Back to Journals » Infection and Drug Resistance » Volume 15
Knowledge of Malaria and Its Uncomplicated Treatment with Argemone mexicana L. in Selected Districts of Jimma Zone, Oromia Regional State, Ethiopia: A Community-Based Cross Sectional Survey
Authors Tekassa T, Hasen G , Merga H , Cavin AL, Graz B, Suleman S
Received 22 March 2022
Accepted for publication 10 June 2022
Published 16 June 2022 Volume 2022:15 Pages 3087—3095
DOI https://doi.org/10.2147/IDR.S367524
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Tamirat Tekassa,1 Gemmechu Hasen,1 Hailu Merga,2 Anne-Laure Cavin,3 Bertrand Graz,3 Sultan Suleman1
1School of Pharmacy, Institute of Health, Jimma University, Jimma, Oromia Regional State, Ethiopia; 2Department of Epidemiology, Institute of Health, Jimma University, Jimma, Oromia Regional State, Ethiopia; 3Medicines Unit, Antenna Foundation, Geneva, Switzerland
Correspondence: Gemmechu Hasen; Tamirat Tekassa, Email [email protected]; [email protected]
Background: With the problems of increasing levels of drug resistance and difficulties to afford and access effective antimalarial drugs in poor and remote areas, herbal medicines could be an important and sustainable source of treatment. Argemone mexicana L. (AM) is a medicinal plant known long ago in several countries for treatment of numerous diseases including malaria. The aim of this study was to conduct a survey on the use of AM in the prevention and treatment of uncomplicated malaria in selected districts of Jimma Zone, Oromia Regional state, Ethiopia.
Methods: A community-based cross-sectional study was conducted in two selected districts in Jimma Zone, southwest Ethiopia. In total, 552 participants from 17 kebeles (villages/communities) and 18 traditional healers of the districts were interviewed. Data collection was conducted from April 27 to May 18, 2020 using pre-tested structured questionnaires. The data were analyzed using Epi Info 7.0 and the descriptive statistics were used to summarize the results.
Results: The study indicated that AM is available, known by 39.8% of the respondents and used for prevention and treatment of malaria by 5.7% of the population. All traditional healers interviewed knew the plant, and 44.4% use it for treatment of malaria. In addition, AM is especially used to treat malaria, amoebiasis, diarrhea, cough, and tuberculosis.
Conclusion: The availability and use of AM to treat malaria was verified in both community and traditional healers. AM, which was found effective as antimalarial plant in high Plasmodium falciparum endemicity in Mali, is also well known and accepted in these areas of Ethiopia for the treatment of malaria. Further research is needed to assess wether AM is also effective against malaria in Ethiopia where P. vivax and P. falciparum coexist.
Keywords: Argemone mexicana L., traditional medicine, antimalarials, Malaria
Corrigendum for this paper has been published
Introduction
Malaria is still a disease of public health concern in developing countries. It is estimated that there were over 200 million cases and over 4,50,000 related deaths in 2019 alone. Of all malaria cases reported in 2019, around 94% were in sub-Saharan African countries.1 In Africa, pregnant women and children are the most commonly affected segment of the population.2 In Ethiopia, more than 68% of the people live in areas are at risk of malaria transmission, primarily at altitudes below 2000m.3 Ethiopia is afflicted by two types of malaria species: Plasmodium falciparum (60%) and Plasmodium vivax (40%).3–5 More than 80% of the population living in rural areas of Ethiopia primarily use traditional medicine to treat their ailments.5 Studies show that Ethiopia has about 200 medicinal plants that have been in use against malaria across various parts of the country, and a higher number of these medicinal plants are found in the western and southwestern parts of the country.5–8
