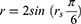Back to Journals » Nature and Science of Sleep » Volume 14
Laryngopharyngeal Reflux in Obstructive Sleep Apnea-Hypopnea Syndrome: An Updated Meta-Analysis
Received 28 September 2022
Accepted for publication 4 December 2022
Published 15 December 2022 Volume 2022:14 Pages 2189—2201
DOI https://doi.org/10.2147/NSS.S390272
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Jie He,1,2,* Chunmao Wang,1,2,* Wancheng Li1,2
1Clinical Medical College of Chengdu Medical College, Chengdu, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Chengdu Medical College, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wancheng Li, The First Affiliated Hospital of Chengdu Medical College, BaoGuang Street 278, Chengdu, People’s Republic of China, Tel +86-028-83016637, Fax +86-27-83016637, Email [email protected]
Abstract: Laryngopharyngeal reflux (LPR) is a common disorder in patients with obstructive sleep apnea-hypopnea syndrome (OSAHS). This meta-analysis was carried out to evaluate the LPR prevalence in individuals with OSAHS and to analyze the correlation of LPR positivity with the clinical features of patients with OSAHS. A detailed review of the English and Chinese literature on the occurrence of LPR in patients with OSAHS was performed by employing online search tools such as PubMed, EMBASE, Web of Science, VIP, CNKI, WanFang, etc. Two researchers analyzed the studies for quality according to the STROBE standard checklist. The acquired data were analyzed using Stata 11.0 and R 3.6.1 software. The effect size was estimated and calculated using weighted mean difference (WMD) and correlation coefficients. Moreover, a combined analysis was performed by employing either a random- or fixed-effects model. Ultimately, 27 studies met our inclusion criteria. Our study revealed that the LPR prevalence in OSAHS patients was 49%. We carried out subgroup analyses as per OSAHS severity, ethnicity, and body mass index (BMI). The results suggested that the probability of LPR in European and American patients with OSAHS was higher, and the prevalence of LPR was higher in obese individuals and patients with severe OSAHS. Moreover, apnea-hypopnea index (AHI) and BMI were higher in LPR-positive OSAHS patients than in LPR-negative OSAHS patients, but no significant variation in age was observed in the two groups. Moreover, the reflux symptom index (RSI) scores and the reflux finding score (RFS) exhibited a positive correlation with AHI. The current literature shows a higher incidence of LPR in individuals with OSAHS (49%). The severity of AHI in individuals with OSAHS is associated with the presence of LPR. Patients with OSAHS accompanied by LPR showed higher BMI and AHI as compared to those patients with LPR-negative OSAHS.
Keywords: LPR, obstructive sleep apnea-hypopnea syndrome, meta-analysis, correlation, reflux symptom index score, reflux finding score
Introduction
Laryngopharyngeal reflux (LPR) is a highly prevalent clinical condition that affects nearly 10% of the US population, showing an upward trend in published papers in recent years.1 LPR is a disease of the pharynx characterized by morning hoarseness, nocturnal cough, sore throat, excessive mucus in the pharynx, and pharyngeal foreign body sensation. It is recognized as a clinical disorder that differs from the classic gastroesophageal reflux disease (GERD).2 Furthermore, LPR occurs mainly in the upper respiratory tract, mainly in the hypopharynx and larynx region, whereas GERD is due to gastric and duodenal reflux into the esophagus, resulting in acid reflux and heartburn.3,4
OSAHS is reported to be a commonly occurring sleep-breathing disorder, with about 3–7% of general adults suffering from it.5 The pathogenesis of this disease is unclear, and clinical manifestations are mostly snoring during sleep, disrupted sleep architecture, increased nocturia, daytime sleepiness, memory loss, and neuropsychiatric symptoms.6 Its pathophysiology is characterized by the collapse of the upper airway structures resulting in partial or complete obstruction of airflow and decreased oxygen saturation during sleep.7 Several studies on LPR in patients with OSAHS have been published in the last few years. Many authors consider LPR to be common comorbidity in patients with OSAHS, and it may be closely linked with the severity of the disease. Payne et al8 evaluated the inflammatory manifestations in the pharynx of patients with OSAHS by fiberoptic laryngoscopy and the reflux finding scale (RFS) and found that 90% of OSAHS patients had features consistent with LPR, and a positive association was observed between apnea-hypopnea index (AHI) and RFS scores in OSAHS patients. During the pathophysiological process, OSAHS and LPR influence and promote each other, forming a vicious circle. However, common factors contributing to both diseases may involve other risk factors, including elevated body mass index (BMI), alcohol consumption, and smoking.
The relationship between LPR and OSAHS remains controversial as of this date. Iannella et al9 had reported in a study that there was no association between the severity of OSAHS and the degree of salivary pepsin reflux. However, Gouveia et al10 revealed a significant correlation between RSI scores and AHI (r=0.37, P=0.0078) and also concluded LPR symptoms may be related to the presence and severity of OSAHS. Furthermore, Magliulo et al11 conducted a meta-analysis in 2018 to evaluate the LPR prevalence in patients suffering from OSAHS and the variations in clinical characteristics between individuals that are LPR positive and negative.
