Back to Journals » Drug Design, Development and Therapy » Volume 17
Knowledge Mapping of Drug Repositioning’s Theme and Development
Authors Lang X , Liu J, Zhang G, Feng X, Dan W
Received 25 January 2023
Accepted for publication 11 April 2023
Published 18 April 2023 Volume 2023:17 Pages 1157—1174
DOI https://doi.org/10.2147/DDDT.S405906
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Xiaona Lang,1 Jinlei Liu,2 Guangzhong Zhang,3 Xin Feng,1 Wenchao Dan3
1Pharmacy Department, Tianjin Hospital, Tianjin, People’s Republic of China; 2Cardiology Department, Guang ‘anmen Hospital, Chinese Academy of Traditional Chinese Medicine, Beijing, People’s Republic of China; 3Dermatological Department, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Wenchao Dan, Dermatological Department, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, People’s Republic of China, Tel +86 13652001152, Email [email protected]
Background: In recent years, the emergence of new diseases and resistance to known diseases have led to increasing demand for new drugs. By means of bibliometric analysis, this paper studied the relevant articles on drug repositioning in recent years and analyzed the current research foci and trends.
Methodology: The Web of Science database was searched to collect all relevant literature on drug repositioning from 2001 to 2022. These data were imported into CiteSpace and bibliometric online analysis platforms for bibliometric analysis. The processed data and visualized images predict the development trends in the research field.
Results: The quality and quantity of articles published after 2011 have improved significantly, with 45 of them cited more than 100 times. Articles posted by journals from different countries have high citation values. Authors from other institutions have also collaborated to analyze drug rediscovery. Keywords found in the literature include molecular docking (N=223), virtual screening (N=170), drug discovery (N=126), machine learning (N=125), and drug-target interaction (N=68); these words represent the core content of drug repositioning.
Conclusion: The key focus of drug research and development is related to the discovery of new indications for drugs. Researchers are starting to retarget drugs after analyzing online databases and clinical trials. More and more drugs are being targeted at other diseases to treat more patients, based on saving money and time. It is worth noting that researchers need more financial and technical support to complete drug development.
Keywords: drug repositioning, drug discovery, bibliometric, citation analysis, trend analysis
Introduction
Drug repositioning is the identification of new indications for already developed and marketed drugs to treat new areas of disease not mentioned in the current specifications.1 The discovery and development of novel drugs are estimated to cost hundreds of millions of dollars, take many years to develop and market and have a low success rate.2,3 It is a high-risk, high-investment process, and the pace of pharmaceutical R&D is failing to keep up with the needs of society.4 A drug target may play a role in several different diseases,5 or the drug may have multiple targets, each of which is associated with a different disease.6 Drug repositioning was first proposed in 2004 to increase the efficiency of drug development for pharmaceutical companies, finding new uses for and improving existing drugs.7 As research continues, the definition of drug repositioning has been expanded to include active substances that do not enter the clinical stage due to toxicity or poor efficacy and drugs that are withdrawn from the market for safety reasons.8 It is an emerging area of drug development that attracts an increasing number of scholars.
Drug repositioning has made significant progress in recent years of research. Many drugs have been repurposed from their original indications to new indications. For example, in recent years, due to the rapid spread of COVID-19, many anti-AIDS medicines have been repurposed to fight the virus. In addition, for rare diseases, tumors, tuberculosis, and other difficult-to-cure diseases, drugs on the market are screened from the gene bank for clinical trials to save development time. New uses for various types of drugs have been listed in the literature and are summarized below.
