Back to Journals » Cancer Management and Research » Volume 12
Large Scale, Multicenter, Prospective Study of Apatinib in Advanced Gastric Cancer: A Real-World Study from China
Authors Peng W, Zhang F, Wang Z , Li D , He Y, Ning Z, Sheng L, Wang J, Xia X, Yu C, Wang Z, Zhao Y, Liang H, Hu B, Sun C, Wang D, Cheng Y, Pan M, Xia L, Guo X, Zhang Y, Hu Z, Li X, Lu L, Zhang J, Qian H, Xie H, Sun G
Received 10 February 2020
Accepted for publication 20 May 2020
Published 6 August 2020 Volume 2020:12 Pages 6977—6985
DOI https://doi.org/10.2147/CMAR.S249153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Wanren Peng, 1,* Fenglin Zhang, 2,* Zishu Wang, 3,* Dongliang Li, 4,* Yifu He, 5,* Zhongliang Ning, 6 Lili Sheng, 7 Jidong Wang, 8 Xiaoyang Xia, 9 Changjun Yu, 10 Zian Wang, 3 Yong Zhao, 11 Hui Liang, 12 Bing Hu, 13 Cuiling Sun, 14 Daoqin Wang, 15 Yunsheng Cheng, 16 Ming Pan, 17 Liming Xia, 18 Xinglai Guo, 19 Yanshun Zhang, 20 Zhiqiang Hu, 21 Xinzhong Li, 22 Lin Lu, 23 Jun Zhang, 24 Hong Qian, 8 Hua Xie, 25 Guoping Sun 1,*
1Department of Oncology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui 230022, People’s Republic of China; 2Department of Oncology, People’s Hospital of Maanshan City, Maanshan, Anhui 243000, People’s Republic of China; 3Department of Oncology, The First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui 233004, People’s Republic of China; 4Department of Gastrointestinal Surgery, People’s Hospital of Lu’an City, Lu’an, Anhui 237005, People’s Republic of China; 5Department of Oncology, The First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Cancer Hospital), Hefei, Anhui 230022, People’s Republic of China; 6Department of Gastrointestinal Surgery, The First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Cancer Hospital), Hefei, Anhui 230022, People’s Republic of China; 7Department of Oncology, Yijishan Hospital of WanNan Medical College, Wuhu, Anhui 340202, People’s Republic of China; 8Department of Oncology, The Navy Anqing Hospital, Anqing, Anhui 246003, People’s Republic of China; 9Department of Oncology, The First People’s Hospital of Chuzhou City, Chuzhou, Anhui 239000, People’s Republic of China; 10Department of Gastrointestinal Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui 230022, People’s Republic of China; 11Department of Oncology, People’s Hospital of Lu’an City, Lu’an, Anhui 237005, People’s Republic of China; 12Department of Oncology, Lu’an Hospital of Traditional Chinese Medicine, Lu’an, Anhui 237006, People’s Republic of China; 13Department of Oncology, The First Affiliated Hospital of University of Science and Technology of China, Hefei, Anhui 230022, People’s Republic of China; 14Department of Oncology, People’s Hospital of Fuyang City, Fuyang, Anhui 236047, People’s Republic of China; 15Department of Gastrointestinal Surgery, Wanbei Coal-Electricity Group General Hospital, Suzhou, Anhui 234000, People’s Republic of China; 16Department of Oncology, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui 230601, People’s Republic of China; 17Department of Oncology, People’s Hospital of Chizhou City, Chizhou, Anhui 247000, People’s Republic of China; 18Department of Oncology, The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui 230031, People’s Republic of China; 19Department of Oncology, Fuyang Cancer Hospital, Fuyang, Anhui 236033, People’s Republic of China; 20Department of Oncology, Huainan First People’s Hospital, Huainan, Anhui 232000, People’s Republic of China; 21Department of Oncology, Huaibei Miners General Hospital, Huaibei, Anhui 235000, People’s Republic of China; 22Department of Oncology, People’s Hospital of Huaibei, Huaibei, Anhui 235000, People’s Republic of China; 23Department of Oncology, The 901 Hospital of the Joint Logistic Support Force of the People’s Liberation Army of China, Hefei, Anhui 230033, People’s Republic of China; 24Department of Oncology, The Second People’s Hospital of Wuhu, Wuhu, Anhui 241001, People’s Republic of China; 25Department of Oncology, The People’s Hospital of Xuancheng City, Xuancheng, Anhui 242000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guoping Sun Email [email protected]
Background: In China, gastric cancer (GC) ranks second in incidence and mortality. Over 80% of patients with GC were diagnosed at an advanced stage with poor clinical outcome. Chemotherapy was the mainstream treatment with limited benefit. Apatinib, an inhibitor of targeting vascular endothelial growth factor receptor 2 (VEGFR2), has been approved for third-line treatment of advanced gastric cancer. However, the data of apatinib treatment in the real-world setting are limited. In this real-world study, we aimed to understand the current treatment pattern of apatinib, investigate the effectiveness and safety of apatinib in real-world settings, and explore the potential factors associated with the clinical outcomes.