However, the incidence of LPR may vary between different OSAHS subgroups, while the latest research work has reported the link between LPR and OSAHS. The conclusions reached by Magliulo et al11 remain to be further validated by observational studies. Therefore, it is essential to perform a meta-analysis by utilizing all existing studies to quantitatively assess the relationship between the occurrence of LPR and OSAHS. In this study, the pathogenesis of LPR in OSAHS patients and the correlation between the two diseases have been analyzed and discussed. Meanwhile, an updated meta-analysis has been conducted to evaluate the prevalence of LPR in patients suffering from OSAHS and associate their characteristics.
Methods and Materials
Method Used for Literature Search
Our meta-analysis was registered at the prospective register of systematic reviews (PROSPERO, the website is https://www.crd.yorl.ac.uk/PROSPERO/, and the ID is CRD4202235462). A computer search of PubMed, EMBASE, Web of Science, VIP, CNKI, and WanFang was performed to comprehensively collect literature on the onset of LPR in patients with OSAHS, and the search time frame was set between the database running time and August 1, 2022. The keywords and subject terms used included “laryngopharyngeal reflux” or “LPR” and “Obstructive Sleep Apnea-Hypopnea Syndrome” or “Obstructive Sleep Apnea” or “Obstructive Sleep Apnea Syndrome” or “OSA” or “OSAHS” or “OSAS.”
Inclusion and Exclusion Criteria
The criteria for eligibility are stated below:
- Case-control, cohort, Randomized controlled trial, or cross-sectional study.
- Diagnostic criteria for LPR: RSI questionnaire score ≥ 13 and/or RFS score ≥ 7 or diagnosed by 24-hour oropharyngeal Dx-pH probe system.12,13 The Ryan score is generated automatically by the system, which is a composite score, and the calculations of which are based entirely on the given pH thresholds of upright and supine positions. Scores more than 9.41 in the upright position and/or 6.80 in the supine position were considered indicative of LPR.14
- Subjects fulfilled the diagnostic criteria for OSAHS as per polysomnography (PSG) (adults: AHI ≥ 5/h; children: AHI ≥ 1/h). OSAHS severity was described by means of traditional definitions (AHI < 5, normal; AHI 5–14, mild OSAHS; AHI 15–29, moderate OSAHS; and AHI ≥ 30, severe OSAHS).15
The criteria of exclusion are stated below:
- Editorials, reviews, letters, other sorts of literature reviews, or case reports.
- Unable to extract enough information from the original article or contact the corresponding author for more information.
- Studies that were not conducted in humans.
- Studies included patients with OSAHS with a history of chronic airway disease, cerebrovascular disease, chronic cardiac failure, endocrine disease, and malignancies.
- LPR has not been categorized as a different disorder from gastroesophageal reflux. Publications on gastroesophageal reflux disease (GERD) in individuals with OSAHS were not considered for this study.
- Research reporting controls with AHI ≥ 5 events/h in adults and AHI ≥ 1 events/h in children.
- Overlapping studies and data from the research conducted by the same authors.
Literature Screening
Based on the above literature screening criteria, two researchers independently searched relevant articles from the above-mentioned databases and read their titles and abstracts, screened potentially eligible articles and obtained their full text, then read the full text in detail and evaluated again, and if the two researchers disagreed on the inclusion or exclusion of articles, a third researcher would analyze the disagreement and consult the data to determine whether to include or exclude. The PRISMA flow diagram below (Figure 1) summarizes the results of the article selection processes.
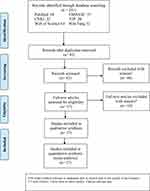 |
Figure 1 Flow diagram indicating the literature selection process and results based on the preferred reporting items for the meta-analysis. |
Data Extraction and Management
The quality of the selected research papers was assessed independently by two researchers utilizing the STROBE standard checklist.16 The minimum and maximum scores on this list were between 0 and 44, respectively, and the studies that achieved no less than 16 scores were selected to proceed with meta-analysis.17 The data extraction and evaluation from the literature were done independently by two other researchers, and the following information was achieved: 1. Basic information about the article: country, publication date, and first author; 2. Prevalence and incidence of LPR-positive patients with OSAHS; 3. Baseline characteristics of the study population: age, BMI, AHI, and lowest oxygen saturation comparison data; 4. OSAHS measurements and their types. 5. Study quality. If the included literature lacked the necessary content, the researchers contacted the article author no less than twice by phone or email, and they were requested to provide the missing data.