The most famous example is that of Sildenafil, which was repositioned as a critical drug for erectile dysfunction; initially, its side effect since its primary purpose had been the treatment of hypertension and angina.9,10 Rifampicin, an anti-tuberculosis drug, has been repositioned as an analgesic to relieve pain in joint cavities.1 Eculizumab, initially indicated for uremia, has also been repositioned to treat myasthenia gravis.11 Minoxidil has been repositioned for hypertension and hair loss prevention.12–14 Everolimus has been repositioned from transplant rejection prevention to treating tuberous sclerosis and inhibiting tumor growth.15 Verapamil and cimetidine are both inhibitors of CYP enzymes that inhibit the direct effects of Pgh1 and increase antimalarial activity.16,17 Even though they do not have antimalarial activity alone, they show synergistic effects when combined with antimalarials.18 Drug associations increase the success of repositioning. The use of combinations of drugs can provide synergistic effects, thereby reducing the risk of cytotoxicity being triggered when drugs are used alone; while also reducing the concentration of drugs being used.19 For example, the combination of Prednisone and Perphenazine, 6-Thiourea, can be used against histiocytosis.20 Some studies have shown that Propranolol, used as a beta-blocker in combination with etodolac, reduces surgical and inflammatory stress responses and promotes tumor metastasis.21 The most notable example currently includes repositioning drugs initially used to treat diseases such as HIV and malaria to treat novel coronaviruses.22–24 Due to the global outbreak of the novel coronavirus, Hydroxychloroquine, combined with Azithromycin, has been proposed as a potential therapy.25
Citespace is a literature visualization and analysis software that uses econometric analysis to create visual maps of particular literature, allowing analysis of the current state of research and inference of trends.26 HistCite is a citation analysis tool that quickly maps trends in a given field and organizes the number of times articles are cited to identify critical research and scholars.27 By analyzing a lot of data, it was found that these literature analysis tools could effectively show the correlation between articles, and obtained reliable data and clear pictures. They could summarize and analysis the literature with a large amount of data, which was beneficial for researchers to explore related fields.
There are many articles on drug repositioning. Most of the content provides examples of repurposing drugs or describes the methods and significance of drug repurposing. However, the use of bibliometric tools to analyze the content of recent articles has not yet been widely implemented. Therefore, this paper reviews thousands of pieces of literature, including keywords, authors, and research institutions. This step is essential. From the perspective of visual analysis, the associations between literature can be displayed through tables and figures, providing a scientific basis and data for future researchers studying this field.
Materials and Methods
Data Sources
Web of Science (WOS) is a primary source of citation information in bibliometric research. Associations between citations can be analyzed using this database. The total number of papers published in WOS exceeds those in Scopus, PubMed, China National Knowledge Infrastructure (CNKI), Chinese Social Science Citation Index (CSSCI), and other well-known databases. WOS also provides bibliometric software for general statistics and citation analysis. Many software and online sites only accept data exported from WOS, therefore our data were also filtered from the WOS database.
The core collection of the Web of Science database was searched with “drug repositioning” as the subject term and “repositioning drug;” “drug repurposing;” “repurposing, drug;” “rescue, drug” and “drug rescue” as synonyms for the search. The period was from January 1, 2001, to July 31, 2022. The investigation was conducted in August 2022, and 6215 articles were retrieved. The articles that met the requirements were obtained after removing those that did not.
Analytical Tools
Data from countries/regions, institutions, and journals were analyzed statistically and then visually, using HistCite software and the Journal Impact Factor (IF) from Journal Citation Report (2021). Using CiteSpace, keywords, related institutions, authors and journal citations can be parsed. By analyzing information about drug repositioning and constructing co-occurrence networks, the research foci and trends in related research in recent years can be determined. When constructing the keyword and institution-related graphs for this study, CiteSpace 6.1.R2 was set to Slice=3, g-index k=25, to ensure that each time slice gets the same amount of node information. Set Purning to “Pathfinder;” “Purning sliced networks” and “Pruning the merged network”. When constructing the literature co-citation network, the software parameters were set as Slice=1, Top N=30. No cropping was done to the network, The settings of other parameters are the same as those of the keyword.
Results
Literature Statistics
As shown in Figure 1, a total of 3814 documents were included through screening, classified into six types, from 102 countries and regions, 4670 institutions, 20,207 authors, and 993 journals, containing 6853 keywords written in seven languages. By calculating the global citation scores, 279 documents were found to have been cited more than 100 times.
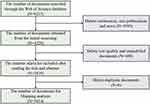 |
Figure 1 The process of literature screening. |
As shown in Figure 2, no relevant research articles were published from 2001 to 2003, while the number of published articles increased annually since 2004, with 50 articles published before 2011 and 993 articles published in 2021, which is 19.86 times more than the total number before 2011. The first article was published in 2004 with a high Total Global Citation Score (TGCS), indicating that its content will be helpful for future research. The number of articles in 2011 and 2013 was not significant; TGCS was high due to the high scores of the articles, indicating that these two years were critical for research in this area. After 2019, although the number of articles was rising, the TGCS value was low, and this decline could be due to a lack of content innovation or minimal research significance.
 |
Figure 2 Yearly output and score. |
Geographical Distribution of the Literature
The United States, China, India and the United Kingdom are relatively active in this area. Figure 3 shows that the United States has the highest number of publications, with 1097, accounting for 29.43% of the total publications retrieved. The top ten countries involved in the study are listed in Table 1, and it is evident that research institutes in Asia have contributed to many publications. Four Asian countries have 1522 publications, accounting for 39.90% of the total publications referred. The top three countries in terms of Average Citation Index (ACI) value were the USA, China, and India, which indicates that these three countries are at a more mature stage of development in this area with more valuable research content than other countries in this field.