Methods: This was a prospective, multicenter observational study in a real-world setting. Patients aged ≥ 18 years with histologic diagnosis of advanced GC were eligible for enrollment. The eligible patients received either apatinib monotherapy or apatinib plus chemotherapy by physician’s discretion. Apatinib treatment could be used as first-line, second-line, or third-line and above therapy. The primary endpoint was progression-free survival (PFS). The secondary endpoints were overall survival (OS), ORR, DCR, and safety profile.
Results: A total of 737 patients with advanced gastric cancer treated with apatinib were included in the FAS population. A total of 54.9% patients used apatinib monotherapy and 45.1% patients used apatinib combination therapy. A total of 44.1% patients received apatinib in first-line treatment, 28.2% in second-line, and 27.7% in third-line and above. In first-line treatment, the objective response rate (ORR) was 9.09% and 16.42% in apatinib monotherapy and combination therapy groups, and disease control rate (DCR) was 78.41% and 89.29%, respectively. Patients who received combination therapy achieved significantly longer median progression-free survival (mPFS; 6.18 vs 3.52 months, p< 0.01) and median overall survival (mOS; 8.72 vs 5.92 months, p< 0.01) compared with monotherapy. In second-line and third-line therapy, combination therapy showed a better trend in tumor response and survival outcomes compared with monotherapy. For all patients, apatinib combined with paclitaxel were associated with longer mPFS compared with other combinations (8.88 vs 6.62 months). Multivariate analysis showed that combination with paclitaxel (p=0.02) and experience of apatinib-related specific AEs (p< 0.01) were independent predictors for PFS and OS. The safety profile was tolerable and no unexpected adverse events were reported.
Conclusion: In a real-world setting, apatinib showed a favorable effectiveness and safety profile in patients with advanced gastric cancer. Apatinib combination therapy, especially combined with paclitaxel, might lead to better survival benefit in first-line treatment. Combination with paclitaxel and the occurrence of apatinib-specific AEs were independent factors associated with better survival outcomes.
Trial Registration: NCT03333967.
Keywords: apatinib, combination therapy, real-world, advanced gastric cancer
Corrigendum for this paper has been published
Introduction
Gastric cancer (GC) is one of the most common malignant tumors and the third leading cause of cancer-related deaths worldwide.1 In China, GC ranks second in incidence and mortality.2–5 Over 80% of patients with GC are diagnosed at an advanced stage with poor clinical outcome and a low 5-year survival rate of <20%.6,7 Chemotherapy is the recommended treatment in China but the benefits are limited. Consequently, it is crucial to explore and optimize treatment strategy to improve the survival benefit of patients with GC.