Traditional herbal medicines are the source of two main groups of modern anti-malarial medicines: artemisinin and quinine derivatives. With the problems of increasing levels of drug resistance and difficulties to afford and access effective antimalarial drugs in poor areas, traditional herbal medicines could be an important and sustainable source of treatment.9 AM, a pantropical weed originating from Mexico, belongs to the Papaveraceae family with a long history of use in traditional medicine.10,11 The plant has been used in Indian folk medicine to treat a wide variety of diseases including malaria.12 It is also being used in several African countries as an antimalarial agent.13 A population survey conducted in 2005 in Mali found that 87% of uncomplicated episodes of malaria were first treated at home with either traditional or modern medicines, or a combination of both. The best outcome was associated with the use of AM decoction, which is a traditional recipe for curative and preventive treatment in the villages.14 After the population survey, AM was selected as the most promising treatment. It was further assessed in a prospective, dose-escalating clinical trial. The remedy was prescribed in three regimens in order to determine the optimal dose. At D14, the proportion of adequate clinical response (ACR) was the highest (73%) with the group taking decoction twice daily for 7 days. Another observation was that the overall proportions of ACR were lower in children aged <1 year (45%) and higher in patients aged >5 years (81%). Very few patients had complete parasite clearance, but 67% of the patients with ACR had a parasitaemia <2000/l.15
A second clinical trial was conducted in Mali in 301 patients with presumed uncomplicated malaria with AM decoction versus ACT (Artemisinin Combination Therapy) standard treatment (artesunate-amodiaquine). Both treatments were well tolerated. Over 28 days, second-line treatment was not required for 89% of the patients on AM, versus 95% on ACT. Deterioration to severe malaria was 1.9% in both groups in children aged ≤5 years and 0% had coma/convulsions. The 3-month follow-up showed that the residual parasitemia at D28 was not associated with a subsequent malaria episode and/or anemia.14 There was thus no statistical difference in efficacy between ACT and AM, and the latter is now government-approved in Mali, in order to improve access to validated antimalarials and also to reduce the drug pressure for development of resistance to ACT.16 In Ethiopia, AM is locally known as korehare17; Yahyaeshoh5 and Kolcolich.8,18 It is mainly reported to grow in the northern and southern parts of the country.5,7,18,19
In some districts of Amhara Regional State, the plant is mainly recognized as an invasive alien weed.18,20 However, in those areas, the plant leaf is also traditionally used to treat wounds19,21 and rabies.22 Furthermore, in southern Ethiopia, in the Sidama Region, decoction of AM roots is used to treat malaria.6,8 The plant is also well known in Bale, Oromia Regional State,17 but, to date the literature does not have any ethno-botanical report of AM in the Jimma area. This paper aims to present a preliminary survey study on the use of AM in the prevention and treatment of uncomplicated malaria in selected districts of Jimma Zone, Oromia Regional state, Ethiopia.
Methods and Materials
Study Area and Source Population
The Jimma Zone consists of 20 rural districts that belong to Oromia Regional State in southwestern Ethiopia with its capital, Jimma town. It is located 357 km southwest of Addis Ababa. The zone generally lies between 1000 and 3500 m above sea level. The annual rainfall is between 1300 mm and 2100 mm.7,23 This cross-sectional survey was conducted in two selected districts of Jimma Zone. The districts were Shebe-sombo and Seka-chekorsa. Shebe-sombo is located 50 km away from Jimma town. The district has 21 kebeles (the smallest administrative unit in Ethiopia) and has an estimated population of 159,110 (unpublished, Shebe-Sombo district office, 2020). Seka-Chekorsa is located 10 km from Jimma town. The district has 36 kebeles and a population of 280,741 (unpublished, Seka Chekorsa district office, 2020).
Sample Size
The required sample size of the survey was estimated by a single proportion formula using the following assumptions: malaria prevalence (P=20.7%) from a recently published study in the nearby Asendabo in Jimma Zone, southwest Ethiopia,24 5% margin of error and 95% confidence interval. Finally, after considering 10% of non-response rate and design effect of 2, the final sample size became 554.
Sampling Procedures
The total sample size was allocated to kebeles selected from the two districts. A total of 17 kebeles (more than 30%) were selected using simple random sampling from each district. Then, specific households were selected using systematic random sampling technique by using house numbers from selected kebeles. Mothers and/or caretakers and/or members of the household whose age was greater than 18 years were interviewed every 17th house after selection of the first house by lottery method. To support the quantitative findings, 18 traditional healers or knowledgeable persons found in the community were also interviewed using the snowball technique.