Statistical Analysis
R (version 3.6.1) and Stata software (version 11.0) were used to summarize and examine the extracted data. We normalized and expressed the continuous variables as the WMD with a 95% confidence interval (95% CI). The current meta-analysis used Spearman’s CORs to examine the associations between RSI/ reflux finding score (RFI) score and AHI score in patients with OSAHS. According to the standard error, which is generally dependent on the importance of the rank COR, the dependence of Spearman’s product-moment COR on the sampling distribution is not indicated. Therefore, the Fisher transformation was used to compare each COR, and an investigation was subsequently conducted with the transformed values as the input before converting them back to CORs. Cohen’s criteria were used to examine the measured effect size (small, ≤ 0.3; moderate, 0.3–0.5; and large, > 0.5).18 Moreover, Pearson’s COR was employed to study the correlation between the AHI score and RSI/RFI score. In accordance with the above description, several studies have reported a method for converting Pearson’s to Spearman’s COR using the following formula:
Where r and rs represent Pearson’s and Spearman’s CORs, respectively,19 Cochran’s Q and chi-square tests were used to examine data heterogeneity. The I2 statistic was used to detect heterogeneity (25%, 50%, and 75% represented low, moderate, and high heterogeneity, respectively; I2 < 50% and I2 ≥ 50% indicated low and high heterogeneity among studies, respectively). In the case of zero heterogeneity among studies, we employed fixed- and random-effects models.
Descriptive analysis, subgroup analysis, and meta-regression were employed to investigate the source of heterogeneity. The overall population was categorized according to disease severity, ethnicity, and BMI in order to conduct subgroup analyses. One study at a time was removed for the sensitivity analysis, which was conducted to explore how each study impacted the combined effect size. Begg’s, Egger’s tests, and linear regression were carried out to evaluate publication bias.
Results
Publications Retrieved and Included in the Study
Overall, 241 related research articles were collected from the databases. Out of which 228 duplicated studies were excluded by filtering the abstracts and titles, leaving 48 articles. We downloaded these 48 articles and thoroughly reviewed the complete text; 14 papers were discarded following a review of the inclusion and exclusion criteria. These papers were excluded for the following reasons: four publications were review articles, two were letters to the editor, four did not include a control group of healthy people, two lacked relevant data, and two were animal experiments. Finally, 27 articles were considered for the meta-analysis, as shown in Figure 1. We identified 26 studies8,9,20–43 involving LPR incidence in OSAHS patients, as shown in Table 1. Ten articles9,22,24,26,29,33,36,39,42,43 compared the clinical characteristics of patients with LPR-positive OSAHS and LPR-negative OSAHS (Table 2). Six studies10,25,27–29,36 provided Spearman’s or Pearson’s CORs between RSI score and AHI score. Four studies8,25,27,28 provided Spearman’s or Pearson’s CORs between RSI and AHI scores. Figure 1 represents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for selecting and screening articles from the literature. Tables 1–3 present the fundamental data of the included studies.
 |
Table 1 Characteristics of Included Studies |
 |
Table 2 Participants’ Characteristics of Included Studies |
 |
Table 3 Correlation Coefficients (Cor‐values) of Included Studies |
Prevalence of LPR Among Patients with OSAHS
The meta-analysis on the prevalence of LPR in individuals with obstructive sleep apnea included 26 studies (Table 1). A total of 2419 patients in total were involved, out of which 973 were LPR positive and 1446 were LPR negative. In these meta-analyses, the overall incidence of LPR-positive among OSAHS patients was calculated to be 49% (95% CI = 0.40–0.58, P < 0.001, I2 = 96.1%) (Figure 2).
 |
Figure 2 Prevalence of laryngopharyngeal reflux in OSAHS patients based on a random effects model. |
Subgroup Analysis
Subgroup analysis according to ethnicity showed that the overall incidence of LPR-positive OSAHS patients was 59% in the Caucasian population (95% CI = 0.42–0.77, P < 0.001, I2 = 94.2%); in the Latin Americans, the overall incidence of LPR-positive OSAHS patients was 56% (95% CI = 0.31–0.82, P < 0.001, I2 = 98.7%); while, in the Asians, it was 43% (95% CI = 0.33–0.53, P < 0.001, I2 = 93.0%). In subgroup analysis according to BMI, the overall incidence of LPR-positivity in individuals with OSAHS was 59% with a mean BMI ≥ 30 (95% CI = 0.43–0.75, P < 0.001, I2 = 97.4%) and 41% in those with a mean BMI < 30 (95% CI = 0.32–0.51, P < 0.001, I2 = 93.4%). In subgroup analysis according to a mean AHI, the overall incidence of LPR-positivity among individuals with OSAHS was 55% in those with a mean AHI ≥ 30 (95% CI = 0.41–0.70, P < 0.001, I2 = 96.7%) and 44% in those with a mean AHI < 30 (95% CI = 0.31–0.57, P < 0.001, I2 = 96.5%). (Supplementary Table 1).
Age Variations Between Patients with Negative LPR and Positive LPR
Eight studies provided data on the age of patients that were LPR negative and those with positive laryngeal reflux, and the results suggested that there was no variation in age between the LPR+ and LPR- groups (WMD = −0.80, 95% CI = −2.77–1.17, P = 0.425, I2 = 0.0%). (Supplementary Table 2). (Supplementary Figure 1).
Differences in BMI Between Patients with Negative LPR and Positive LPR
Eight studies provided data on the BMI of patients that were LPR negative and LPR positive, and the results suggested that individuals in the LPR- group had a lower BMI in comparison to those in the LPR+ group (WMD = −1.61, 95% CI = −3.10--0.12, P = 0.034, I2 = 81.5%). (Supplementary Table 2). (Supplementary Figure 2).