 |
Table 1 The Top 10 Productive Countries in the Studies |
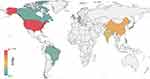 |
Figure 3 Distribution of global publications in the field of drug repositioning. |
As shown in Table 2, the top institution in terms of the number of published articles, the Chinese Acad Sci (N=79, 2.07%), belonged to China, followed by Harvard Med Sch (N=57, 1.49%). The Case Western Reserve Univ had higher ACI values than the other institutions, indicating good article content and a high overall rating. Next were the NIH and Harvard Med Sch, with ACI values of 43.76 and 39.65, respectively. It is worth noting that although the number of publications from Chinese institutions is high, the average citation score is low, whereas the quality of literature published by institutions in the US is superior.
 |
Table 2 The Top 10 Institutions (Based on Records and TGCS, Respectively) |
Analysis of Journals
Table 3 lists the top ten journals with the highest total global citation score (TGCS) in drug repurposing research. NUCLEIC ACIDS RESEARCH had the highest TGCS, but the number of its publications was small. This indicates that the articles published by NUCLEIC ACIDS RESEARCH were the most cited and studied, and their quality was more valuable. It may be that due to the recent spread of COVID-19, more researchers have paid attention to the virus and increased their research efforts in nucleic acids. Among these journals, SCIENTIFIC REPORTS published the total number of papers (N=122, 3.20%), followed by JOURNAL OF BIOMOLECULAR STRUCTURE & DYNAMICS (N=107, 2.81%). The highest ACI value was for the JOURNAL OF BIOMOLECULAR STRUCTURE & DYNAMICS (4.43), followed by BBRIEFINGS IN BIOINFORMATICS (2.79) and the JOURNAL OF CHEMICAL INFORMATION AND MODELING (2.75). NATURE published only four relevant articles, but its high number of citations indicates that the content of the articles in this journal is good and valuable for research.
 |
Table 3 The Top 10 Journals |
Figure 4 shows a double map overlay of journals. The left of the diagram represents citing journals, and the right side of the graph represents the relationship between the referenced journals. The orange and green lines indicate that articles published in Molecular/Biology/Immunology are mainly cited in Molecular/Biology/Genetics journals. The longer the horizontal axis of the ellipse, the more articles are published in the corresponding journal. The longer the vertical axis of the ellipse, the more authors there are. We found that articles in similar journals frequently cited each other, and the relationship between molecular biology and immunology, and genetics was closely connected, which provided the basis for drug exploration and discovery.
 |
Figure 4 The dual-map overlay. |
Analysis of Cooperative Relationships
Figure 5 illustrates the collaborations between authors, institutions and countries. Figure 5A illustrates collaboration between countries, with the US having the most collaboration with other countries, followed by the UK and China. Figure 5B represents the collaborations between institutions. Each node on the plot represents an institution, with the size of the node indicating the number of articles published by the institution. The connecting lines between the nodes represent the collaborations between different institutions, with the thickness of the lines indicating the strength of those collaborations. The nodes are colored differently based on the publication periods. A higher centrality of a node indicates a greater level of cooperation with other institutions. Nodes with a centrality greater than or equal to 0.1 are enclosed by purple circles, such a node is considered to have high centrality. Currently, the highest centrality is Massachusetts General Hospital (N=16, Centrality=0.35), followed by NCI (N=25, Centrality=0.34) and Shanghai Jiaotong University (N=38, Centrality=0.32). Although these institutions do not publish many articles, they collaborate extensively with other institutions. The majority of the top-ranked institutions are university institutions or research institutes, with hospitals being rare. This indicates a bias toward fundamental research in the drug repositioning area. Figure 5C represents the relationship between authors using VOSviewer.28 Due to the large number of authors, only nodes with a high degree of collaboration are selected. The authors were selected based on the number of publications and the level of collaboration.