Data Collection
Data collection was conducted from April 27 to May 18, 2020 using validated structured questionnaires, and participants were interviewed in Afaan Oromo and Amharic languages. Answers were recorded on paper, and then entered into Epi Info software.
Data Quality Assurance and Analysis
During data entry, to ensure accuracy, data were coded and fed into Epi Info 7.0 (CDC, Atlanta, USA) by two individuals, and the data entered was double-checked for its validity, accuracy and completeness. Finally, data analysis were done using Epi info 7.0 and presented with tables, frequency, and percentages.
Results
Characteristics of Study Participants
A total of 554 participants have been interviewed in this survey, but 552 of them were included in the final analysis as two participants interrupted the interview. Participants of the study were either a mother or a caretaker from a given household. Mean age was 37.5 years, and most of participants were females (Table 1). Eighteen traditional healers and knowledgeable persons in the villages were also interviewed. Malaria is locally known as abusa, busa, and sakera (in the Afaan Oromo language) or woba (in the Amharic language) in the districts selected for this specific study.
 |
Table 1 Characteristics of Respondents in Selected Districts of Jimma Zone, from April 27 to May 18, 2020 |
The most common symptoms cited for malaria were high-grade fever 552 (100%), headache 545 (98.7%), shivering 550 (99.6%), loss of appetite 547 (99.1%), vomiting 476 (86.2%) and diarrhea (47.6%). Traditional healers cited the same symptoms at very similar percentage: high-grade fever (100%), headache (100%), shivering (100%), loss of appetite (94.4%), vomiting (88.9%), diarrhea (55.6%), and fatigue (62.5%). The characteristics, mode of treatment, and outcomes of patients who had history of malaria in the past 3 months are presented in Table 2. Among participants who had malaria episode, all went to health facility and received modern medicines, while 4.3% also used home remedies in addition to modern treatment. Papaya and Amygdalina were mentioned by one patient as traditional herb. Prayer or other actions were not mentioned as a mode of treatment. Results about malaria prevention methods are presented in Table 3. Almost all study participants (99.1%, n=547) believed that malaria was a preventable disease. The prevention measures used are insecticide treated bed nets (ITN, 99.3%), indoor residual spraying (IRS, 99.6%), home treatments (35.5%) and smoke (30.6%) (Figure 1). Amongst the home treatments, 98.9% were medicinal plants used while one participant witnessed his use of coffee slaughter – it is a traditional ceremony held using coffee when an individual is infected with malaria. After the ceremony in the study districts, people believe that an infected person would get healed. After public health malaria prevention measures, the large majority of participants (98.9%) reported that malaria episodes were less frequent in their area. Participants also reported that they had not observed significant problems associated with those prevention measures, but one of them complained that following measures like indoor residual spraying, for example, had observed increased flies in unusual rate in the house. All the traditional healers beleived that malaria is a preventable disease, and 94.4% use ITN and IRS, most of them (77.8%) additionally used home treatments or smoke (55.6%). Among those who undertook home treatments, 92.8% used medicinal plants. All the traditional healers interviewed perceived that malaria is less frequent while applying those prevention methods and none found any new problem following their uses. Among participants, 509 (92.2%) beleived that it is also possible to prevent malaria episodes during pregnancy. Of the 315 women who responded to the questionnaire, 5 of them only reported using antimalarials prophylaxis during their pregnancy.
