Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
L-Cystine-Containing Hair-Growth Formulation Supports Protection, Viability, and Proliferation of Keratinocytes
Authors Riegel K, Hengl T, Krischok S, Schlinzig K, Abts HF
Received 17 April 2020
Accepted for publication 5 June 2020
Published 3 August 2020 Volume 2020:13 Pages 499—510
DOI https://doi.org/10.2147/CCID.S254720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Kristina Riegel,1,2 Thomas Hengl,2 Saskia Krischok,2,3 Kim Schlinzig,2 Harry F Abts2
1Cell Biology Unit, University Medical Center Mainz, Mainz 55131, Germany; 2Pharmaceutical Development, Merz Pharmaceuticals GmbH, Frankfurt Am Main 60438, Germany; 3Co.faktor GmbH, Berlin 10178, Germany
Correspondence: Harry F Abts
Merz Pharmaceuticals GmbH, 60438, Frankfurt Am Main, Alfred-Wegener-Straße 2, FIZ 2, F08.10.014, Frankfurt Am Main 60438, Germany
Tel +49 69 15 03 1324
Fax +49 69 15 03 9152
Email [email protected]
Purpose: Clinical studies have confirmed that the hair-growth-promoting effect of approved oral drug combinations is beneficial for the treatment of diffuse telogen effluvium, which is characterized by the excessive loss of telogen club hairs. Since data elucidating the mode of action of such combinations are limited, our study focused on the identification of cellular processes potentially supporting the treatment of hair loss.
Materials and Methods: A minimal growth culture system (MGM) was used to mimic in vitro the reduced activity of human hair follicular keratinocytes (HHFKs). The effect of four core compounds (L-cystine, thiamine, calcium D-pantothenate, and folic acid) of a marketed oral combination (Panto[vi]gar®), which are approved for the treatment of diffuse hair loss, was examined by comparing HHFKs cultured either with or without the compounds. After determining their impact on metabolic activity and proliferation, we conducted a comparative whole-genome gene expression study with subsequent functional grouping of differentially expressed genes to identify cellular processes influenced by the tested compounds.
Results: The four core compounds of an oral hair-growth formulation enhanced proliferation and metabolic activity of HHFKs compared to HHFKs cultivated in MGM only. Functional grouping of differentially expressed genes confirmed the regulation of cell cycle-/proliferation-associated genes (cdk1, HJURP) and revealed regulation of cell death- and oxidative stress-associated gene groups. A supportive effect of the compounds on cell viability was demonstrated by lower sensitivity to solar-simulated UV-radiation and increased protection against oxidative stress. We established a central role for L-cystine, as changes in the expression of the anti-oxidative gene hmox1 were L-cystine-dependent. However, to reach a maximal stimulating effect on proliferation, the combination of all four compounds was necessary.
Conclusion: The tested compound combination had positive effects on metabolic activity, cell viability, and proliferation of keratinocytes. Furthermore, this study suggested that L-cystine primarily contributes to the observed protection against endogenous oxidative stress.
Keywords: telogen effluvium, protection, genetic analysis, keratinocytes, oxidative stress
Introduction
During hair-growth the basal cellular part, the hair follicle continuously progress through the hair cycle stages: growth (anagen), involution (catagen), and rest (telogen). In each cycle a new hair shaft is formed, while the old hair mostly falls out during an actively regulated process termed exogen.1 A new follicular cycle is initiated through the regeneration of the lower follicle, which is mediated through the interaction of the dermal papilla with the hair-specific epithelial stem cells in the hair follicle bulge.2 The mature (anagen) hair follicle can be divided into a permanent upper segment and a regenerating lower segment. The lower part contains the dermal papilla (formed by special fibroblasts), which is framed by an enlarged bulb consisting of rapidly proliferating matrix keratinocytes, melanocytes and outer root sheath keratinocytes.3 As stated in the review by Cotsarelis, it is still not clear whether the bulge cells migrate towards the follicle during the anagen phase in order to provide the matrix cells with new cells, or whether the matrix cells self-renew and maintain their compartment throughout the anagen phase.4 Regarding the actual hair shaft growth, it has been demonstrated that the highly proliferative hair matrix keratinocytes give rise to the hair shaft and the inner root sheath. During the upward movement of the hair matrix keratinocytes, they are differentiating into the hair shaft, which consists of the cuticle, the cortex, and the medulla.3 A major regulator of the hair shaft growth is the dermal papilla, which controls the hair bulb size, hair shaft size and anagen duration.3 In the subsequent catagen phase, hair matrix cells reduce their proliferating and stop differentiating so that the hair shaft growth is terminated. Finally, the hair follicle regresses and reaches a state of rest (telogen).
