Back to Journals » International Medical Case Reports Journal » Volume 15
Lacrimal Duct Obstruction and Infection Associated with Non-Traumatic Corneal Perforation: A Case Series
Authors Nitta K , Mukai R , Todokoro D, Akiyama H
Received 18 February 2022
Accepted for publication 8 June 2022
Published 23 June 2022 Volume 2022:15 Pages 313—322
DOI https://doi.org/10.2147/IMCRJ.S363034
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Keisuke Nitta, Ryo Mukai, Daisuke Todokoro, Hideo Akiyama
Department of Ophthalmology, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan
Correspondence: Keisuke Nitta, Department of Ophthalmology, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan, Tel +81-27-220-8338, Fax +81-27-220-3841, Email [email protected]
Purpose: To report a case series of lacrimal duct obstruction and infection associated with non-traumatic corneal perforation.
Case Series: This study included 6 eyes in 6 patients with non-traumatic corneal perforation treated between April 2019 and March 2021. All 6 cases were associated with lacrimal duct obstruction and infection. Purulent discharge caused by lacrimal duct infection was observed in all 6 patients (100%). However, three of the 6 patients (50%) did not show purulent discharge at initial examination and lacrimal duct obstruction was therefore not initially recognized. Dry eye was observed in five of the 6 patients (83%) and may have caused corneal deterioration, increasing susceptibility to perforation. Further, dry eye masks symptoms of lacrimal duct obstruction and infections, such as epiphora and regurgitation of purulent discharge, making the association with lacrimal duct obstruction and infection difficult to determine. All patients were treated for both corneal perforation and lacrimal duct disease, and conditions improved, with no recurrence of either corneal perforation or lacrimal duct disease.
Conclusion: In patients with a combination of lacrimal duct disease and corneal perforation, treatment of both diseases resulted in stabilization of patient condition. Dry eyes may mask symptoms of lacrimal duct diseases, such as epiphora and purulent discharge, and lacrimal duct disease may thus be underdiagnosed.
Keywords: corneal perforation, lacrimal duct obstruction, lacrimal duct infection, dacryocystitis, purulent discharge, dry eye, case series
Introduction
Corneal perforation is associated with significant ocular morbidity and warrants prompt intervention, both to restore globe integrity and to minimize the risk of secondary complications, such as endophthalmitis, choroidal hemorrhage, and glaucoma. Causes of corneal perforation include trauma, microbial keratitis, ocular surface diseases like dry eye or Sjögren syndrome and autoimmune disorders like rheumatoid arthritis or Mooren’s ulcer.1–5 Although reports have described corneal perforation caused by canaliculitis6,7 or dacryocystitis,8,9 the relationships of lacrimal duct obstruction or lacrimal duct infection and corneal perforation have not been examined in a large number of cases, so much remains unclear. This study aimed to report cases of lacrimal duct obstruction and infection associated with non-traumatic corneal perforation and investigation of the characteristics of such cases.
Methods
A total of 27 eyes in 26 consecutive patients (15 men, 11 women; mean age, 68.7 years; range, 5–93 years) who were treated due to non-traumatic corneal perforation from April 2019 to March 2021 at Gunma University Hospital were identified. The medical records for these 27 eyes were reviewed and 6 eyes of 6 patients were associated with nasolacrimal duct disease and infection. The present case series included these 6 patients, for whom we reviewed the systemic and ocular complications, symptoms, status of lacrimal duct, causative organism, treatment of lacrimal duct disease and corneal perforation, and visual outcomes.
When lacrimal duct obstruction was suspected, the lacrimal syringing test and dacryoendoscopy (FT-203F MD10; FiberTech Co., Tokyo, Japan) tests were conducted to diagnose lacrimal duct obstruction or lacrimal duct infection. Dacryoendoscopy was performed as previously reported10 to check the status inside the lacrimal duct. A lacrimal stent (Lacrifast®; Kaneka Co., Osaka, Japan) was used for nasolacrimal duct intubation.
Bacteriological examinations, including smear test and culture, were performed when purulent discharge was observed. Pathological examinations were performed when lacrimal concretion was obtained.