 |
Table 2 Characteristics, Mode of Treatment, and Outcome of Respondents Who Had History of Malaria in the Past 3 Months in Two Selected Districts of Jimma Zone from April 27 to May 18, 2020 |
 |
Table 3 Respondents Understanding About Method of Prevention and Diagnosis of Malaria in Two Selected Districts of Jimma Zone from April 27 to May 18, 2020 |
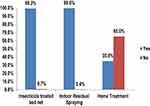 |
Figure 1 Method of malaria preventions used by respondents of Jimma Zone from April 27 to May 18, 2020. |
Malaria Diagnosis
In this study, malaria diagnosis was based on symptoms (98.8%), microscopy (98%), and RDTs (18.5%), respectively. Similarly, traditional healers reported that they too or members of their families also went to the Health Centers. Amongst the traditional healers or their families, nine (100%) of the respondents who claimed that they suspected malaria were tested by microscopy, two respondents (2.2%) by RDT, and eight respondents (88.9%) were based on symptoms when they or their family had malaria episode.
Knowledge and Practice Related to AM
About 40% (219/551) of participants recognized the plant either fresh or on picture. Out of this sample, 37.5% did not know its name. Some knew it as white thorn (“Kore adi” or “asangira adi”), hyena thorn (“kore maja”), or medicine for amoebiasis (“Qoricha didda”) or medicine for evil eye (“Qoricha nyaattuu”) or “Aksir” in Arabic. Almost all of the traditional healers (n = 17/18) knew AM, and they name it. Among the participants, 31/552 (5.7%) have already used AM to treat a disease for themselves or for others. Among the traditional healers, 16/17 (94.1%) reported that they used it or saw somebody using it for its medicinal value in their community, as in Table 4. Participants using AM reported that they got the plant either from nearby street (17/19), their own garden (5/21) or from herbalists (4/21) in their community. It is not a plant sold in the market. Similar frequencies were given by traditional healers: nearby street (n=7/16), from own garden (n=4/16) and from herbalist (n=4/16). One traditional healer mentioned that he collected the plant from another region (Asosa). Amongst the six participants who used AM in the treatment of malaria, five used the root while one used the aerial part. Five use it fresh and one dried while three of them use it in a powdered form. Five participants also boil it with water while one let it soak in cold water. All participants using the plant for treatment of malaria recommended the daily dose of one cup of coffee (about 60mL) once per day. The duration of treatment of malaria according to study participants was 3–10 days. Of the nine respondents, all would volunteer to be part of a future study if there is any study on AM in the prevention or treatment of malaria. Eight traditional healers (TH) who reportedly used AM to treat or prevent malaria, all mainly use the roots. Leaves, stem, whole plant and whole aerial part were also used by one of the TH. The plant is used fresh or dry and reduced into powder. TH, all boil it with water except for a single TH who prepared a cold maceration. They all recommended drinking a unit dose, a cup of coffee. Five of the TH recommended taking a single dose per day while three of the TH recommended taking a cup three times per day. The duration of the treatment recommended (3–10 days) is not dependent on the daily dosage. One traditional healer reported nausea, vomiting, and diarrhea as new symptoms occurring after the use of AM, while the others did not mention any new symptom. One traditional healer recommended drinking milk as a precaution while AM is used. One traditional healer prescribed AM as an adjuvant of modern malaria treatment, and 12/12 traditional healers said they would be pleased to take part in the future research that may involve AM, while the answers of the six others were missing.
 |
Table 4 Medical Uses of A. mexicana in Selected Districts of Jimma Zone from April 27 to May 18, 2020 |
Discussion
Malaria used to be known locally as abusa/busa/sakera among Oromo people or as woba among Amharic-speaking inhabitants in the districts selected. In this survey, almost all participants could articulate common malaria signs and symptoms like high-grade fever, headache, shivering, loss of appetite and vomiting. In a similar study in Uganda, respondents reported the same clinical presentations of malaria.25
In the current study, 99.1% of the participants stated that malaria can be prevented. Moreover, the majority of participants (92.2%) and traditional healers (77.8%) believed that malaria prevention is possible during pregnancy, either with traditional medicines or with modern treatment. In particular, malaria prevention in pregnant women is done via the provision of a package through antenatal care, in order to reduce maternal and fetal malaria episodes.2,26
Moreover, antenatal care service and use of other prevention techniques like insecticide-treated bed nets (ITN) are well practiced according to national set targets in the study area:27 According to Ethiopian Health Sector Plan of 2020; 90% goal was set for ITN and 95% for ANC for malaria prevention and in Jimma, the coverage reached was comparable to the one planned. All participants used several methods for malaria prevention: the one recommended by the authorities like insecticide treated bed nets and indoor residual sprayings (IDS), but also home treatments and smoke. These strategic prevention measures are mainly practiced in study districts and are linked to the current declining pattern of malaria prevalence in the Jimma area.