A pathological hair loss exists when the physiological degree of daily hair loss is exceeded for prolonged time. The hair cycle disorder telogen effluvium is a form of pathologic hair loss, which is characterized by an increase in telogen hair follicles.5 This type of hair loss is a diffuse, reversible form that affects the whole scalp.6 Causes of telogen effluvium include iron deficiency, thyroid disease, and metabolic or endocrine disorders.5 Dietary deficiencies7 or general stress can also trigger its onset. Treatment of telogen effluvium includes L-cystine-containing oral combinations, which exhibit some variations in their composition. A meta-analysis has confirmed successful treatment effects of these combinations against diffuse hair loss through increasing the anagen rate, reflecting a normalization of the hair cycle disturbance.8 But the precise mode of action underlying the clinical efficacy of these oral combinations is still not fully understood.
Here, we investigate how active components of an oral combination (Panto[vi]gar®, Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) affect cellular processes, which might be relevant for hair-growth and through which the compounds could potentially contribute to a hair-growth-promoting effect. We unveil that the in vitro correlate (IC) of the aforementioned oral formulation consisting of L-cystine, thiamine, calcium D-pantothenate, and folic acid (an assumed metabolite of p-aminobenzoic acid (PABA))9 has a positive impact on the proliferation and viability of human hair follicular keratinocytes (HHFKs) and contributes to a higher protection against endogenous oxidative stress.
Materials and Methods
Cell Culture and Treatment
Human hair follicular keratinocytes (HHFKs; Provitro GmbH, Berlin, Germany; ScienCell, Carlsbad, CA, USA) were propagated in serum-free growth media (PromoCell; Heidelberg, Germany) at 37°C in the presence of 5% CO2 in a humidified atmosphere. Preparation of HHFKs from hair follicles isolated from human scalp tissue involved proprietary protocols of ScienCell. Briefly: After removal of the dermal papilla of isolated hair follicles, hair follicle derived keratinocytes were allowed to grow out in submersed cell culture to form a population of keratinocytes form different regions. These keratinocytes named HHFKs are capable to proliferate and can be expanded over at least 10 passages. The nature of the keratinocytes is confirmed by their appearance in cell culture (cobblestone morphology in light-microscopy) and by the immunohistological detection of keratin 18. We expanded the HHFKs to generate a frozen master cell bank from which cells were used for the described experiments in defined passages.6–8 Cells were seeded into flat-bottom microplates (NunclonTM; Sigma-Aldrich®; Munich, Germany) or cell culture flasks at a density of 5700–6700 cells cm−2. After overnight incubation, cells were switched to minimal growth medium (MGM; basal medium without L-cystine, thiamine, calcium D-pantothenate, folic acid and biotine; customer formulation; PromoCell; Heidelberg, Germany) or MGM plus a combination of L-cystine, thiamine, calcium D-pantothenate, and folic acid (termed IC). IC serves as an in vitro correlate of the oral formulation Panto[vi]gar®, which was developed depending on the expected systemic bioavailability at the hair follicle and the usability of compounds in an in vitro cell culture system.10 The following experiments for characterization of compound effects were carried out after an incubation time of 72 h.
Preparation of the Medium Serving as in vitro Correlate of the Oral Formulation
The four compounds selected as in vitro correlate (IC) of a marketed, oral hair-growth formulation (Panto[vi]gar®) were prepared as stock solutions in dimethyl sulfoxide (DMSO), except for L-cystine, which was directly prepared in MGM. These stock solutions were further diluted in assay media to obtain the required test compound concentration and to restrict DMSO to a maximal concentration of 1% (v/v). Unused portions of the stocks were stored at −20°C. The composition of IC consisted of 100 µM thiamine, 50 µM L-cystine, 1 µM calcium D-pantothenate, and 1 µM folic acid. In certain experiments cells were cultured with different concentrations of IC. This was achieved by diluting IC in MGM: IC/10 refers to IC diluted 10 times in MGM, resulting in a concentration of 10 µM thiamine, 5 µM L-cystine, 0.1 µM calcium D-pantothenate, and 0.1 µM folic acid.
Metabolic Activity, Proliferation, De Novo DNA Synthesis
Metabolic activity, proliferation and apoptosis of HHFKs were detected 72 h after cultivating them in 96-well plates under the indicated conditions in a final volume of 100 µL per well in 4–6 replicates. Background controls were included in each case.
Metabolic activity was determined by a resazurin solution (resazurin sodium salt [0.1 mg/mL in phosphate-buffered saline; PBS], Sigma-Aldrich®; Munich, Germany). 10 µL of the resazurin solution was added to the treated cells and incubated for 90 min at 37°C. Metabolically active cells convert resazurin to the fluorescent resofurin. The resulting fluorescence was measured on a plate reader (SynergyTM H4, BioTek®; Bad Friedrichshall, Germany) at 560 nm excitation and 590 nm emission.