Although no consensus has been reached on uniform criteria for the diagnosis of dry eye, this study diagnosed dry eye according to the criteria of Holland et al11 Briefly, a combination of a questionnaire on symptoms, checking the status of the corneal epithelia by fluorescent staining and checking the status of lacrimal secretion by the Schirmer test were used. The questionnaire included items regarding foreign body sensation, itching, eye pain, epiphora, photophobia, discharge, vision difficulties, hyperemia and any additional symptoms.
This study was approved by the ethics committee of the Gunma University Graduate School of Medical Science (approval no. HS2021-154) and all protocols were conducted in accordance with the tenets of the Declaration of Helsinki. Consent for publication was obtained from all patients.
Case Presentations
Mean age of the 6 patients (1 male, 5 females) with corneal perforation associated with lacrimal duct disease was 76.7 years (range, 68–88 years). Detailed information including age, sex, laterality, systemic and ocular complications, presence of epiphora, presence of purulent discharge and timing of the discharge being noticed, prescription of antimicrobial eye drops at the time of referral, results of both smears and culture of purulent discharge, pathology of lacrimal concretion, status and treatment of the lacrimal duct, treatment of the corneal perforation, corneal status of the other eye, visual acuity before and after treatment, and causes of corneal perforation are summarized in Table 1.
 |
Table 1 Summary of Patient Data Related to Lacrimal Duct Disease and Corneal Perforation |
Case 1
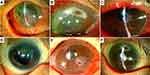
An 82-year-old woman was referred to our department with recurrent corneal perforation in the right eye. Three years earlier, her right eye had developed a corneal perforation. At that time, severe dry eye due to Sjögren’s syndrome had been diagnosed as the cause of corneal perforation. She was treated with amniotic membrane transplantation and conjunctival flap. Upper and lower lacrimal punctum coagulation to close the lacrimal duct had then been conducted by a local ophthalmologist for severe dry eye. At the referral, opacity, and vascular invasion due to previous corneal perforation from the center to the lower part of the cornea were observed in the right eye, and a new corneal perforation was identified on the temporal side of the previous corneal perforation (Figure 1A). Since visual acuity was poor with light perception, she was administered ofloxacin eye ointment to stop leakage without surgery. Ten days after referral, the leakage had not stopped, so a conjunctival flap was applied. Eleven days after referral, the reflux of purulent discharge from the reopened lacrimal punctum in the right eye was noticed. Nasolacrimal duct obstruction in the middle to lower part and chronic dacryocystitis were confirmed on dacryoendoscopy, and nasolacrimal duct intubation was conducted. After 1.5 months, dacryocystitis subsided. The lacrimal stent was then removed, and the lacrimal punctum and lacrimal canaliculi were cauterized to completely close the lacrimal duct.
Case 2
An 88-year-old woman reported difficulty seeing in the right eye for the past 4 days and sudden loss of vision the day before. She visited her local ophthalmologist and was referred to our department for corneal perforation of the right eye. She had been visiting the ophthalmologist for uveitis and dry eye, but had stopped seeing the doctor a year earlier. However, she had continued to use steroid eye drops once a day in both eyes. Peripheral corneal thinning was observed from the 8 o’clock to 1 o’clock position and corneal perforation was evident at 1 o’clock in the right eye (Figure 1B). Filamentary keratitis and superficial punctate keratopathy (SPK) were observed in the left eye. The Schirmer I test showed 9 mm in the right eye and 0 mm in the left eye, although results for the right eye were considered inaccurate because of the presence of corneal perforation. Blood tests were positive for SS-A antibody, SS-B antibody, and rheumatoid factor. Based on these results, Sjögren’s syndrome was suspected. A family history of rheumatoid arthritis in her daughter and peripheral corneal ulcer suggested that she also had rheumatoid arthritis, but since the patient had no joint symptoms, a definitive diagnosis of rheumatoid arthritis could not be made. In the right eye, direct examination of vitreoretinal tissue was not possible due to corneal edema and anterior chamber inflammation. B-mode ultrasonography showed neither vitreous opacity nor choroidal detachment. Lacrimal syringing test indicated nasolacrimal duct obstruction and chronic dacryocystitis in the right eye. Gatifloxacin eye drops, cefmenoxime eye drops and betamethasone eye drops were prescribed. Therapeutic soft contact lenses (SCL) were also applied. Four days after the first visit, dacryoendoscopy of the right eye revealed obstruction of the nasolacrimal duct just below the lacrimal sac and chronic dacryocystitis, and nasolacrimal intubation was conducted. Six days after referral, cellulitis developed around the right lacrimal sac, with a blood temperature of 38.5°C. She was admitted to hospital and received intravenous administration of antimicrobial agents. Her condition gradually improved, and intravenous infusion ended after a week. Finally, the cellulitis and lacrimal duct infection subsided. Perforated cornea also stabilized, with corrected visual acuity reaching 20/250.