Reports indicated that declining malaria positivity rate might be due to intensive prevention interventions and raising awareness in the last decades.23,27
Malaria diagnosis techniques reported in this study are in line with the national malaria diagnosis guideline.4 Specificity of clinical diagnosis of malaria depends on the age group, time, place, and epidemiology of the disease. In particular, a parasitological test is crucial in low malaria transmission setting, unlike high malaria transmission area where the pre-test probability is high and therefore rapid test or clinical diagnosis more likely to be correct.28
Malaria is still the major health problem in the study area even though its prevalence is declining.24 The expected malaria incidence was 7% and 16% in the two selected districts. In the present study, only 4% of the respondents reported malaria episode. This might be because the census covered the last 3 months only and not included the year as is the case in the official incidence measures. Additionally, the time of study (April–May) corresponds to the minor malaria episode, September to December being the major one in Jimma area.29–32 The other reason for low prevalence and declining pattern of malaria could also be linked to the activities of Health Extension Workers (HEWs) in the community and various malaria control interventions.24
Even though high use of traditional medicine is expected in rural area, most study participants who had malaria episode preferred modern health institutions for treatment. A similar study in Uganda also found that modern health care was more sought than herbal medicine.30 Several factors affect treatment seeking behaviors of traditional medicine users like perceived efficacy, safety, age, availability of modern medicine, and cost.9 In another study in Mali, respondents preferred a modern health facility when they had malaria.31 However, it can be assumed that if the population has access to information on the proper use of a clinically validated plant against malaria and available locally for free, this could enable people to treat themselves in the case of a non-complicated episode, even when standard treatment is not available, and this should avoid progress to severe episode in most cases. In the case of traditional healers, 77.8% of them used home treatments additionally to ITN and IRS as preventive methods. AM was known by 219 participants (39.8%), and 31 of them (5.7%) reported that they either used the plant or knew someone who used it for medicinal purpose in their community. Moreover, this survey revealed that three respondents as well as one traditional healer interviewed use AM in prevention of malaria in pregnant women. In other studies AM was, as here, used to treat several diseases such as malaria, wound, and rabies.11,12,18,19,25 Although the exact mechanism of action and active compounds of AM remains unknown, the aerial part of the plant can be used as a first alternative treatment when conventional antimalarial medicines are unavailable due to its good safety profile and proven clinical efficacy.31 Looking to the phytochemistry of the plant, a bioguided fractionation of AM on P. falciparum led to the isolation of three active alkaloids: berberine, protopine and allocryptopine, with a respective IC50 of 0.32 µg/mL; 1.46 µg/mL and 0.32 µg/mL.32 However, those measured activities, even if high, are too weak to explain the powerful activity of AM decoction on human. The antimalarial activity of the AM decoction in human could be due to synergic effects of biomolecules, or pro-drugs or another explanation could be that the in vitro test used in the bio-guided fractionation process could not have been suitable as the plant decoction may not have a direct toxicity on the parasite but any other biological activity involved in the overall clinical effect.16,33 Qualitatively traditional healers or knowledgeable persons in the community were interviewed in order to support quantitative study. However, since it was a retrospective study, it may introduce recall bias, and the cross-sectional nature of the method that considers a single study setting may also be regarded as another limitation.
Conclusion
Both community and traditional healers revealed the availability and use of AM to treat malaria, with no report of adverse reaction and with good satisfaction regarding efficacy. Thus, this population survey allowed us to see that this plant is known, and culturally accepted in this region of Ethiopia, in particular for the treatment of malaria. Future research is needed to assess whether AM is effective against malaria in the Ethiopian context, and especially with a low endemicity and with the combined occurrence of P. vivax and P. falciparum.