AHI Variations Between Patients with Negative LPR and Patients with Positive LPR
Ten studies provided AHI data between individuals with negative LPR and individuals with positive LPR, and the results suggested that individuals in the LPR- group had a lower AHI index than the LPR+ group (WMD = −5.61, 95% CI = −9.51- −1.70, P = 0.005, I2 = 65.1%). (Supplementary Table 2). (Supplementary Figure 3).
Differences in Minimum Oxygen Saturation Between Negative LPR and Positive LPR Patients
Five studies provided data on minimum oxygen saturation in patients with negative laryngeal reflux versus those with positive laryngeal reflux, and the findings indicated that individuals in the LPR- group had higher minimum oxygen saturation than those in the LPR+ group (WMD = 3.82, 95% CI = 2.81–4.83, P = 0.007, I2 = 0%). (Supplementary Table 2). (Supplementary Figure 4).
Meta-Analysis of Correlation Between RSI Score, RFS Score, and AHI Score
Six studies reported Pearson’s or Spearman’s CORs for the association between RSI scores and AHI scores. AHI score is an important criterion for evaluating OSAHS, and it directly correlates with OSAHS severity. We conducted a meta-analysis on RSI scores and AHI scores in patients with OSAHS using the “meta” R package. The analysis revealed an effect size for the RSI score and AHI score of 0.32 (95% CI = 0.24–0.39, P < 0.001, I2 = 27%) (Figure 3A). Three studies reported Pearson’s or Spearman’s CORs for the association between RFS and AHI scores in patients with OSAHS. The analysis revealed an effect size for RFS and AHI scores of 0.45 (95% CI= 0.01–0.74, P=0.047, I2 = 91%) (Figure 3B).
 |
Figure 3 Funnel plot of effect sizes measured as correlations RSI score, RFS score and AHI. (A) RSI score; (B) RFS score. |
Meta-Regression and Sensitivity Analysis
A combined analysis of the incidence of LPR in all OSAHS patients exhibited a high degree of heterogeneity (I2=96.1%, P<0.001). Hence, a meta-regression analysis was performed to identify the source of the high heterogeneity. Meta-regression analysis showed that the P values for the ethnicity, AHI, and BMI were 0.090, 0.195, and 0.054, respectively, indicating that the factors such as race, AHI, and BMI did not have a significant effect on heterogeneity. In sensitivity analyses, each of the 26 studies was removed one by one in turn, and the remaining studies were subjected to meta-analysis, and the results were compared with the results before the previous exclusion, which showed that the item-by-item removal of each study had no significant effect on the combined results. The Figure 4 summarized the sensitivity analysis for meta-analysis of incidence. Less literature was included for meta-inclusion of the remaining continuous variables, and meta-regression and subgroup analyses were not performed.
 |
Figure 4 Sensitivity analysis of studies on prevalence of laryngopharyngeal reflux in OSAHS patients in meta-analysis. |
Publication Bias
In the meta-analysis regarding the incidence of laryngeal reflux, publication bias was not suggested in our combined analysis based on Egger’s (P = 0.058) and Begg’s (P = 0.053). (Figure 5). In the meta-analysis on the comparison of age in patients with OSAHS (negative and positive laryngeal reflux), publication bias was not significant in this combined analysis based on Egger’s (P = 0.067) and Begg’s (P = 0.083). In the meta-analysis on the comparison of BMI in patients with OSAHS (negative and positive laryngeal reflux), publication bias was not significant in this combined analysis according to Egger’s (P = 0.321) and Begg’s (P = 0.386). In the meta-analysis on the comparison of AHI in patients with OSAHS (negative and positive laryngeal reflux), publication bias was not significant in this combined analysis according to Egger’s (P = 0.530) and Begg’s (P = 0.788). In the meta-analysis on the comparison of minimum oxygen saturation in patients with OSAHS (negative and positive laryngeal reflux), publication bias was not significant in this combined analysis according to Egger’s (P=0.488) and Begg’s (P = 0.462). In the meta-analysis regarding the correlation between the RSI score and AHI index, the symmetry of the funnel plot according to linear regression analysis (t = 1.53, P = 0.224) suggested that the publication bias was not significant in this study.