Case 3
A 74-year-old woman was referred for corneal perforation of the right eye. Four months earlier, nasolacrimal duct intubation of the right eye had been performed by a local ophthalmologist. However, ocular discharge had continued, and the lacrimal stent had been removed 2 months earlier. Ocular hyperemia and discharge had continued, and corneal perforation was identified in the central part of the cornea (Figure 1C). Treatment with therapeutic SCL, levofloxacin eye drops, and vancomycin eye drops was prescribed, since methicillin-resistant Staphylococcus aureus (MRSA) had been detected in a culture by the previous ophthalmologist. Dacryoendoscopy of the right eye revealed obstruction of the lower nasolacrimal duct and infection of the whole length of the nasolacrimal duct. Nasolacrimal duct intubation was then conducted. The corneal perforation was closed spontaneously soon after applying the therapeutic SCL, and gradually increased in corneal thickness. The ocular discharge tended to decrease but did not disappear completely. The patient therefore underwent dacryocystorhinostomy as a fundamental treatment.
Case 4
A 78-year-old woman with a history of rheumatoid arthritis was referred to our department with corneal perforation in the right eye. Three months earlier, a periapical corneal ulcer had been identified in the right eye and had once improved with antimicrobial and steroid eye drops. However, the peripheral ulcer recurred 2 months earlier, resulting in corneal perforation despite the use of antimicrobial and steroid eye drops. The patient was referred to our department after wearing a therapeutic SCL. The patient also had dacryocystitis and the lacrimal sac was punctured by her local ophthalmologist. At the time of referral to our department, the anterior chamber had been formed with a therapeutic SCL. The cornea of the right eye showed peripheral thinning from the 6 o’clock to 10 o’clock positions, and perforation was observed on the inferior nasal area (Figure 1D). Cefmenoxime and betamethasone eye drops were prescribed. Dacryoendoscopy of the right eye revealed obstruction in the upper nasolacrimal duct and lacrimal concretion in both the inferior canaliculus and lacrimal sac. Nasolacrimal intubation was conducted. Two days later, although the discharge was reduced, hardness around the lacrimal sac was observed, and antibacterial medication was prescribed. Four days later, the site of lacrimal sac puncture became a lacrimal cutaneous fistula, and the surrounding area became infected. Since MRSA was detected in the culture of discharge at the first visit, intravenous vancomycin was initiated. Eight days later, the inflammation was resolved. Dacryocystectomy of the right eye was performed, and the upper and lower canaliculi and lacrimal puncta of both eyes were ablated to close the lacrimal ducts.