The CyQUANT® direct assay kit (InvitrogenTM; Darmstadt, Germany) was performed to measure the DNA content of vital cells following the manufacturer’s instructions. Fluorescence of the DNA intercalating dye was measured at λEx/λEm = 480 nm/535 nm on the plate reader (SynergyTM H4).
De novo DNA synthesis was determined by the BrdU cell proliferation ELISA kit (Roche Diagnostics, Mannheim, Germany). During an incubation time of 4 h, the labeled thymidine analog BrdU is incorporated into the newly synthesized DNA. Following the manufacturer’s instructions, de novo DNA synthesis was detected indirectly using a luciferase-coupled antibody directed against BrdU. Luminescence was measured on a plate reader (SynergyTM H4).
Microarray
Four replicates of TRIzol® (Sigma-Aldrich®; Munich, Germany) cell lysates of MGM- and IC-treated HHFKs were used for the whole-genome gene expression study carried out with a one-color Agilent whole human genome oligo microarray 8 x 60K (Miltenyi Biotec B.V. and Co. KG, Bergisch Gladbach, Germany). After background correction, normalization, and correlation analysis, the whole data set was processed by differential gene expression analysis. Significant expression differences of the genes – also referred to as reporters – were selected by applying a P-value ≤ 0.01 as selection threshold. In addition to the P-value, the reliable candidates for differentially expressed genes were required to show at least a 4-fold average expression difference between the sample groups. Additionally, a functional grouping analysis based on the ‘gene ontology biological processes function’ was performed (available at: http://geneontology.org/).
Quantitative Polymerase Chain Reaction (qPCR) Analysis
Differential expression of selected genes was confirmed using quantitative polymerase chain reaction (qPCR) analysis (GenXPro, Frankfurt, Germany). A Taqman®-qPCR was performed with 15–20 ng total RNA per reaction using the StepOneTM real-time PCR system (Applied Biosystems, Darmstadt, Germany). The genes rpl13a, actb and b2m, coding for ribosomal protein L13a, beta actin and beta-2-microglobulin, respectively, served as housekeeping genes.
SDS-PAGE and Western Blotting
Total cell lysates were prepared from HHFKs cultivated in the indicated culture conditions. After separating 5–10 µg protein by using NuPAGE® 4–12% Bis-Tris gels in the XCell SureLockTM electrophoresis cell system, proteins were transferred onto a polyvinylidene fluoride membrane and were blocked with 2% bovine serum albumin in 1 x Tris-buffered saline + 0.05% Tween 20 for 4–16 h. The following primary antibodies were used: mouse anti-β-actin (Sigma-Aldrich®; Munich, Germany), rabbit anti-CHAC1, rabbit anti-CDC2, rabbit anti-HJURP, and rabbit anti-HO1 (all from Santa Cruz Biotechnology; Heidelberg, Germany). Proteins were visualized indirectly using horseradish peroxidase-conjugated secondary antibodies (anti-mouse and anti-rabbit; Jackson ImmunoResearch; PA, USA) and enhanced chemiluminescence immunodetection. Western Blots were quantified by densitometry (ImageJ software). Total protein levels were normalized to the levels of a housekeeping gene. The protein levels after IC treatment are shown in relation to levels detected in HHFKs cultured only in MGM. CDK1 protein levels after treatment with L-cystine were related to levels detected in IC-cultured HHFKs.
Solar-Simulated UV-Irradiation
HHFKs were treated for 24 h with MGM or IC and subsequently UV-irradiated with 25 mJ cm−2 using a solar simulator (290––800 nm; Suntest® CPS, Atlas, Illinois, USA). Non-irradiated control cells were covered with UV-impermeable plates during irradiation in the solar simulator. Metabolic activity was determined 24 h after irradiation using resazurin solution (see above). Apoptosis was analyzed 14 h after irradiation using the Caspase-Glo®-3/-7 assay (Promega, Mannheim, Germany). Cleavage activity of caspase-3 and −7 was determined according to manufacturer’s instructions.
Glutathione Levels
Glutathione (GSH) levels in HHFKs were measured 72 h after MGM or IC incubation using a GSH assay kit (Fluorometric, Sigma-Aldrich®, Munich, Germany), which allows the determination of intracellular GSH. After a wash step using PBS, treated HHFKs were incubated with the detection solution (1:40 dilution of the substrate in assay buffer) for 40 min at room temperature. Subsequently, the detection solution was removed and replaced by MGM. Fluorescence images were taken by an inverted fluorescence microscope (Axiovert 200M, Zeiss, Jena, Germany) using the filter set 49 (Zeiss, G365, FT 395, BP445/50). Total cell fluorescence was determined by using the ImageJ software.