 |
Figure 5 Funnel plots were employed to assess the publication bias among the included studies examining prevalence of laryngopharyngeal reflux of OSAHS patients. |
Discussion
In 1995, Janson et al44 observed that patients with reflux symptoms were more likely to experience daytime sleepiness or fatigue in a large epidemiological survey of 2202 individuals, and this suggested that there may be a high co-morbidity between reflux and OSAHS. Early studies did not distinguish between typical GERD and disease resulting from reflux of gastric contents beyond the upper esophagus. As research progressed, the general term for the set of signs and symptoms due to reflux of gastric contents above the upper esophageal sphincter became known as LPR and was considered a separate disease from gastroesophageal reflux disease (GERD).45 All included studies in this study confirmed the high rate of LPR in patients with OSAHS. Caparroz et al22 found a 59.7% LPR prevalence in patients with OSAHS using the RSI and RSF questionnaires. These results were confirmed by our meta-analysis, which showed a 49% incidence of LPR in patients with OSAHS. The high co-morbidity of OSAHS and LPR may be due to the presence of common risk factors such as alcohol consumption, smoking, obesity, and a high-fat diet. Furthermore, subgroup analysis suggested that the positivity rate of LPR was slightly higher in OSAHS patients with a mean AHI greater than 30; high-level of LPR positivity rate was observed in European and American OSAHS patients than in Asian and Latin American populations; similarly, it was higher in obese OSAHS patients. This suggests that OSAHS, obesity, and LPR exist in a vicious circle that affects each other. Secondly, the geographical and dietary habits differ between different ethnic groups, leading to a different prevalence of LPR in OSAHS patients. Europeans and Americans are likely to consume more high-fat foods than Asians, which leads to a higher prevalence of LPR. We further analyzed the age difference between LPR+ and LPR- patients, and the findings revealed no variation in age between the two groups, suggesting that age may not be an influencing factor for LPR. Lechien et al46 showed a higher probability of LPR in the elderly, which was associated with the relaxation of the upper esophageal sphincter. The studies we included did not have articles specifically evaluating the LPR profile of elderly OSAHS. Therefore, it was not possible to confirm the effect of age on pharyngeal reflux in patients with OSAHS.
The relationship between OSAHS severity and the incidence of LPR has always been controversial, and no substantial evidence is found in the literature for its confirmation. Lee et al37 studied 88 patients with OSAHS to analyze this point, and they reached a conclusion that OSAHS severity was independent of the severity of the clinical parameters associated with LPR. However, the findings of Elhennawi et al34 were observed to be different in this regard. They studied a group of 62 OSAHS patients and observed that the mean reflux symptom index was greater than 13 scores in all patients with severe OSAHS. In addition, they noticed a considerably higher incidence of nocturnal LPR in patients with severe OSA in comparison to patients with mild OSA. Wang et al23 illustrated that severe OSAHS condition led to lower mean nocturnal pharyngeal pH, and CPAP treatment was effective in reducing LPR episodes. AHI and minimum oxygen saturation are important indicators for evaluating the severity of OSAHS. We compared AHI and minimum oxygen saturation in LPR+ and LPR- OSAHS patients and combined these two indices for analysis. The results suggested that LPR+ patients had higher AHI and lower minimum oxygen saturation as compared to LPR- patients; meanwhile, the RSI index and AHI were weakly correlated with r = 0.32, whereas RFI was moderately correlated to AHI (r = 0.45). All these results suggest that OSAHS patients with LPR may be under a more severe condition. The original cause of this phenomenon may be as follows: firstly, pharyngeal reflux not only affects the mucosal damage of the upper airway but also causes inflammatory edema in the pharynx and aggravates the obstruction of the upper airway, it also affects the lower airway and pulmonary function, and every time pharyngeal reflux occurs, the inhalation of acidic reflux can directly stimulate the nerve endings of the tracheal mucosa and cause spasm of the trachea;47 on the other hand, the reflux acidic material stimulates chemoreceptors in the lower esophagus, and the chemoreceptors cause a vagal reflex that further aggravates the spasm of the airway. Trace amounts of inhaled acidic reflux stimulate the secretion of inflammatory substances from the tracheal mucosa, and these inflammatory substances can directly induce airway hyperresponsiveness.48 Some scholars believe that micro-inhalation of acidic reflux further destroys the active substances on the airway and alveolar surface, which will further lead to alveolar atrophy and pulmonary atelectasis.49 This vicious cycle caused by pharyngeal reflux directly causes increased nocturnal microarousals and breath-holding in patients with OSAHS, further aggravating the severity of OSAHS. Steward et al50 treated 27 patients with co-morbid OSAHS and LPR with pantoprazole for up to 3 months and compared the patients’ post-treatment and pre-treatment assessments of daytime sleepiness and reflux symptom assessment, roommate evaluation of their snoring, and sleep disorder degree index, and found that daytime sleepiness, reflux symptoms, and AHI were significantly improved in patients treated with acid suppression. Therefore, we hypothesize that frequent episodes of pharyngeal reflux may exacerbate the severity of OSAHS.
Another factor to consider that is related to both diseases, LPR and OSAHS, is the BMI of the patients, as OSAHS patients usually have a higher BMI. Our study revealed that the BMI of LPR+ patients was higher as compared to LPR- patients, suggesting that OSAHS patients with a high BMI may have a greater probability of having LPR. This result is consistent with a previous meta-analysis.11 Rodrigues et al38 evaluated LPR-related clinical indicators in 105 OSAHS patients (39 obese and 66 non-obese) and found that LPR-related clinical indicators were higher in obese OSAHS patients. Xavier et al32 also indicated that 89% of OSAHS patients had signs and symptoms of LPR, and obese OSA patients had a higher probability of LPR. The increase in intra-abdominal pressure is mainly caused by obesity, a mechanism that has significant pathophysiological implications in LPR and OSAHS. In OSAHS, obesity leads to increased respiratory effort, decreased thoracic range of motion, increased negative intrathoracic pressure, slowed esophageal clearance of reflux, and increased incidence of reflux episodes.51 However, obesity is not the sole causative factor of LPR. Other factors such as age, gender, psychiatric factors, environmental factors, diet, smoking, and alcohol consumption can also cause LPR. We believe that further in-depth studies on this topic are essential to elucidate the role of BMI in the pathogenesis of LPR in patients with or without OSA.