Case 5
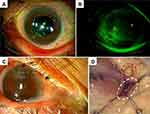
A 68-year-old man presented to our department with recurrent corneal perforation in the right eye. He had a history of bone marrow transplant for chronic myelogenous leukemia and graft-versus-host disease (GVHD) with severe dry eye, as well as corneal perforation in the right eye and right dacryocystectomy with lacrimal punctum coagulation for chronic dacryocystitis 3 years earlier. Since then, he had been using steroid eye drops. His left eye also had a corneal perforation and a history of lacrimal duct treatment, although the details were unclear. Opacity and vascular invasion in the lower nasal cornea of the right eye due to previous corneal perforation, and recurrent corneal perforation in the same area were observed during referral (Figure 1E). The patient was admitted to the hospital the same day and was prescribed gatifloxacin eye drops and steroid eye drops. Anterior chamber formation occurred soon after the application of therapeutic SCL. Twelve days after admission, therapeutic SCL was removed and no leakage from the anterior chamber was observed. Thirteen days after admission, reopening of the right inferior punctum and reflux of discharge from the punctum was observed with pressure on the right lacrimal sac. A dead space was identified at the site of the previously resected lacrimal sac, causing infection-like dacryocystitis. The patient underwent dacryocystectomy with drainage of discharge and complete closure of the lacrimal duct.
Case 6
A 70-year-old woman showed corneal perforation of the right eye. She had systemic lupus erythematosus, mixed connective tissue disease and hypertrophic cardiomyopathy, and was taking oral prednisolone at 6 mg/day. She had started dry eye treatment 3 years earlier. One month earlier, the patient had developed peripheral corneal ulcer in the right eye. She was prescribed steroid eye drops, steroid ointment, levofloxacin eye drops and cyclosporine eye drops. Three weeks earlier, upper and lower lacrimal plugs had been inserted into the right eye. Two days earlier, she developed corneal perforation and was referred to our department wearing a therapeutic SCL. At the time of the initial visit to our department, peripheral corneal perforation in the supranasal area was observed in the right eye, and the anterior chamber had flattened despite wearing the therapeutic SCL (Figure 1F). In the left eye, no thinning or perforation of the peripheral cornea was observed (Figure 2A). However, fluorescein staining revealed severe SPK (Figure 2B). She was directly admitted the same day. Lamellar keratoplasty (LKP) of the right eye was performed the next day. Eleven days after LKP, reflux of purulent discharge from the lacrimal duct of the right eye was observed (Figure 2C). Dacryoendoscopy revealed an obstruction just below the lacrimal sac, a large lacrimal concretion in the lacrimal sac, and purulent discharge in the lacrimal sac and lacrimal canaliculi. Dacryocystectomy and removal of the lacrimal concretion were then performed (Figure 2D), and the lacrimal punctum and canaliculi were closed by coagulation.
Discussion
Yokogawa et al7 reported that 2 of 31 eyes (6.4%) with perforated corneas showed diseases of the lacrimal drainage system. These 31 cases were exclusively severe cases requiring corneal surgery and cases treated with therapeutic SCL were excluded. In the present study, 4 of the 6 cases (Cases 2–5) were small perforations that could be treated with therapeutic SCL, and those cases had been excluded from the report by Yokogawa et al. However, even a small corneal perforation can lead to an urgent condition such as endophthalmitis, so investigation of the exact cause of corneal perforation and the presence of lacrimal duct obstruction or lacrimal duct infection that would contribute to corneal perforation is important.
Corneal ulcers result from lacrimal duct obstruction and lacrimal duct infection.12,13 The mechanism is postulated to involve direct invasion by pathogenic organisms that flow back to the ocular surface from the lacrimal sac,6 backflow of toxins, matrix metalloproteinases and lysosomes produced in the lacrimal duct,8 or allergies against toxins produced by pathogenic organisms.7 On the other hand, lacrimal canaliculitis and dacryocystitis rarely lead to corneal perforation, presumably because of the need for a combination of factors, not simply the single factor of lacrimal duct infection, but also host factors.8
As a complication of systemic and ocular diseases, five of the six cases (83%) had dry eye. Two patients (Cases 1 and 2) had primary Sjögren’s syndrome dry eye, and the other two (Cases 4 and 6) had secondary Sjögren’s syndrome associated with collagen diseases. The remaining case (Case 5) had non-Sjögren’s syndrome dry eye, a secondary lacrimal gland deficiency due to GVHD. Epiphora was observed in three of the six (50%) patients (Cases 2–4) and not in the other three. Purulent discharge was observed in all six patients but was not apparent at the time of initial examination and was only noticed during hospitalization in the three patients who did not show epiphora (Cases 1, 5 and 6). All five patients with SPK in the cornea of the other eye (Cases 1, 2, 4–6) had dry eye, and two patients with peripheral corneal thinning (Cases 2, 4) had confirmed and suspected rheumatoid arthritis, respectively.