Data Analysis
Mean values of relative fluorescence/luminescence units and standard deviation (SD) of replicates were calculated using Gene5 software (Biotek®; Bad Friedrichshall, Germany). Statistical significance was assessed with Student’s t-test for independent groups.
Results
Phenotypic Characterization of HHFKs in a Minimal Growth System
An established in vitro growth-limiting system, which mimics the reduced activity of keratinocytes associated with diminished hair-growth,10 was employed to investigate the influence of hair-growth-promoting combinations on cellular processes, aiming to elucidate the compound effects at a molecular level. The minimal growth medium (MGM) in this culture system omits all compounds provided by a marketed hair-growth formulation. As in vitro correlate (IC) of this oral hair-growth formulation a combination of L-cystine, thiamine, calcium D-pantothenate, and folic acid (a presumed metabolite of PABA)9 was supplemented to MGM thus providing a system to analyze compound effects in an in vitro cell model. In this study, we choose to determine compound affected cellular processes in the hair follicle-derived keratinocytes, the HHFKs.
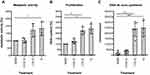
Initially, we performed a phenotypic characterization of cultured cells by analyzing metabolic activity, proliferation, and de novo DNA synthesis of HHFKs after treatment with IC. When compared to HHFKs cultivated in MGM it was found that cultivation of HHFKs in IC promoted metabolic activity (Figure 1A), DNA content (Figure 1B), and BrdU incorporation (Figure 1C) in a concentration-dependent manner. The treatment with the tested compounds caused a 1.4–2.3-fold increase in metabolic activity, a 1.9–2.9-fold increase in DNA content, and a 3.6–41.0-fold increase in de novo DNA synthesis (Figure 1). The enhanced proliferative and metabolic activities reflect a robust characteristic of the IC treatment and confirm that the use of the chosen in vitro model system is useful to show significant compound-dependent effects on keratinocytes derived from hair follicles.
Impact of IC on Gene Expression
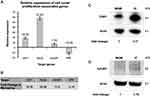
In order to elucidate the cellular processes that may underlie the effects of IC on HHFKs on a molecular level, we conducted a whole-genome gene expression study by employing an Agilent whole human genome oligo microarray. The microarray data revealed differential expression of 1278 reporters, of which 707 were induced and 571 were repressed after IC treatment. Moreover, 153 of the induced and 83 of the suppressed reporters revealed a more than 10-fold difference in expression (Figure 2A). Subsequently, IC-regulated reporters were then clustered into functional groups to identify biological processes and signaling pathways that are affected by the IC compounds. This functional grouping revealed that the majority of differentially expressed genes are associated with cell proliferation, cell viability and cell stress (Figure 2B). The regulation of the functional group associated with proliferation and cell cycle agrees well with the observed increased cell proliferation caused by IC treatment (Figure 1).
Cell Proliferation Enhancing Effect of IC
Since the functional grouping of differentially expressed genes supports our initial finding that IC has an influence on the proliferation/cell cycle of HHFKs, we additionally validated our expression data using selected genes coding for proliferation-promoting (cyclin-dependent kinase 1 (CDK1),11 Holliday junction recognition protein (HJURP),12 centromere protein A (CENPA))13 and proliferation-inhibiting proteins (Kruppel-like factor 4 (KLF4))14.
We confirmed the enhanced expression of cdk1 (18.36-fold), HJURP (48.88-fold) and cenpA (14.8-fold) after IC treatment, which was demonstrated in the gene expression study, by qPCR analysis (Figure 3A and B). In contrast to these examples of induced genes, the gene coding for KLF4, a negative regulator of the cell cycle,14 showed a decrease in expression in the presence of IC (Figure 3A and B). Additional immunoblot analysis of CDK1 and HJURP confirms that the increase in mRNA levels after IC treatment correlates with enhanced protein levels (Figure 3C, D and Supplementary Figure 2). In summary, our data show that IC treatment has a positive impact on proliferation and cell cycle of hair follicle-derived keratinocytes.
Positive Influence of IC on Cell Viability
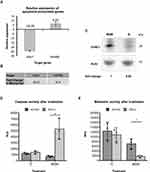
Besides the regulation of proliferation, the functional grouping also revealed an influence of IC on the gene group ‘cell death’. For confirmation of this finding, the differentially expressed genes chac1 and tnfrsf6b detected in the microarray analysis were selected because of their known regulatory roles in programmed cell death (apoptosis). While the pro-apoptotic protein cation transport regulator-like protein 1 (CHAC1) can modulate the AIF-PARP apoptotic pathway,15 tumor necrosis factor receptor superfamily member 6B (TNFRSF6B) is an anti-apoptotic protein.16 In comparison to HHFKs treated with MGM alone, the microarray analysis revealed a higher expression of tnfrsf6b and a lower expression of chac1 in IC-treated HHFKs, which was confirmed by qPCR (Figure 4A and B). Immunoblot analysis demonstrated that the differential expression of chac1 is reflected on the protein level as well (Figure 4C and Supplementary Figure 3). Together these data underline the positive role of the IC compounds in improving the general cell viability at different levels (promotion of proliferation and prevention of programmed cell death).