In general, heterogeneity of results in meta-analyses is linked to several factors, such as the quality of included studies, population characteristics, and experimental methods. In our study, most of the results of the combined analyses were highly heterogeneous. To explore potential sources of heterogeneity, we performed subgroup analyses and meta-regression analyses with the largest inclusion sample with regards to the prevalence of LPR. Meta-regression analyses revealed no heterogeneity in terms of race, disease severity, and weight. Regardless of the meta-regression results, we still performed subgroup analyses by race, disease severity, and weight. However, no obvious sources of heterogeneity were found. Some unknown confounding factors may increase the heterogeneity of the meta-analysis and decrease its reliability.
Nevertheless, this meta-analysis has several strengths. This study indicates that OSAHS patients have a higher probability of developing LPR in combination and that clinicians should also consider patients’ pharyngeal reflux when assessing OSAHS disease, as treatment of pharyngeal reflux may help control OSAHS disease; this is the largest meta-analysis of the relevant literature, and subgroup analysis was performed to present more reliable conclusions. Furthermore, newly published studies in Chinese were also included in this meta-analysis. Although a previous meta-analysis reported the relationship between LPR and OSAHS, this study included more literature, and the conclusions were different from that. Moreover, only the research papers of medium to high quality were considered for this study, which makes this meta-analysis more reliable.
However, there are still some potential limitations of this study. The dietary diversity and mental-emotional factors of the population may also cause LPR and may be one of the sources of heterogeneity, which can lead to statistical errors. Furthermore, due to the lack of a validated longitudinal cohort study in this study, we were unable to infer a causal relationship between OSAHS and LPR. There could be reciprocal or interdependent effects between LPR and OSAHS.
Conclusion
Laryngopharyngeal reflux is a common symptom in patients with OSAHS. The overall incidence of positive LPR in patients with OSAHS was calculated to be 49% in this meta-analysis; OSAHS patients with LPR have higher AHI and BMI scores, and RSI and RFI scores for LPR are positively associated with AHI. Finally, more detailed study plans are needed in the future to determine the correlation between LPR and the risk of OSAHS.
Data Sharing Statement
The original contributions presented in this meta-analysis are included in the article, further inquiries and R code can be directed to the corresponding authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Doukas PG, Vageli DP, Sasaki CT, et al. Pepsin promotes activation of epidermal growth factor receptor and downstream oncogenic pathways, at slightly acidic and neutral pH, in exposed hypopharyngeal cells. Int J Mol Sci. 2021;22:8. doi:10.3390/ijms22084275
2. Horvath L, Fostiropoulos K, Burri E, et al. Value of transnasal esophagoscopy in the workup of laryngo-pharyngeal reflux. J Clin Med. 2021;10:14. doi:10.3390/jcm10143188
3. Liu Q, Feng CC, Wang EM, et al. Efficacy of mosapride plus proton pump inhibitors for treatment of gastroesophageal reflux disease: a systematic review. World J Gastroenterol. 2013;19(47):9111–9118. doi:10.3748/wjg.v19.i47.9111
4. Eryilmaz F, Ahmed F, Rehmani AK, et al. Scoliosis and gastroesophageal reflux disease in adults. Cureus. 2021;13(5):e15359. doi:10.7759/cureus.15359
5. Msaad S, Chaabouni A, Marrakchi R, et al. Nocturnal Continuous Positive Airway Pressure (nCPAP) decreases high-sensitivity C-reactive protein (hs-CRP) in obstructive sleep apnea-hypopnea syndrome. Sleep Disord. 2020;2020:8913247. doi:10.1155/2020/8913247
6. Kang JM, Kim ST, Mariani S, et al. Difference in spectral power density of sleep EEG between patients with simple snoring and those with obstructive sleep apnoea. Sci Rep. 2020;10(1):6135. doi:10.1038/s41598-020-62915-x