Ethical Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki. The ethical clearance was obtained from Jimma University with a letter dated 11/03/2020 and reference number “JU/R/122/12”. As such, the verbal informed consent process was acceptable and approved by the Jimma University Ethics Committee. Then, an official letter was sent to respective administrators of each district. Verbal informed consent was obtained from all participants (mothers and/or care takers and/or members of the household whose age was greater than 18 years) enrolled in the study. Participants’ names and other personal identifiers were not included to maintain confidentiality.
Acknowledgments
We would like to acknowledge leaders of each district of Seka Chekorsa and Shebe Sombo as well as each selected kebele leaders for their cooperation during data collection on AM. We are also grateful to study participants and traditional healers for their willingness to participate in this study. Moreover, we appreciate and acknowledge Antenna Foundation for collaboration during this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Malaria; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria.
2. World Health Organization. World Malaria Report 2019. World Malar Rep. 2019: 1–232. Available from. https://www.who.int/publications-detail/world-malaria-report-2019.
3. FMOH. Malaria Prevention & Control Program; 2020.Available from: http://www.moh.gov.et/ejcc/en/malaria-prevention-control-program.
4. Lo E, Hemming-Schroeder E, Yewhalaw D, et al. Transmission dynamics of co-endemic Plasmodium vivax and P. falciparum in Ethiopia and prevalence of antimalarial resistant genotypes. PLoS Negl Trop Dis. 2017;11(7):1–25. doi:10.1371/journal.pntd.0005806
5. Alebie G, Urga B, Worku A, Smith T, Penny MA. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malar J. 2017;16:1–13. doi:10.1186/s12936-016-1650-6
6. Asnake S, Teklehaymanot T, Hymete A, et al. Survey of medicinal plants used to treat malaria by Sidama people of Boricha district, Sidama Zone, South Region of Ethiopia. Evid Based Complement Alternat Med. 2016;2016. Doi:10.1155/2016/9690164
7. Suleman S, Beyene T, Kebebe D, et al. Treatment of malaria and related symptoms using traditional herbal medicine in Ethiopia. J Ethnopharmacol. 2018;213:262–279. doi:10.1016/j.jep.2017.10.034
8. Tefera BN, Kim Y. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomedicine. 2019;7:1–2.
9. Willcox ML, Bodeker G. Traditional herbal medicines for malaria. In: British Medical Journal. Vol. 329. BMJ Publishing Group; 2004:1156–1159.
10. Argemone mexicana (PROTA) Plant Use English; 2020. Available from: https://uses.plantnet-project.org/en/Argemonemexicana (PROTA).
11. Factsheet Argemone mexicana (Mexican Prickly Poppy); 2020. Availablefrom: https://keys.lucidcentral.org/keys/v3/eafrinet/weeds/key/weeds/Media/Html/Argemone mexicana (Mexican Prickly Poppy).
12. Brahmachari G, Gorai D, Roy R. Argemone mexicana: chemical and pharmacological aspects. Brazilian J Pharmacogn. 2013;23(3):559–575. doi:10.1590/S0102-695X2013005000021
13. Sinha S, Medhi B, Sehgal R. Challenges of drug-resistant malaria. Parasite. 2014;21:61. doi:10.1051/parasite/2014059
14. Willcox ML, Graz B, Falquet J, et al. A reverse pharmacology approach for developing an anti-malarial phytomedicine. Malar J. 2011;10. Doi:10.1186/1475-2875-10-S1-S8
15. Willcox ML, Graz B, Falquet J, et al. Argemone mexicana decoction for the treatment of uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 2020;101(12):1190–1198. doi:10.1016/j.trstmh.2007.05.017
16. Graz B, Willcox ML, Diakite C, et al. Argemone mexicana decoction versus artesunate-amodiaquine for the management of malaria in mali: policy and public-health implications. Trans R Soc Trop Med Hyg. 2010;104(1):33–41. doi:10.1016/j.trstmh.2009.07.005
17. Bussmann RW, Swartzinsky P, Worede A, et al. Plant use in odo-bulu and demaro, Bale region, Ethiopia. J Ethnobiol Ethnomedicine. 2011;1–21.