We further investigated the beneficial effect of the IC compounds on cell viability by challenging the HHFKs with solar-simulated UV-radiation, thus providing a ubiquitous, environmental stress factor to the cells. The irradiation-induced apoptosis of MGM- and IC-treated HHFKs was quantified by determining caspase-3/-7 activity. Based on preliminary tests showing a high sensitivity of HHFKs to solar-simulated UV-radiation (data not shown) a dose of 25 mJ solar simulated UV was chosen for following irradiation tests. Measuring caspase-3/-7 activities 14 h after irradiation revealed a significant induction of UV-induced apoptosis in MGM-cultivated HHFKs, but not in IC-cultivated HHFKs (Figure 4D). The protective effect of IC was also reflected in the smaller post-irradiation decrease in metabolic activity in cells treated with IC compared with those cultured in MGM alone (Figure 4E). While the metabolic activity of IC-cultivated HHFKs was reduced after UV-irradiation by only around 12 ± 4%, MGM-cultivated HHFKs showed an average metabolic activity reduction of 75 ± 2% after UV-irradiation.
Higher Protection Against Oxidative Stress After Treatment with IC
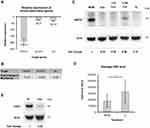
The gene expression study also revealed the regulation of genes associated with the ‘response to oxidative stress’, which displays another gene group being crucial for cell protection. In general, several genes encoding anti-oxidative proteins can be induced in response to oxidative stress. Among others, hmox1, which codes for heme oxygenase 1 (HMOX1), is an example of such inducible genes and represents one mechanism in eukaryotic cell to increase their anti-oxidative capacity.17 The microarray and qPCR analysis revealed a strong induction of hmox1 expression in HHFKs only cultured in MGM compared with IC-treated HHFKs. Consequently, these data demonstrate that the IC compounds are linked to a prevention of hmox1 induction (Figure 5A and B).
We screened the microarray data for further candidates being potentially involved in cell protection and identified the regulation of the genes encoding for the L-cystine transporter SLC7A11 and of the enzyme cystathionase (CST), which converts cystathionine to L-cystine and L-homoserine.18 Due to their function both genes are contributing to the regulation of intracellular L-cystine levels. Interestingly, the expression of slc7a11 is again associated with oxidative stress, which is in this particular situation caused by L-cystine deficiency resulting in an intracellular glutathione (GSH) depletion.19 The significant reduced expression of slc7a11 (14.93-fold reduction) and cst (15.89-fold reduction) in IC-treated HHFKs compared with MGM-treated HHFKs observed in the whole-genome gene expression study was consistent with the differential expression detected by qPCR analysis (Figure 5A and B).
Since modulation of slc7a11 expression has already been correlated to oxidative stress caused by L-cystine deficiency, we decided to investigate how the compound L-cystine contributes to protection against oxidative stress in our system. To analyze this presumed influence of L-cystine on oxidative stress, we chose the determination of HMOX1 protein levels in HHFKs cultivated in MGM, IC or with L-cystine alone. While HMOX1 was undetectable in IC-treated HHFKs, HMOX1 was clearly present in MGM-treated HHFKs (Figure 5C and Supplementary Figure 4). Interestingly, the treatment of HHFKs with L-cystine alone suppressed HMOX1 expression comparable to the IC treatment (Figure 5C and Supplementary Figure 4). Moreover, serial dilution of L-cystine (10-fold, 100-fold) showed that suppression of HMOX1-expression by L-cystine was concentration dependent (Figure 5C and Supplementary Figure 4).
Since we found that genes associated with oxidative stress are induced in the absence of the IC compounds, we wanted to provide further evidence of a protective potential of the IC compounds by determining the direct effect of IC on an intrinsic anti-oxidant system like GSH, a major anti-oxidant. GSH depletion is known to be associated with oxidative stress,20 thus cellular GSH-levels provide an additional marker of this process of endogenous stress. Additionally, there is a published positive correlation between L-cystine availability and GSH levels.21,22 In this study, we measured intracellular GSH levels indirectly using a fluorescence-based assay after culturing HHFKs only with MGM and after IC supplementation. The measured fluorescence of IC-treated HHFKs displayed a broader distribution and show higher GSH levels on average (Figure 5D and Supplementary Figure 1). Mean levels of GSH in HHFKs only cultured in MGM were reduced by 45% compared to those treated with IC (Figure 5D).