7. Zaffanello M, Piacentini G, Sacchetto L, et al. Sleep-disordered breathing in children with rare skeletal disorders: a survey of clinical records. Med Princ Pract. 2018;27(5):451–458. doi:10.1159/000491391
8. Payne RJ, Kost KM, Frenkiel S, et al. Laryngeal inflammation assessed using the reflux finding score in obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006;134(5):836–842. doi:10.1016/j.otohns.2006.01.012
9. Iannella G, Vicini C, Polimeni A, et al. Laryngopharyngeal reflux diagnosis in obstructive sleep apnea patients using the pepsin salivary test. Int J Environ Res Public Health. 2019;16:11. doi:10.3390/ijerph16112056
10. Gouveia CJ, Yalamanchili A, Ghadersohi S, et al. Are chronic cough and laryngopharyngeal reflux more common in obstructive sleep apnea patients? Laryngoscope. 2019;129(5):1244–1249. doi:10.1002/lary.27557
11. Magliulo G, Iannella G, Polimeni A, et al. Laryngopharyngeal reflux in obstructive sleep apnoea patients: literature review and meta-analysis. Am J Otolaryngol. 2018;39(6):776–780. doi:10.1016/j.amjoto.2018.09.006
12. Lapeña JFF
13. Vance D, Alnouri G, Shah P, et al. The validity and reliability of the reflux finding score. J Voice. 2020. doi:10.1016/j.jvoice.2020.11.008
14. Mesallam TA, Farahat M. Self-perception of swallowing-related problems in laryngopharyngeal reflux patients diagnosed with 24-hour oropharyngeal pH monitoring. Biomed Res Int. 2016;2016:7659016. doi:10.1155/2016/7659016
15. Li X, He J. The association between serum/plasma leptin levels and obstructive sleep apnea syndrome: a meta-analysis and meta-regression. Front Endocrinol (Lausanne). 2021;12:696418. doi:10.3389/fendo.2021.696418
16. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi:10.1016/j.ijsu.2014.07.013
17. Kazemzadeh M, Shafiei E, Jahangiri K, et al. The preparedness of hospital emergency departments for responding to disasters in Iran; a systematic review and meta-analysis. Arch Acad Emerg Med. 2019;7(1):e58.
18. Du L, Shi HY, Qian Y, et al. Association between social support and suicidal ideation in patients with cancer: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2021;30(2):e13382. doi:10.1111/ecc.13382
19. Wang Y, Huang S, Yu P. Association between circulating neuregulin4 levels and diabetes mellitus: a meta-analysis of observational studies. PLoS One. 2019;14(12):e0225705. doi:10.1371/journal.pone.0225705
20. Erdem D, Yılmaz YF, Özcan M, et al. Correlation of sleep-disordered breathing and laryngopharyngeal reflux: a two-channel triple-sensor pHmetry catheter study. Eur Arch Otorhinolaryngol. 2018;275(10):2585–2592. doi:10.1007/s00405-018-5107-0
21. Pilakasiri A, Mahakit P. Prospective study of the prevalence and co-morbidities of obstructive sleep apnea in active-duty army personnel in the three southernmost provinces of Thailand using questionnaire screening. Mil Med Res. 2018;5(1):39. doi:10.1186/s40779-018-0186-1
22. Caparroz F, Campanholo M, Stefanini R, et al. Laryngopharyngeal reflux and dysphagia in patients with obstructive sleep apnea: is there an association? Sleep Breath. 2019;23(2):619–626. doi:10.1007/s11325-019-01844-0
23. Wang L, Han H, Wang G, et al. Relationship between reflux diseases and obstructive sleep apnea together with continuous positive airway pressure treatment efficiency analysis. Sleep Med. 2020;75:151–155. doi:10.1016/j.sleep.2020.07.024
24. Yue R, Xing D, Qin J, et al. The effect of obstructive sleep apnea surgery on laryngopharyngeal reflux with obstructive sleep apnea. Acta Otolaryngol. 2020;140(8):697–701. doi:10.1080/00016489.2020.1755448
25. Tang X, Tang Q, Li S, et al. Changes in laryngopharyngeal reflux after uvulopalatopharyngoplasty for obstructive sleep apnea: an observational study. Laryngoscope Investig Otolaryngol. 2022;7(1):266–273. doi:10.1002/lio2.718
26. Campanholo MAT, Caparroz FA, Vidigal TA, et al. Assessment of laryngopharyngeal reflux and obstructive sleep apnea: a population-based study. Laryngoscope. 2022;132(9):1877–1882. doi:10.1002/lary.30061
27. Yang XP, Xie Q, Xie J, Chen X. Analysis of upper airway morphology and laryngopharyngeal reflux in obese patients with OSA. Chin J Otorhinolaryngol Head Neck Surg. 2022;57:874–879.