Based on these results, corneal perforation was attributed to lacrimal duct disease alone in one patient (Case 3), to a combination of lacrimal duct disease and dry eye in three patients (Cases 1, 5 and 6), and to a combination of lacrimal duct disease, dry eye, and autoimmune disorders such as rheumatoid arthritis in two patients (Cases 2 and 4). This result is consistent with previous speculation that corneal perforation associated with lacrimal duct obstruction or lacrimal duct infection is complicated by factors other than lacrimal duct disease.
In the present study, steroid eye drops had been used for 4 of 6 eyes (66%) prior to corneal perforation. Some reports have suggested that the use of steroid eye drops contributes to corneal ulcer or perforation.14,15 However, those reports were based on a relatively small number of cases and did not conduct statistical analyses. On the other hand, studies with larger numbers of patients and statistical analyses have shown that steroid use is not a risk factor for causing corneal perforation.16,17 Further, steroids are frequently used to promote quiescence in cases of severe ocular surface inflammation, so steroid use may be a marker of heightened disease severity.18 In fact, for cases in which steroid eye drops were prescribed to treat peripheral corneal ulcer (Cases 4 and 6), steroid eye drops may have contributed to preventing corneal perforation. Although determining how steroid eye drop use affects each case of corneal perforation in this study is difficult, it should be noted that while corticosteroids can decrease ocular surface inflammation, they may also increase the risk of corneal ulceration or perforation, so corticosteroids should be used judiciously.18
Epiphora and elevation of tear meniscus height are usually observed in lacrimal duct obstruction, and purulent discharge is observed in lacrimal duct infections such as dacryocystitis. These epiphora, elevated tear meniscus and purulent discharge can be easily noticed both subjectively and objectively, leading to diagnosis of lacrimal duct disease. However, three patients (Cases 1, 5 and 6) who were noticed to have been discharged during hospitalization showed neither epiphora nor discharge at the time of initial examination, while the other three patients (Cases 2–4) showed both epiphora and discharge at the time of initial examination. The reduced tear meniscus due to severe dry eye was thought to have masked epiphora and discharge, and obstruction or infection of the lacrimal duct went unnoticed. Therefore, in cases of corneal perforation in a patient with dry eye, even in the absence of epiphora or discharge, the presence of lacrimal duct obstruction and lacrimal duct infection should be suspected, and the patient should be checked by compression of the lacrimal sac, lacrimal syringing test and dacryoendoscopy. Patients could possibly have already been prescribed antimicrobial eye drops at the time of referral, and therefore did not show discharge. However, in Cases 3 and 4, ocular discharge was observed from the time of initial examination even though antimicrobial eye drops had been prescribed, so this hypothesis was not supported.
Although Sjögren’s syndrome and rheumatoid arthritis are known to cause corneal perforation, these conditions are basically bilateral.3 Further, while cases of corneal perforation caused by dry eye alone have been reported,2,3 no studies have provided data on large numbers of dry eye cases allowing rough calculation of the rate of corneal perforation. Dry eye may be complicated by corneal ulceration, particularly in patients with Sjögren’s syndrome, and corneal perforation may occasionally develop in such cases.2,3 Corneal perforation occurred in 17 of 1838 patients (0.9%) with Sjögren syndrome4 and in 2 of 243 patients (0.8%) with ocular GVHD.18 The incidence of corneal perforation associated with rheumatoid arthritis is estimated as 0.234–9.82/million/year.5 Of course, these factors may also be involved in corneal perforation, but do not represent the sole cause. We believe that the influence of unilateral lacrimal duct disease on the side of the corneal perforation was significant in all six cases of corneal perforation in this study.
In the case of corneal perforation, thorough examination of the perforated eye as well as the condition of the other eye can provide insights into the factors that led to the perforation. In fact, five of the six patients with dry eye in our study had SPK in the other eye, and one patient with rheumatoid arthritis showed thinning of the peripheral cornea in the other eye.