Keywords
The top five keywords in terms of frequency were “drug repositioning”, “molecular docking”, “virtual screening”, “molecular dynamics” and “drug discovery.” From the above statements, we can identify the main elements of the research direction as well as other important research areas of the central nodes. The visualization of high-frequency co-occurring words is presented in Figure 6A. Figure 6B shows the top ten most cited terms, which include “inflammatory bowel disease”, “systems biology”, “gene expression” and “drug-target interaction prediction.” These words represent areas of research that have received significant attention and are indicative of trends and developments in this field. They are marked with red dots in Figure 6A. The information related to the top 20 high-frequency keywords are summarized in Table 4, which reflects the main keywords explored in recent literature.
 |
Table 4 The Top 20 Keywords in the Studies |
 |
Figure 6 (A) networks of keyword in the studies; (B) The top 10 strongest strength citation burst. |
Literature Co-Citation Analysis
Our aim was to identify the latest research trends by analyzing articles from recent years. When we searched the database to count the number of citations in the past three years, we found that the top ten articles were all published in 2020. Nine out of ten articles were related to the SARS-CoV-2 virus. Only one article explores the possibility of reusing antimicrobials as drugs by creating deep neural network models. The disease outbreak in late 2019 made the articles consistent in their research focus, with all of them eager to investigate the most urgently needed treatment options. The basic information of these articles is presented in Table 5.
 |
Table 5 The Top 10 Most Cited Articles |
To understand the connections between the articles, we conducted a co-citation analysis on them. During this analysis, we identified the top three most cited articles in the field of drug repositioning. We then performed a content analysis of these three articles and found that all three were representative of the field. The first article was related to the initiation of drug repositioning, while the second and third articles were focused on treatment methods and the type of medication used for the SARS-CoV-2 virus. These articles continue to be highly influential.
Co-citation analysis of the included literature and co-citation network was obtained and clustered to obtain Figure 7.2,3,29–64 The different colors represent the different clustering blocks, including nine significant clusters: #0 predicting drug-disease association; #1 CoV-2 main protease; #2 chemical-protein interactome; and #3 CoV-2 infection. Table 6 shows the representative articles in each clustering block,38,65–72 that are highly cited or have high TGCS scores. It also shows the importance of these articles for the researchers. The representative articles employ different approaches, such as bioinformatic analysis or deep learning for retargeting of drugs. Potential drugs are discovered through analysis of drug and disease targets. Re-dosing for the treatment of the novel coronavirus and drug combinations to improve therapeutic outcomes have also been foci of research in recent years.
 |
Table 6 Key Articles in Each Cluster |
 |
Figure 7 The cluster map of document co-citation. |
Discussion
Based on the literature analysis, it is clear that among the 3814 articles, there has been a significant increase in the quality and quantity of articles published after 2011. Furthermore, there is cooperation between countries, particularly between China and the United States, and the papers are highly cited. Most of the research is carried out through collaborations between schools and institutes. However, most co-authors reside in the same country, and there are not many collaborations between domestic and foreign scholars. The main focus of the literature revolves around drug repositioning, discoveries, and gene expression, which are all current research topics in this field. There are many citations between journals, and the top cited articles are periodic, with excellent research significance, deserving the attention of researchers.
We used data analysis software to identify the top three cited articles, which have had a significant impact on the field of drug repositioning and contain high-quality content that will be useful for future research.
Ashburn TT and Thor KB published “Drug Repositioning: Identifying and Developing New Uses for Existing Drugs” in 2004. This article was cited the most (N=1729) and was the first one to be published in this field. It analyzed the major pharmaceutical companies at the time, which were trying to improve productivity and create new uses for existing drugs. This resulted in shortened development times and reduced risk. The article also provided several examples of successful drug repositioning, including duloxetine for stress-related urinary incontinence and depression, dapoxetine for premature ejaculation, and thalidomide, which was found to be a tumor necrosis factor inhibitor once again.7 The article analyzes the obstacles that may be encountered and suggests ways to overcome them. It marks a significant beginning for the field of drug repositioning, as a SARS-CoV-2 protein interaction map has revealed potential targets for drug repurposing. In the citation index, the article by Gordon DE, Jang GM, Bouhaddou et al which targets the SARS-CoV-2 virus, came in second place (N=1323). Mass spectrometry identified 332 high-confidence protein interactions with human proteins, as well as sixty-six druggable human proteins or host factors from 69 compounds. Two sets of pharmacological agents that show antiviral activity to combat the virus were also identified.37 In 2020, Wu CR, Liu Y, Yang YY, Zhang P, Zhong et al published an analysis of therapeutic targets for SARS-CoV-2 and discovered potential drugs using computational methods. Their article was the third most cited (N=1070) on the treatment of the SARS-CoV-2 outbreak in Wuhan, China, in late 2019. The authors systematically analyzed the genetic code of the virus, predicted the target structure, and screened eligible drugs using currently available databases of antiviral drugs. One application of drug repositioning is to use already-marketed drugs to treat sudden unknown viruses or novel diseases.57
Table 5 summarizes the top 10 articles based on the number of citations. The reason for the high number of citations and the seriousness with which these articles have been taken is the rapid spread of the SARS-CoV-2 virus at the end of 2019, as well as the extremely harmful nature of the virus. As people are pressed for time, a more effective and faster treatment method involves retargeting and screening the existing antiviral drugs to identify the most appropriate ones. In subsequent results, we found that this method was effective in curbing the spread of the virus. Researchers are still fighting viruses through various research methods, such as mass spectrometry analysis, protein data screening, and molecular docking simulation binding in drug libraries. By combining existing drugs with new scientific and technological means, more updated discoveries in the field of new virus research are expected.