In recent years, anti-angiogenic therapy has become a main treatment choice for cancer patients, and some angiogenesis inhibitors have shown favorable efficacy in lung, breast, colon and gastric cancers.8,9 Apatinib is a small-molecule tyrosine kinase inhibitor (TKI) that highly selectively binds and inhibits vascular endothelial growth factor receptor 2 (VEGFR2). The Phase II and Phase III clinical trials have shown that apatinib exhibits promising efficacy and tolerable safety profile in patients with chemotherapy-refractory advanced or metastatic gastric carcinoma,10,11 and apatinib has been approved in advanced gastric cancer for third-line treatment in China. In addition, many other clinical studies have also investigated the efficacy and safety of apatinib in many cancers including GC, and also proposed some concerns such as the optimal treatment dosage of apatinib, combined regimens, the incidence of apatinib specific adverse events, and the biomarkers for prognosis prediction, which are difficult to demonstrate by conducting randomized clinical trials (RCTs) with large sample sizes.
Recently real-world study has become an important tool in generating evidence for safety and effectiveness to support further study design of RCTs. However, the data of apatinib treatment in the real-world setting are limited. Therefore, we conducted this real-world study to understand the current treatment pattern of apatinib in advanced gastric cancer, investigate the effectiveness and safety of apatinib in real-world settings, and explore the potential factors that are related to the clinical outcomes.
Patients and Methods
Patients
Patients with histologically confirmed diagnosis of advanced gastric cancer and age ≥18 years old were eligible for enrollment. If the patients had allergy to apatinib or other excipients, were pregnant or lactating, or another contraindication for apatinib, they were not enrolled in the current study. In addition, the patients who were not eligible for this study according to the physician’s discretion were not enrolled in this study.
Study Design and Treatment
This was a prospective, multicenter observational study in a real-world setting. The eligible patients received apatinib monotherapy or apatinib plus chemotherapy. The chemotherapy regimens included docetaxel, paclitaxel, capecitabine and oxaliplatin (XELOX), 5-fluorouracil (5-Fu), epirubicin+oxaliplatin+capecitabine (EOX), docetaxel+cisplatin+fluorouracil (DCF). Based on physician’s discretion and patients’ approval, apatinib could be used as first-line, second-line, or third-line and above therapy. Apatinib was administered (250 mg or 500 mg) once daily. One cycle of treatment consisted of 28 days. And the dosage was adjusted by the physician based on the patient’s individual conditions.
Progression-free survival (PFS) was the primary endpoint and defined as time from the date of entry to the date of disease progression or death from any cause. The secondary endpoints were overall survival (OS), objective response rate (ORR), disease control rate (DCR) and safety profile. The OS was defined as the time from entry to the date of death or the last follow-up. The ORR included the complete response (CR) and partial response (PR); the DCR included the CR, PR, and stable disease (SD). For safety profile, the incidence of adverse events (AEs), treatment-related AEs, and apatinib-related specific AEs were assessed.
Demographic and baseline data were collected. All patients were followed up for at least 1 year. Subgroup analyses were conducted according to possible survival relevant factors (such as combination regimens, and numbers of metastasis). Multivariate analysis was used to identify independent prognostic factors for survival outcomes.
Statistical Analyses
The safety population comprised all patients who received at least one dose of apatinib and had available case report form data. The effectiveness population comprised all patients in the safety population. Rates were compared using the χ2 test. PFS, OS and survival rates were estimated using Kaplan-Meier methodology. The multivariate backward Cox regression analysis was used to identify independent prognostic factors for survival outcomes. Statistical analyses were performed using Statistical Analysis Software version.
Results
Patient Characteristics
Between September 1, 2017 and April 15, 2019, 737 patients with gastric cancer treated with apatinib were enrolled and included in the FAS population. Of all patients, 54.9% were treated with apatinib monotherapy and 45.1% with apatinib combination therapy. A total of 44.1% patients received apatinib as first-line treatment, 28.2% as second-line and 27.7% as third-line and above. The patient characteristics are shown in Table 1. The median age was 64 years (IQR 54–71), 540 were male and 197 were female patients. Most patients (95.2%) were with ECOG PS 0–1. All the patients were diagnosed with advanced gastric cancer, and 58.63% of patients were identified to have metastases. According to the treatment regimens and treatment lines, we divided the patients into six groups (Table 1). The patients who received apatinib monotherapy as the first-line treatment were regarded as the F-A group; those who received apatinib plus chemotherapy as the first-line treatment were regarded as the F-C group; those who received apatinib monotherapy as the second-line treatment were regarded as the S-A group; those who received apatinib plus chemotherapy as the second-line treatment were regarded as the S-C group; those who received apatinib monotherapy as the third-line and above treatment were regarded as the T-A group; those who received apatinib plus chemotherapy as the third-line and above treatment were regarded as the T-C group. There were no significant differences in baseline characteristics between the apatinib monotherapy and combination therapy groups in different treatment lines.