Since the data obtained so far indicate a pivotal role of L-cystine in the protection against oxidative stress, we additionally tested to what extent L-cystine alone contributes to the beneficial effects of IC on the proliferation by using CDK1 as a proliferation marker. Immunoblot analysis showed that CDK1 expression was lower in HHFKs, which were only cultured with L-cystine, compared to HHFKs, which were cultured with all compounds of IC (Figure 5E and Supplementary Figure 4). However, CDK1 expression was higher in L-cystine-treated HHFKs than in HHFKs treated with MGM alone indicating a positive influence of L-cystine on proliferation (data not shown).
Discussion
Several oral hair-growth combinations are available on the market and though they vary slightly in their overall composition, most of them contain L-cystine. In this study, the impact of a hair-growth-promoting, L-cystine-containing combination on cellular processes was investigated by employing a growth limiting in vitro system, which was published recently.10 The selected core compounds of the hair-growth-promoting combination (Panto[vi]gar®) L-cystine, thiamine, calcium D-pantothenate, and folic acid (as an assumed metabolite of PABA) were the main focus of this work. While in the study of Hengl et al the minimal growth system was used with normal human epidermal keratinocytes (NHEKs),10 we evaluated the compound effects using HHFKs, which represent a mixed population of keratinocytes derived from the hair follicle, aiming to use a simplified in vitro model, which is closer to the hair follicle physiology. Applying growth-limiting conditions to these cells allowed us to test the impact of the hair-growth-promoting combination and obtain hints on basic cellular processes that might be involved in improving hair-growth.
Consistent with the NHEK-based study of Hengl et al,10 measuring metabolic activity and proliferation of HHFKs cultivated in the minimal growth system revealed reduced cell activity. In the clinics, it is believed that diminished hair-growth is correlated to a reduced activity of hair follicle cells. The addition of the four selected core compounds to MGM led to a restoration of cell activity, indicated by an increase in metabolic activity, proliferation, and DNA synthesis demonstrating that the tested compounds, in general, have a positive influence on the physiologic activity of human keratinocytes (Figure 1).
In the present study, our whole-genome gene expression study provided further insights into the underlying molecular mechanisms by showing that tested core compounds are predominantly affecting cell cycle-, cell death-, and oxidative stress-associated gene groups in HHFKs (Figure 2). As judged by induced expression of growth-promoting and repressed expression of growth-inhibiting genes, the finding is supported that the tested compounds have a positive influence on proliferation and viability of HHFKs (Figures 3 and 4). Proliferation plays, in general, a pivotal role in hair-growth. During the anagen phase of the hair cycle, the hair shaft elongates as a result of the high proliferative and differentiating hair matrix keratinocytes.3 Measurement of the cell proliferation kinetics of hair matrix cells revealed a maximum turnover rate that confirmed very high proliferative activity as a characteristic of these cells.23 Moreover, impaired hair-growth is associated with premature initiation of the catagen phase as well as with reduced proliferative activity of hair matrix cells. Functional loss of proteins (eg P-cadherin), which results in a significant inhibition of proliferation and transition into the catagen phase,24 manifests clinically as sparse, short hair.25 Because the tested compounds have a positive impact on the proliferation of cells from hair follicles, this underscores their ability to support a cellular process that is in general crucial during hair-growth.
In addition with our study, we provide evidence of the beneficial effects of the compounds under stress-related (endogenous cellular and environmental stress) conditions. UV-radiation is a crucial exogenous stressor for hair and scalp, since they are exposed to solar UVR on a daily basis. Irradiation of human anagen hair bulbs results in a premature transition into the catagen phase, reduced hair shaft elongation, and decreased proliferation of hair matrix keratinocytes.26 Moreover, there are case reports where high levels of UV rays caused telogen effluvium.27 Interestingly, our study revealed that IC-treated HHFKs are more resistant against UV-radiation-induced apoptosis as those cultured in MGM alone (Figure 4). In contrast to the NHEK-based study by Hengl et al,10 we found that the required UV-dose for inducing apoptosis in HHFKs was significantly lower (25mJ vs 200 mJ). A higher sensitivity of HHFKs to UV-radiation compared to human skin keratinocytes has already been reported in a study that examined the response of human hair follicles to radiation.26 Thus the higher general sensitivity towards UV-irradiation of our HHFKs underscores their origin from the hair follicle.