Our analysis of the top 10 cited articles revealed that the majority were related to the SARS-CoV-2 virus. However, drug repositioning is applicable to multiple diseases, not only viral infections. Therefore, we selected ten articles from the top 100 cited articles, based on disease type and citation count. All of these articles focus on the process and significance of drug repurposing in various conditions. Figure 8 displays the articles and their basic information. These articles provide valuable insights into the field of drug repurposing.
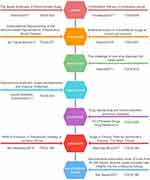 |
Figure 8 Different diseases with the strongest impact of TCGS in the top 100 literature. |
In terms of cancer, repositioning of combination enhanced tumor treatment and prevention and reduced drug resistance and toxicity.73 Target profiles of kinase inhibitors were analyzed by proteomics, suggesting potential therapeutic uses. Cabozantinib could be used to treat acute leukemia.74 In terms of enteropatia, many targeted drugs have been proven to have a particular effect on intestinal bacteria, reusing non-antibiotic treatment, broadening the research field of antibiotic resistance, and effectively avoiding side effects.75 The authors used gene expression databases to reintegrate drugs for inflammatory bowel disease. Topiramate was used as an example because of its high score after the screening, and it was experimentally proved to have the potential to be used in inflammatory bowel disease.76 In terms of tuberculosis (TB), through whole-cell screening methods, new anti-TB drug candidates could be identified, anti-TB drugs could be redirected from existing antibacterial drugs, the efficiency of drug production and development could be improved, and the reused drugs could be used in combination to increase efficacy.77 In terms of osteoporosis, before 2000, osteoporosis drugs were discovered mainly based on animal experiments or clinical observation. In recent years, driven by basic bone biology experiments, new drugs have been explored through treatment schemes such as rare bone diseases.78 In terms of parasitosis, due to the increasing drug resistance of parasitic diseases in recent years, existing drugs were insufficient to control the global spread of the disease. High-throughput screening of compounds in the database, such as antineoplastic drugs, antibiotics, and antifungal drugs. Repurposing drugs or developing new drug combinations was the best approach.29 Regarding cerebrosis, MMP-9 inhibitors had essential significance in treating ischemic strokes, such as neuroprotection and extension of the thrombolytic window. These included biotherapies using viral vectors, an endogenous inhibitor of MMP-9, repurposing of old drugs such as Minocycline, new chemical entities like DP b99, and other therapeutic approaches like therapeutic hypothermia.79 There were three methods for treating Alzheimer’s Disease (AD): one was to develop molecular target drugs corresponding to the pathogenesis, the second was to treat AD as a combination of multi-target medications, and the third was to reposition existing drugs in AD to promote the success of clinical trials.80 In terms of psychological illness, genome-wide association studies have been conducted on thousands of people to screen genes and drug targets related to bipolar disorder through the identified genomic loci. These new data may allow investigators to select the drugs available to treat bipolar disorder and perform different drug combinations.81
We have learned that there are five broad pathways for drug repositioning. The first one is when a drug is found to be effective in treating another disease by chance. For example, Aspirin was originally marketed as an analgesic, but it was later discovered by researchers to also have anti-platelet coagulation properties in serendipitous discovery;82 Dimethyl Fumarate was initially used as a treatment for psoriasis, but it has now also been repurposed to treat autoimmune diseases,83 and Thalidomide has been redirected to treat multiple myeloma.8 The second pathway is the discovery of drugs with a new activity, such as Nelfinavir, an HIV protein inhibitor that is now a potent broad-spectrum antitumor agent.84 The third approach is where pathways are re-identified. For example, Duloxetine was initially used as an antidepressant. However, the discovery that Serotonin and Norepinephri. The second pathway involves discovering drugs with new activities. For instance, Nelfinavir was originally developed as an HIV protein inhibitor, but it has now been repurposed as a potent broad-spectrum antitumor agent.85,86 The fourth pathway involves discovering that the protein targeted by a drug is also implicated in treating another disease. For example, Everolimus has been repurposed to treat breast cancer based on the discovery that the protein it targets is also involved in the development of this disease;87 and a fifth pathway is the unexpected discovery of side effects in clinical trials, such as what occurred with Sildenafi.10,88,89
Drug repositioning examples were summarized employing in Figure 9 by Figdraw. The selected drugs were searched in databases, and the top-ranked drugs were presented according to the number of relevant studies and citations.