 |
Table 1 Patient Characteristics |
Effectiveness
When apatinib was used in first-line treatment, the ORR was, respectively, 9.09% and 16.42% for apatinib monotherapy and combination therapy groups, and DCR was 78.41% and 89.29%, respectively. Although there was no statistical significance, a trend was observed that patients who received combination therapy could achieve higher ORR and DCR. There were four patients who achieved CR including one in the monotherapy group and three in the combination group. Most patients achieved SD (69.3% vs 72.9%) in the monotherapy and combination therapy groups.
In second-line and third-line and above treatment, no patients achieved CR. And most patients achieved SD (70.4% vs 74.6%) in second-line treatment for the monotherapy and combination therapy groups, and (70.1% vs 81.8%) in third-line and above treatment. In second-line treatment, no significant difference was identified between the monotherapy and combination therapy groups with the ORR of 12.35% vs 9.52% and the DCR of 82.72% vs 84.13%. In third-line and above, the DCR was 81.82% vs 76.59% with no significant difference between the two treatment groups (Table 2).
 |
Table 2 Response of Patients to Monotherapy and Combination Therapy in Different Treatment Lines |
For survival outcomes in all patients, the mPFS of the first-line, second-line, and third-line and above therapy was 5.72, 5.52, and 4.87 months, respectively. The mOS of the first-line, second-line, and third-line and above therapy was 7.63, 7.5, and 7.5 months, respectively. The mPFS of the monotherapy and combination therapy was 4.61 and 6.38, respectively. The mOS of the monotherapy and combination therapy was 6.51 and 8.88 months, respectively (Figure 1). In the first-line treatment, patients who received combination therapy achieved significantly longer mPFS (6.18 vs 3.52 months, p<0.01) and mOS (8.72 vs 5.92 months, p<0.01) compared with monotherapy. And the 6-month PFS rate (38.72% vs 51.28%, p=0.02) and 12-month PFS rate (12.57% vs 26.69%, p<0.01) were also significantly higher in the combination therapy groups. In the second-line treatment, the mPFS (6.42 vs 5.33 months) was longer in the combination therapy group with no significant difference, and the mOS was similar between the two groups (7.30 vs 7.50 months). In third-line and above treatment, a trend was also observed with longer mPFS (6.68 vs 4.47 months) and mOS (10.63 vs 6.51 months) in combination therapy compared with those in monotherapy (Table 3 and Figure 1).
 |
Table 3 Survival Analysis of Patients to Monotherapy and Combination Therapy in Different Treatment Lines |
Exploratory Analysis
The mPFS varied among patients who received different combination regimens. Patients who received apatinib combined with paclitaxel achieved a longer mPFS (8.88 vs 6.62 months) compared with other combinations. For patients with ≤2 metastasis, the mPFS was 6.41 months, and for patients with >2 metastasis, the mPFS was much shorter (3.62 months). Patients who did not experience apatinib treatment suspension achieved a longer mPFS than those who experienced treatment suspension (6.97 vs 4.41 months). Patients without metastasis achieved longer mPFS (7.27 vs 5.56 months) compared with those with metastasis. For patients who experienced apatinib-related AEs, the mPFS was longer (10.03 vs 3.32 months) compared with those who did not experience apatinib-related AEs. The subgroup analysis showed that the combination regimen, number of metastasis, treatment suspension and experience of apatinib-related AEs might be the factors that could affect the survival benefit. All the results are shown in Table 4.