18. Alemayehu K. Prevalence and effects of Argemone mexicana (Papaveraceae) on biodiversity in Ethiopia. Afr J Ecol. 2011;160–166.
19. Araya S. Study of plants traditionally used in public and animal health management in Seharti Samre. J Ethnobiol Ethnomedicine. 2015;11(1):1–25.
20. Assefa A, Tadesse N, Belay TB, Hailu A. Assessment of the invasive alien plant species Argemone ochroleuca in North Gondar and West Gojam Zones, Amhara Region, Ethiopia. Int J Nat Resour Ecol Manag. 2017;2016:107–114.
21. Meragiaw M. Wild useful plants with emphasis on traditional use of medicinal and edible plants by the people of Aba’ala. J Med Plant Herb Ther Res. 2016;4:1–16.
22. Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of LiboKemkem District, northwest Ethiopia. J Ethnobiol Ethnomedicine. 2015;1–38.
23. Dadi L, Berhane M, Ahmed Y, et al. Maternal and newborn health services utilization in Jimma Zone, Southwest Ethiopia: a community based cross-sectional study. BMC Pregnancy Childbirth. 2019;19(1):1–13. doi:10.1186/s12884-019-2335-2
24. Jemal A, Ketema T, A declining pattern of malaria prevalence in Asendabo Health Center Jimma zone, Southwest Ethiopia. BMC Res Notes. 2019;12(1):290. doi:10.1186/s13104-019-4329-6
25. Anywar G, Van’t Klooster C, Byamukama R. Medicinal plants used in the Treatment and Prevention of Malaria in Cegere Sub- County, Northern Uganda. Ethnobot Res Appl. 2016;14:505–516. doi:10.17348/era.14.0.505-516
26. President US, Initiative M. President’s malaria initiative Ethiopia. 2019.
27. Ouedraogo M, Kurji J, Abebe L, et al. Utilization of key preventive measures for pregnancy complications and malaria among women in Jimma Zone, Ethiopia. BMC Public Health. 2019;19(1):1–16. doi:10.1186/s12889-019-7727-8
28. Graz B, Willcox M, Szeless T, et al. “Test and treat” or presumptive treatment for malaria in high transmission situations? A reflection on the latest WHO guidelines. Malar J. 2011;10:1–8.
29. Hawaria D, Getachew H, Zhong G, et al. Ten years malaria trend at Arjo Didessa sugar development site and its vicinity, Southwest Ethiopia: a retrospective study. Malar J. 2019;18:1–11. doi:10.1186/s12936-019-2777-z
30. Lee YJ, Adusumilli G, Kazungu R, et al. Treatment-seeking behavior and practices among caregivers of children aged ≤5 y with presumed malaria in rural Uganda. Trans R Soc Trop Med Hyg. 2019;113(9):525–533. doi:10.1093/trstmh/trz039
31. Ardiet D, Falquet J. Home treatments alone or mixed with modern treatments for malaria in Finkolo AC, South Mali: reported use, outcomes and changes over 10 years. Trans R Soc Trop Med Hyg. 2015;1–5.
32. Simoes-Pires C, Hostettmann K, Haouala A, et al. Reverse pharmacology for developing an anti-malarial phytomedicine. The example of Argemone mexicana. Int J Parasitol Drugs Drug Resist. 2014;4(3):338–346. doi:10.1016/j.ijpddr.2014.07.001
33. Willcox ML, Graz B, Diakite C, et al. Is parasite clearance clinically important after malaria treatment in a high transmission area? A 3-month follow-up of home-based management with herbal medicine or ACT. Trans R Soc Trop Med Hyg. 2011;105(1):23–31. doi:10.1016/j.trstmh.2010.10.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