Mixed infections with a predominance of Gram-positive bacteria are the most common cause of chronic dacryocystitis.19 In this study, Gram-positive bacteria were detected in 5 of the 6 cases, and 3 of the 6 cases were mixed infections, consistent with previous reports.
Treatment-resistant corneal ulcers complicated by chronic dacryocystitis can reportedly be controlled after lacrimal duct treatment,13 and early diagnosis and planning for early surgery are imperative in cases of lacrimal duct obstruction in order to manage corneal infection.19 In this study, all 6 patients received treatments for both corneal perforation and nasolacrimal duct disease, resulting in stabilization of the clinical condition, and no recurrence of corneal perforation has been identified since then. These results indicate that treatment of lacrimal duct infections is important, even in cases of corneal perforation, and may minimize corneal damage.
In lacrimal duct obstruction with dry eye, patients who have not been diagnosed with dry eye before surgery may complain of persistent or more severe ocular discomfort after surgeries such as lacrimal tube intubation or dacryocystorhinostomy.20,21 In such patients, surgical procedures such as dacryocystectomy and lacrimal canalicular closure are good options, as they lead to a higher postoperative tear meniscus.22,23 In the present study, four patients (Cases 1, 4, 5 and 6) underwent dacryocystectomy or lacrimal canalicular closure.
The fact that the lacrimal duct can be reopened even after treatment by obstruction (such as by closure of the lacrimal punctum or dacryocystectomy) is very important, as observed in two of our patients (Cases 1 and 5). Therefore, even if the lacrimal duct has been treated before, lacrimal duct examination is necessary to exclude lacrimal duct obstruction or infection.
Finally, we have reported 6 cases of corneal perforation associated with nasolacrimal duct obstruction and infection. Some important symptoms of nasolacrimal duct obstruction and infection, including epiphora and purulent discharge, might be hidden by dry eye. Checking the condition of the other eye and lacrimal duct is essential to identify the causes of corneal perforation. In particular, the lacrimal duct needs to be checked carefully, as reopening can occur even after treatment to close the duct. In cases of associated lacrimal duct disease, the lacrimal duct should be treated concurrently with treatment of the corneal perforation.
Abbreviations
SPK, superficial punctate keratopathy therapeutic; SCL, therapeutic soft contact lenses; MRSA, methicillin-resistant Staphylococcus aureus; GVHD, graft-versus-host disease; LKP, lamellar keratoplasty.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of the Gunma University Graduate School of Medical Science (approval no. HS2021-154) and all protocols were conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from patients for the publication of data in this case series and the accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Deshmukh R, Stevenson LJ, Vajpayee R. Management of corneal perforations: an update. Indian J Ophthalmol. 2020;68(1):7–14. doi:10.4103/ijo.IJO_1151_19
2. Baranwal VK, Satyabala K, Mishra A, Dutta AK. Sterile corneal perforations in a case of severe dry eyes. Med J Armed Forces India. 2015;71(3):290–292. doi:10.1016/j.mjafi.2013.04.005
3. Deswal J, Arya SK, Raj A, Bhatti A. A case of bilateral corneal perforation in a patient with severe dry eye. J Clin Diagn Res. 2017;11(4):Nd01–nd02. doi:10.7860/jcdr/2017/24149.9645
4. Singh S, Das AV, Basu S. Ocular involvement in sjögren syndrome: risk factors for severe visual impairment and vision-threatening corneal complications. Am J Ophthalmol. 2021;225:11–17. doi:10.1016/j.ajo.2020.12.019