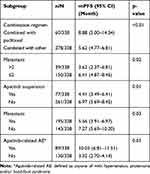 |
Table 4 Subgroup Analysis of mPFS in Combination Therapy |
The multivariate backward Cox regression analysis were also conducted, and results showed that combination with paclitaxel (p=0.02) and experience of apatinib-related specific AEs (p<0.01) were independent predictors for mPFS and mOS (Tables 5 and 6).
 |
Table 5 Multivariate Analysis of mPFS in Combination Therapy |
 |
Table 6 Multivariate Analysis of mOS in Combination Therapy |
For patients who received the combination therapy of apatinib plus paclitaxel, 32 patients were treated with first-line treatment, 9 patients were treated with second-line treatment, and 5 patients with third-line and above treatment. In the first-line treatment, the ORR and DCR were, respectively, 18.75% (95% CI 7.21–36.43) and 93.75% (95% CI 79.19–99.23), and the mPFS and mOS were 8.14 months and 9.17 months, respectively. The 6-month PFS rate was 67.73% (95% CI 50.08–80.29) and 12-month PFS rate was 49.64% (95% CI 29.32–67.04).
Safety
A total of 574 patients experienced adverse events, the incidence of all adverse events was 73.59%, and the incidence of grade≥3 AEs was 18.97%. No unexpected AEs and SAEs were observed. The most common and apatinib-related specific grade≥3 AEs are shown in Table 7. The incidence of grade≥3 AEs was similar between the apatinib monotherapy and combination treatment groups.
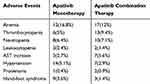 |
Table 7 The Incidence of Grade ≥3 AEs |
Discussion
This large-scale, real-world study added evidence for the effectiveness and safety profile of apatinib in patients with advanced gastric cancer. The results showed that in the real-world clinical setting, 54.9% of patients were treated with apatinib monotherapy and 45.1% with apatinib combination therapy. About 70% of patients received apatinib as the first-line and second-line treatment. In the first-line therapy, patients who received combination therapy achieved longer survival benefit than those who received monotherapy (mPFS 6.18 vs 3.52 months). In second- and third-line and above treatment, the combination regimen also showed a trend of better survival benefit. These findings indicated that apatinib combination therapy could extend survival, especially in first-line therapy.
In China, fluorouracil and cisplatin combination with or without a third drug were considered as standard first-line chemotherapy for advanced gastric cancer. Combination of paclitaxel and capecitabine showed a mPFS of 5.0 months in first-line treatment in advanced gastric patients.12 In the Chinese patient population analysis, the mPFS of capecitabine/cisplatin and 5-fluorouracil/cisplatin were 7.2 and 4.5 months, respectively.13 In another phase III study, docetaxel and cisplatin plus fluorouracil were compared with cisplatin and fluorouracil, and the median time to progression was 5.6 and 3.7 months, respectively.14 Compared to the regimens of cisplatin/S-1 and cisplatin/infusional, the mPFS of 4.8 months and 5.5 months were achieved, respectively.15 In our study, the mPFS was 6.18 months for apatinib combination therapy, which was similar with the results of double or triple chemotherapy. Previous studies revealed that anti-angiogenic drugs combined with chemotherapy showed significant positive effects on PFS and OS in gastric cancer patients.16 First-line treatment with bevacizumab combined with chemotherapy was associated with a longer mPFS compared with chemotherapy (6.7 vs 5.3 months).17 These results were similar with our study, suggesting that anti-angiogenic drugs plus chemotherapy in first-line treatment might lead to better survival benefit.