In addition to the enhanced resistance against exogenous stress factors, we identified a protective potential of the tested compounds also against endogenous oxidative stress. Besides the known inducible expression of the anti-oxidative gene hmox1 during oxidative stress,28–30 HMOX1 is associated with increased cell protection against stress-inducing drugs.31 In this study, the growth-limiting conditions alone appear to result in oxidative stress in the cells as judged by the induced hmox1 expression and the lower intracellular GSH levels that we have detected (Figure 5). In contrast, addition of IC prevented this stress-induced HMOX1 expression. Of the compounds under investigation, we found that L-cystine alone caused a concentration-dependent repression of HMOX1 expression demonstrating the crucial contribution of L-cystine to protection against oxidative stress. As already mentioned, treatment with the IC compounds also correlated with higher intracellular GSH levels compared with HHFKs cultivated in MGM alone (Figure 5). It is well known that L-cystine availability is a factor that maintains higher intracellular GSH levels,21,22 thereby supporting cell survival. Interestingly, Lautier et al showed that a 40% decrease in GSH led to a doubling of hmox1 expression, which then remained constant despite a further decrease in GSH levels.32 The described correlation between GSH-levels and HMOX1 expression is in agreement with observations from our study where in MGM-cultivated HHFKs GSH-levels were clearly reduced while in parallel there was a strong induction of hmox1 gene expression.
While our study demonstrated a central role of L-cystine in the protection against oxidative stress, the data on CDK1 protein levels revealed that the observed positive effect on proliferation is not only based on L-cystine alone, but rather on the combination of the four compounds (Figure 5). The previously published study by Hengl et al, has shown that L-cystine and thiamine are the most important protagonists affecting proliferation, while calcium D-pantothenate and folic acid play a subordinate role.10 A potential area for future studies would, therefore, be to investigate the influence of the single compounds in order to identify further processes, which might be beneficial to support hair-growth. In addition to identifying cellular processes, which are affected by the compounds, it would be also interesting to determine their effects in 3D-models with hair follicle derived keratinocytes and dermal papilla cells. Such 3D-models could take into account cell interaction- and differentiation processes and thus represent an in vitro model that is closer to the hair follicle as mini-organ. Previous approaches to establish 3D models are ranging from the generation of collagen spheroids with derma papilla cells and keratinocytes33 towards the usage of engineered scaffolds in which derma papilla cells and keratinocytes are grown in spatially defined domains.34 By using such models the in vivo situation can be better mimicked and it might be possible to determine compound effects on more specific aspects of hair-growth.
Conclusion
The tested compounds of a hair-growth promoting formulation have a positive effect on cell proliferation, cell viability, and cell protection of hair follicle associated cells (HHFKs) and thus support fundamental cellular processes that are believed to be relevant for healthy hair-growth. Our study elucidates a pivotal role of the compound L-cystine in the protection against oxidative stress as demonstrated by the impact on an anti-oxidative protein HMOX1, whose expression is repressed in the presence of L-cystine. In case of proliferation, it was shown that the combination of all compounds from IC is necessary for a maximal stimulating effect.
Acknowledgments
Chris Parsons is acknowledged for critical reading of the manuscript. Editing services were provided by Scientific Communications & Information, Oxford, UK, and funded by Merz Pharmaceuticals GmbH. Microarray services were performed by Bernhard Gerstmayer and bioinformatics analyses were performed by Jutta Kollet with support from Michail Knauel (all from Miltenyi Biotec, GmbH).
Disclosure
Thomas Hengl, Kim Schlinzig, and Harry F Abts report being employees of Merz Pharmaceuticals GmbH during the conduct of the study and at present. Harry F Abts reports a ceased patent : EP2677026A1. Kristina Riegel worked temporarily at Merz Pharmaceuticals GmbH as a student (internship; masters-thesis) and is currently an employee of University Medical Center Mainz. Saskia Krischok worked temporarily at Merz Pharmaceuticals GmbH as a student (internship) and is currently an employee of co.faktor GmbH. The authors report no other possible conflicts of interest in this work.
References
1. Van Neste D, Leroy T, Conil S. Exogen hair characterization in human scalp. Skin Res Technol. 2007;13(4):436–443. doi:10.1111/j.1600-0846.2007.00248.x
2. Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–1337. doi:10.1016/0092-8674(90)90696-C
3. Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132142. doi:10.1016/j.cub.2008.12.005
4. Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126(7):1459–1468. doi:10.1038/sj.jid.5700376
5. Headington JT. Telogen effluvium. New concepts and review. Arch Dermatol. 1993;129(3):356–363. doi:10.1001/archderm.1993.01680240096017
6. Kligman AM. Pathologic dynamics of human hair loss. I. Telogen effluvium. Arch Dermatol. 1961;83:175–198. doi:10.1001/archderm.1961.01580080005001
7. Dawber R, Simpson N, Barth J. Diffuse Alopecia: Endocrine, Metabolic and Chemical Influences on the Follicular Cycle. Dawber RPR, ed. Oxford: Blackwell Science; 1997.