5. Timlin HM, Hall HN, Foot B, Koay P. Corneal perforation from peripheral ulcerative keratopathy in patients with rheumatoid arthritis: epidemiological findings of the British ophthalmological surveillance unit. Br J Ophthalmol. 2018;102(9):1298–1302. doi:10.1136/bjophthalmol-2017-310671
6. Ishikawa S, Kato N. A case with corneal perforation due to bacterial concretion derived from lacrimal canaliculitis. Am J Ophthalmol Case Rep. 2018;9:116–118. doi:10.1016/j.ajoc.2018.01.004
7. Yokogawa H, Kobayashi A, Yamazaki N, Masaki T, Sugiyama K. Surgical therapies for corneal perforations: 10 years of cases in a tertiary referral hospital. Clin Ophthalmol. 2014;8:2165–2170. doi:10.2147/opth.S71102
8. Hattori T, Shibata M, Minezaki T, et al. A case of corneal perforation caused by dacryocystitis in patient with long-term indwelling of lacrimal intubation. Atarashii Ganka. 2016;33(1):
9. Nagasato D, Tabuchi H, Yamauchi T, Imamura H, Shimizu Y. Severe corneal melting and perforation secondary to chronic dacryocystitis due to delayed ophthalmology consultation. Oxf Med Case Rep. 2021;2021(4):omab009. doi:10.1093/omcr/omab009
10. Matsumura N, Suzuki T, Goto S, et al. Transcanalicular endoscopic primary dacryoplasty for congenital nasolacrimal duct obstruction. Eye. 2019;33(6):1008–1013. doi:10.1038/s41433-019-0374-6
11. Krachmer JH, Mannis MJ, Holland EJ. Cornea: Fundamentals, Diagnosis and Management.
12. Nayak A, Mitra Basu S, De A, Mallick A, Das S, Rath S. Concurrent microbial keratitis and nasolacrimal duct obstruction: concordance, etiopathogenesis, and outcome. Cornea. 2019;38(1):84–88. doi:10.1097/ico.0000000000001767
13. Li G, Guo J, Liu R, et al. Lacrimal duct occlusion is associated with infectious keratitis. Int J Med Sci. 2016;13(10):800–805. doi:10.7150/ijms.16515
14. Gebauer A, McGhee CN, Crawford GJ. Severe microbial keratitis in temperate and tropical Western Australia. Eye. 1996;10(5):575–580. doi:10.1038/eye.1996.133
15. Scott IU, Flynn HW
16. Hsu HY, Ernst B, Schmidt EJ, Parihar R, Horwood C, Edelstein SL. Laboratory results, epidemiologic features, and outcome analyses of microbial keratitis: a 15-year review from St. Louis. Am J Ophthalmol. 2019;198:54–62. doi:10.1016/j.ajo.2018.09.032
17. Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130(2):143–150. doi:10.1001/archophthalmol.2011.315
18. Stevenson W, Shikari H, Saboo US, Amparo F, Dana R. Bilateral corneal ulceration in ocular graft-versus-host disease. Clin Ophthalmol. 2013;7:2153–2158. doi:10.2147/opth.S51180
19. Sharma S, Sahu SK, Rath S, Mallick A, Sharma S, Das S. Microbiological spectrum of microbial keratitis in patients presenting with nasolacrimal duct obstruction. Eur J Ophthalmol. 2021;31(4):1720–1724. doi:10.1177/1120672120940574
20. Kamao T, Takahashi N, Zheng X, Shiraishi A. Changes of visual symptoms and functions in patients with and without dry eye after lacrimal passage obstruction treatment. Curr Eye Res. 2020;45(12):1590–1597. doi:10.1080/02713683.2020.1760305
21. Kang TS, Cho J, Kim J, et al. Modified ocular surface disease index as a screening criteria for dry eye syndrome presenting after successful dacryocystorhinostomy. PLoS One. 2021;16(2):e0247168. doi:10.1371/journal.pone.0247168
22. Galindo-Ferreiro A, Dufaileej M, Galvez-Ruiz A, Khandekar R, Schellini SA. Dacryocystectomy: indications and results at tertiary eye hospital in central Saudi Arabia. Semin Ophthalmol. 2018;33(5):602–605. doi:10.1080/08820538.2017.1375122
23. Murube J, Murube E. Treatment of dry eye by blocking the lacrimal canaliculi. Surv Ophthalmol. 1996;40(6):463–480. doi:10.1016/S0039-6257(96)82013-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.