28. Tamin S, Siregar D, Hutauruk SM, et al. Association between laryngopharyngeal reflux and obstructive sleep apnea in adults. PeerJ. 2022;10:e13303. doi:10.7717/peerj.13303
29. Ji P, Shi L, Xing D, et al. The effect of laryngopharyngeal reflux on arousal in patients with obstructive sleep apnea. Acta Otolaryngol. 2022;142(5):438–442. doi:10.1080/00016489.2022.2075033
30. Eryılmaz A, Erişen L, Demir UL, et al. Management of patients with coexisting obstructive sleep apnea and laryngopharyngeal reflux disease. Eur Arch Otorhinolaryngol. 2012;269(12):2575–2580. doi:10.1007/s00405-012-2062-z
31. Laohasiriwong S, Johnston N, Woodson BT. Extra-esophageal reflux, NOSE score, and sleep quality in an adult clinic population. Laryngoscope. 2013;123(12):3233–3238. doi:10.1002/lary.24236
32. Xavier SD, Moraes JP, Eckley CA. Prevalence of signs and symptoms of laryngopharyngeal reflux in snorers with suspected obstructive sleep apnea. Braz J Otorhinolaryngol. 2013;79(5):589–593. doi:10.5935/1808-8694.20130105
33. Qu Y, Ye JY, Han DM, et al. Esophageal functional changes in obstructive sleep apnea/hypopnea syndrome and their impact on laryngopharyngeal reflux disease. Chin Med J. 2015;128(16):2162–2167. doi:10.4103/0366-6999.162506
34. Elhennawi DM, Ahmed MR, Abou-Halawa AS. Correlation of obstructive sleep apnoea and laryngopharyngeal reflux: phmetry study. Clin Otolaryngol. 2016;41(6):758–761. doi:10.1111/coa.12640
35. Kim SJ, Kim HY, Jeong JI, et al. Changes in the reflux symptom index after multilevel surgery for obstructive sleep apnea. Clin Exp Otorhinolaryngol. 2017;10(3):259–264. doi:10.21053/ceo.2017.00052
36. Altintaş A, Soylu A, Yegin Y, et al. Impact of laryngopharyngeal reflux on the levels of depression and anxiety in patients with obstructive sleep apnea syndrome. J Craniofac Surg. 2017;28(2):e121–e124. doi:10.1097/SCS.0000000000003302
37. Lee JS, Heo SJ, Kim JS, et al. Relationship between the severity of laryngopharyngeal reflux and sleep apnea: using drug-induced sleep endoscopy (DISE). Eur Arch Otorhinolaryngol. 2018;275(1):219–224. doi:10.1007/s00405-017-4812-4
38. Rodrigues MM, Dibbern RS, Santos VJ, et al. Influence of obesity on the correlation between laryngopharyngeal reflux and obstructive sleep apnea. Braz J Otorhinolaryngol. 2014;80(1):5–10. doi:10.5935/1808-8694.20140004
39. Qing C, Wang X, Zeng JM. Clinical analysis of obstructive sleep apnea hypopnea syndrome combined with throat disease. Shanghai Med J. 2019;42(08):454–457.
40. Wang G, Wu W, Wang L, et al. Correlation between obstructive sleep apnea hypopnea syndrome and laryngopharyngeal reflux disease. Chin Arch Otolaryngol Head Neck Surg. 2018;25(11):613–616.
41. Wu SE, Li QM, Huang HS, Dong SQ, Cao HT. Correlation between obstructive sleep apnea hypopnea syndrome and laryngopharyngeal reflux disease. China Prac Med. 2018;13(36):59–60.
42. Wang XY, Han DM, Ye JY. Research on the relationship between obstructive sleep apnea hypopnea syndrome and nocturnal laryngopharyngeal reflux. Chin J Otorhinolaryngol Head Neck Surg. 2008;3:163–168.
43. Zhang CQ, Chen BB, Xiang HJ, Chen RR, Yin S. Analysis of anxiety, depression and quality of life in patients with obstructive sleep apnea hypopnea syndrome with laryngopharyngeal reflux disease. Chin Arch Otolaryngol Head Neck Surg. 2022;29(01):15–19.
44. Janson C, Gislason T, De Backer W, et al. Prevalence of sleep disturbances among young adults in three European countries. Sleep. 1995;18(7):589–597.
45. Lee JS, Jung AR, Park JM, et al. Comparison of characteristics according to reflux type in patients with laryngopharyngeal reflux. Clin Exp Otorhinolaryngol. 2018;11(2):141–145. doi:10.21053/ceo.2017.00577
46. Lechien JR, Carroll TL, Bobin F, et al. Influence of age and sex on clinical and therapeutic features of laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2022;166(3):468–476. doi:10.1177/01945998211020284
47. Konstantinidou SK, Kostaras P, Anagnostopoulos GE, et al. A retrospective study on the evaluation of the symptoms, medications and improvement of the quality of life of patients undergoing robotic surgery for gastroesophageal reflux disease. Exp Ther Med. 2021;21(2):174. doi:10.3892/etm.2020.9605
48. Hurley BP, Jugo RH, Snow RF, et al. Pepsin triggers neutrophil migration across acid damaged lung epithelium. Sci Rep. 2019;9(1):13778. doi:10.1038/s41598-019-50360-4
49. Kahrilas PJ, Howden CW, Hughes N, et al. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143(3):605–612. doi:10.1378/chest.12-1788
50. Steward DL. Pantoprazole for sleepiness associated with acid reflux and obstructive sleep disordered breathing. Laryngoscope. 2004;114(9):1525–1528. doi:10.1097/00005537-200409000-00003
51. Lechien JR, Bobin F, Muls V, et al. Laryngopharyngeal reflux disease is more severe in obese patients: a prospective multicenter study. Laryngoscope. 2021;131(11):E2742–e2748. doi:10.1002/lary.29676
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.