8. Finner AM. Significant improvement of diffuse telogen effluvium with an oral fixed combination therapy a meta-analysis. Int J Trichology. 2011;3(S1):S35S50.
9. Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011;3(1):118–134. doi:10.3390/nu3010118
10. Hengl T, Herfert J, Soliman A, Schlinzig K, Trueb RM, Abts HF. Cystine-thiamin-containing hair-growth formulation modulates the response to UV radiation in an in vitro model for growth-limiting conditions of human keratinocytes. J Photochem Photobiol B. 2018;189:318–325. doi:10.1016/j.jphotobiol.2018.09.005
11. Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185(2):193–202. doi:10.1083/jcb.200812045
12. Foltz DR, Jansen LE, Bailey AO, et al. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137(3):472–484. doi:10.1016/j.cell.2009.02.039
13. Palmer DK, O’Day K, Wener MH, Andrews BS, Margolis RL. A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol. 1987;104(4):805–815. doi:10.1083/jcb.104.4.805
14. Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271(33):20009–20017. doi:10.1074/jbc.271.33.20009
15. Mungrue IN, Pagnon J, Kohannim O, Gargalovic PS, Lusis AJ. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J Immunol. 2009;182(1):466–476. doi:10.4049/jimmunol.182.1.466
16. Pitti RM, Marsters SA, Lawrence DA, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396(6712):699–703. doi:10.1038/25387
17. Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86(1):99–103. doi:10.1073/pnas.86.1.99
18. Fowler B. Transsulphuration and methylation of homocysteine in control and mutant human fibroblasts. Biochim Biophys Acta. 1982;721(2):201–207. doi:10.1016/0167-4889(82)90069-6
19. Bannai S, Kitamura E. Adaptive enhancement of cystine and glutamate uptake in human diploid fibroblasts in culture. Biochim Biophys Acta. 1982;721(1):1–10. doi:10.1016/0167-4889(82)90017-9
20. Chai YC, Ashraf SS, Rokutan K, Johnston RB
21. Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257(4 Pt 1):L163173. doi:10.1152/ajplung.1989.257.4.L163
22. Wang ST, Chen HW, Sheen LY, Lii CK. Methionine and cysteine affect glutathione level, glutathione-related enzyme activities and the expression of glutathione S-transferase isozymes in rat hepatocytes. J Nutr. 1997;127(11):2135–2141. doi:10.1093/jn/127.11.2135
23. Weinstein GD, Mooney KM. Cell proliferation kinetics in the human hair root. J Invest Dermatol. 1980;74(1):43–46. doi:10.1111/1523-1747.ep12514601
24. Samuelov L, Sprecher E, Tsuruta D, Biro T, Kloepper JE, Paus R. P-cadherin regulates human hair growth and cycling via canonical Wnt signaling and transforming growth factor-beta2. J Invest Dermatol. 2012;132(10):2332–2341. doi:10.1038/jid.2012.171
25. Sprecher E, Bergman R, Richard G, et al. Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nat Genet. 2001;29(2):134–136. doi:10.1038/ng716
26. Lu Z, Fischer TW, Hasse S, et al. Profiling the response of human hair follicles to ultraviolet radiation. J Invest Dermatol. 2009;129(7):1790–1804. doi:10.1038/jid.2008.418
27. Camacho F, Moreno JC, Garcia-Hernandez MJ. Telogen alopecia from UV rays. Arch Dermatol. 1996;132(11):1398–1399. doi:10.1001/archderm.1996.03890350142037
28. Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275(21):16023–16029. doi:10.1074/jbc.275.21.16023
29. Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278(14):12029–12038. doi:10.1074/jbc.M211558200
30. Reichard JF, Sartor MA, Puga A. BACH1 is a specific repressor of HMOX1 that is inactivated by arsenite. J Biol Chem. 2008;283(33):22363–22370. doi:10.1074/jbc.M801784200
31. Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94(20):10925–10930. doi:10.1073/pnas.94.20.10925
32. Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992;13(2):227–232. doi:10.1093/carcin/13.2.227
33. Havlickova B, Biro T, Mescalchin A, et al. A human folliculoid microsphere assay for exploring epithelial- mesenchymal interactions in the human hair follicle. J Invest Dermatol. 2009;129(4):972–983. doi:10.1038/jid.2008.315
34. Lim TC, Leong MF, Lu H, et al. Follicular dermal papilla structures by organization of epithelial and mesenchymal cells in interfacial polyelectrolyte complex fibers. Biomaterials. 2013;34(29):7064–7072. doi:10.1016/j.biomaterials.2013.05.068
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.