastric tube after subtotal esophagectomy for intrathoracic esophageal carcinoma. J Am Coll Surg. 2000;190(1):71–77. doi:10.1016/S1072-7515(99)00234-3
22. Lundgren O. The circulation of the small bowel mucosa. Gut. 1974;15(12):1005–1013. doi:10.1136/gut.15.12.1005
23. Bludau M, Vallbohmer D, Gutschow C, Holscher AH, Schroder W. Quantitative measurement of gastric mucosal microcirculation using a combined laser doppler flowmeter and spectrophotometer. Dis Esophagus. 2008;21(7):668–672. doi:10.1111/j.1442-2050.2008.00856.x
24. Pham TH, Perry KA, Enestvedt CK, et al. Decreased conduit perfusion measured by spectroscopy is associated with anastomotic complications. 2011;380–385.
25. Wang X, Pei X, Li X, et al. Predictive value of anastomotic blood supply for anastomotic stricture after esophagectomy in esophageal cancer. Dig Dis Sci. 2019;64(11):3307–3313. doi:10.1007/s10620-018-5451-3
26. Miyazaki T, Kuwano H, Kato H, Yoshikawa M, Ojima H, Tsukada K. Predictive value of blood flow in the gastric tube in anastomotic insufficiency after thoracic esophagectomy. World J Surg. 2002;26(11):1319–1323. doi:10.1007/s00268-002-6366-9
27. Korenaga D, Toh Y, Maekawa S, Ikeda T, Sugimachi K. Intra-operative measurement of the tissue blood flow for evaluating blood supply to the gastric tube for esophageal reconstruction. Hepatogastroenterology. 1998;45(24):2179–2180.
28. Shridhar R, Takahashi C, Huston J, Doepker MP, Meredith KL. Anastomotic leak and neoadjuvant chemoradiotherapy in esophageal cancer. J Gastrointest Oncol. 2018;9(5):894–902. doi:10.21037/jgo.2018.04.09
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