For second-line treatment for advanced gastric cancer in China, there was no standard recommendation except for mono-chemotherapy. Some clinical trials had shown that VEGFR-2 antagonist combined with chemotherapy could significantly improve survival benefit compared with chemotherapy alone in the second-line treatment. A phase III study showed that mPFS with ramucirumab plus paclitaxel was significantly longer than placebo plus paclitaxel (4.4 vs 2.9 months).18 Another study showed that patients who received ramucirumab monotherapy achieved a mPFS of 2.1 months in second-line treatment.19 In a study of apatinib in combination with chemotherapy as second-line, progression-free survival was 3.72 months.20 In our study, the mPFS was 5.33 and 6.42 months in apatinib monotherapy and combination therapy groups, respectively, which was better than the results of ramucirumab and previous study of apatinib, suggesting that apatinib monotherapy or combined with chemotherapy might be a treatment alternative for second-line treatment. For third-line treatment, apatinib monotherapy had been the standard treatment recommended by the guideline in China based on the results of the phase III trial of apatinib, which showed that heavily pre-treated patients who received apatinib monotherapy achieved a mOS of 6.5 months and mPFS of 2.6 months and the mediation analysis found the prolonged progression-free survival of apatinib could mediate overall survival of patients.10,21 In this real-world study, the mPFS was 4.47 months and mOS was 6.51 months in apatinib monotherapy as third-line treatment, which also further validated that even in heavily pre-treated patients, apatinib monotherapy could really bring survival benefit in clinical practice. In addition, several previous studies have shown that apatinib combination therapy markedly increased the DCR and prolonged the PFS compared with chemotherapy alone in gastric cancer patients who failed first-line treatment.22,23 However, the combination regimen of second- and above-line treatment in our study only exhibited a trend of better ORR, DCR and survival outcomes, and no significant difference was identified, which might be explained by the heterogeneity of the large sample sizes in a real-world setting.
Taxanes were considered as alternative first-line and second-line chemotherapy options, and paclitaxel showed good efficacy and tolerance. In the subgroup analysis of this study, apatinib combined with paclitaxel showed obviously prolonged mPFS compared with other chemotherapy (8.88 vs 5.62 months). The multivariate analysis also confirmed that combination with paclitaxel was an independent factor for PFS and OS, which indicated that patients who received apatinib plus paclitaxel therapy could benefit from longer mPFS. The prolonged mPFS and mOS in apatinib combined with taxel/docetaxel regimen might be explained by the fact that apatinib significantly increased the intracellular accumulation of substrate drugs by reversing the multidrug resistance.24 Additionally, a retrospective study had shown that early presence of anti-angiogenesis-related AEs including hypertension, proteinuria, or hand and foot syndrome during the first cycle of apatinib treatment were viable biomarkers of antitumor efficacy in patients with metastatic GC.25 Consistently, the results of the multivariate analysis in this study showed that the occurrence of apatinib-specific AEs were independent factors for longer PFS and OS.
A total of 574 patients reported adverse events, the incidence of all adverse events was 73.59%, and the incidence of grade≥3 AEs was relatively low. No unexpected AEs were observed. These results showed the good safety profile of apatinib when used in real-world clinical practice. In this study, PFS but not OS was analyzed as the primary end point due to the limited follow-up time and challenged data collection from the large sample sizes and real-world setting.
Conclusion
This prospective study demonstrated that apatinib had a favorable effectiveness and safety profile in patients with advanced gastric cancer. In a real-world clinical setting, apatinib was used as monotherapy or combination with chemotherapy, and was also used in first-line and second-line treatment. In first-line treatment, apatinib combination therapy, especially combined with paclitaxel, might lead to better survival benefit. In second-line treatment, apatinib monotherapy or combination with chemotherapy might be an alternative treatment. Although apatinib has been recommended as third-line standard treatment, this study further confirmed that apatinib monotherapy as third-line regimen could really bring survival benefit. Combination with paclitaxel and the occurrence of apatinib-specific AEs were independent factors associated with better survival outcomes. Further well-designed studies with larger sample sizes will be needed to confirm the results, and specific treatment regimens also need further exploration.
Abbreviations
GC, gastric cancer; PFS, progression-free survival; mPFS, median progression-free survival; OS, overall survival; mOS, median overall survival; ORR, objective response rate; DCR, disease control rate; FAS, full analysis set; AE, adverse event; F-A, first-line apatinib alone; S-A, second-line apatinib alone; T-A, third-line and above apatinib alone; F-C, first-line apatinib combined with chemotherapy; S-C, second-line apatinib combined with chemotherapy; T-C, third-line or above apatinib combined with chemotherapy; VEGFR2, vascular endothelial growth factor receptor 2.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent
The study was approved by the Ethics Committee for Clinical Medical Research, the First Affiliated Hospital of Anhui Medical University. All patients provided written informed consent to participate in this research, which was in compliance with the Declaration of Helsinki. All participants have given consent for publication.
Acknowledgments
We thank Cunnan Dong and Guoliang Chen for writing and revising this manuscript. Part of the Apatinib was contributed by Hengrui Medicine Co., Ltd. (Lianyungang, People's Republic of China).
Funding
No funding was received.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer. 2017;36(1):66. doi:10.1186/s40880-017-0234-3
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34(11):502–507. doi:10.1186/s40880-015-0042-6
5. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208
6. Wang W, Sun Z, Deng JY, et al. A novel nomogram individually predicting disease-specific survival after D2 gastrectomy for advanced gastric cancer. Cancer Commun. 2018;38(1):23. doi:10.1186/s40880-018-0293-0
7. Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388(10060):2606. doi:10.1016/S0140-6736(16)32226-7
8. De Vita F, Di Martino N, Fabozzi A, et al. Clinical management of advanced gastric cancer: the role of new molecular drugs. World J Gastroenterol. 2014;20(40):14537–14558. doi:10.3748/wjg.v20.i40.14537
9. Wong H, Yau T. Molecular targeted therapies in advanced gastric cancer: does tumor histology matter? Therap Adv Gastroenterol. 2013;6(1):15–31. doi:10.1177/1756283X12453636
10. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. doi:10.1200/JCO.2015.63.5995
11. Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. doi:10.1200/JCO.2013.48.8585
12. Lu Z, Zhang X, Liu W, et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer. 2018;21(5):782–791. doi:10.1007/s10120-018-0809-y
13. Chen J, Xiong J, Wang J, Zheng L, Gao Y, Guan Z. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin in Chinese patients with advanced and metastatic gastric cancer: re-analysis of efficacy and safety data from the ML17032 phase III clinical trial. Asia Pac J Clin Oncol. 2018;14(5):e310–e316.
14. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–4997. doi:10.1200/JCO.2006.06.8429
15. Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28(9):1547–1553. doi:10.1200/JCO.2009.25.4706
16. Zhao TT, Xu H, Xu HM, et al. The efficacy and safety of targeted therapy with or without chemotherapy in advanced gastric cancer treatment: a network meta-analysis of well-designed randomized controlled trials. Gastric Cancer. 2018;21(3):361–371. doi:10.1007/s10120-018-0813-2
17. Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–3976. doi:10.1200/JCO.2011.36.2236
18. Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised Phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi:10.1016/S1470-2045(14)70420-6
19. Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi:10.1016/S0140-6736(13)61719-5
20. Lu B, Lu C, Sun Z, et al. Combination of apatinib mesylate and second-line chemotherapy for treating gastroesophageal junction adenocarcinoma. J Int Med Res. 2019;47(5):2207–2214. doi:10.1177/0300060519827191
21. Huang L, Wei Y, Shen S, et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget. 2017;8(17):29346–29354. doi:10.18632/oncotarget.12897
22. Cheng H, Sun A, Guo Q, Zhang Y. Efficacy and safety of apatinib combined with chemotherapy for the treatment of advanced gastric cancer in the Chinese population: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:2173–2183. doi:10.2147/DDDT.S170678
23. Guo Y, Tang J, Huang XE, Cao J. Efficacy and toxicity of apatinib combined with or without chemotherapy for patients with advanced or metastatic chemotherapy-refractory gastric adenocarcinoma: a prospective clinical study. Medicine. 2019;98(6):e13908. doi:10.1097/MD.0000000000013908
24. Tong XZ, Wang F, Liang S, et al. Apatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cells. Biochem Pharmacol. 2012;83(5):586–597. doi:10.1016/j.bcp.2011.12.007
25. Liu X, Qin S, Wang Z, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol. 2017;10(1):153. doi:10.1186/s13045-017-0521-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