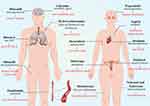 |
Figure 9 Examples of drug repositioning at different sites are presented. |
Lidocaine was initially used as an anesthetic agent for surgical procedures, but it was later discovered also to have the ability to inhibit neuropathic pain. Metformin, developed originally as a classical antidiabetic agent, has been repurposed as an analgesic in treating endometrial cancer. Naloxone and Naltrexone, initially created as opioid antagonists, have also been repurposed as anti-inflammatories that act systemically. Propranolol, Sildenafil, and Minoxidil were all antihypertensive and were later used for migraine prevention, erectile dysfunction, and hair loss prevention. Ritonavir had been used for anti-HIV. Hydroxychloroquine was used to treat lupus erythematosus and rheumatism, and later, it was used to fight the new disease due to the onset of SARS-CoV-2. Sirolimus and Aspirin were later used for cardiovascular diseases. Thalidomide and Methotrexate, formerly antiemetic and folic acid replacement, had been reintroduced as drugs for treating autoimmune diseases. Doxorubicin, an anthracycline drug, was originally used as a chemotherapy agent for cancer treatment. Paclitaxel, on the other hand, was initially used to treat arterial stenosis but was later found to be effective against tumors. Many targeted drugs have been repurposed from drugs that were already on the market, demonstrating the potential of drug repositioning. This strategy is based on the idea that different diseases can be linked together through the drugs used to treat them. Interestingly, many seemingly unrelated diseases can be linked through the drugs used to treat them. Figure 10 provides a visualization of these relationships. The yellow part of the figure represents the main disease types for which the drug is re-utilized. The pink section is the original disease type. Blue represents disease drugs that can be used for a variety of new diseases. The arrow direction is from the old disease type to the new disease type.
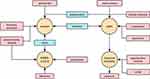 |
Figure 10 Relationships between different diseases. |
Drug repositioning has the potential to treat diseases in various parts of the body, and many drugs that have been repositioned by researchers have played a significant role in treating new disease areas. These drugs deserve focused attention, as they only represent a small fraction of the drugs that have been rediscovered for new uses. As more efforts are focused on repurposing drugs, an increasing number of drugs are in the experimental development stage for new applications. It is expected that more drugs will be redefined for clinical use in the future.
The advantages of drug repositioning are two-fold. Firstly, by using already available drug ADMET data, the preclinical study phase can be omitted, saving both time and money in drug development while reducing risk and increasing revenue. This is because the drug has already undergone preclinical studies during its initial development for another indication. Secondly, experimental validation may not be necessary by analyzing drug targets and screening relevant drug candidates based on available databases, which have been calculated from large amounts of data. This saves time and resources that would have been required for experimental validation.90,91 While drug repositioning has numerous advantages, there are also some shortcomings. One such challenge is the difficulty in patenting drugs that have been repositioned, particularly when the drug is already trademarked based on its initial indication. This can make it challenging for pharmaceutical companies to recoup their investment in drug development and limit the incentive for further research in this area.92 Another challenge with drug repositioning is that developing drugs for new indications requires significant funding and expertise, which can be difficult for small drug development companies to address. Small companies may not have access to the necessary resources or expertise required to conduct the extensive research and clinical trials needed to gain regulatory approval for a repositioned drug. As a result, this may limit the number of drugs that are repositioned, as larger pharmaceutical companies may be better equipped to handle the financial and regulatory demands of drug development.93,94 Many countries do not provide sufficient funding despite their enthusiasm for drug development breakthroughs. This has led to a year-on-year decline in the rate of drug development.
Conclusion
Upon review of the literature, it was observed that many studies employed network analysis to predict new targets, pathways, and localizations for repositioned drugs.95–97 Lotfi Shahreza M et al predicted drug repositioning from different aspects by analyzing other databases and utilizing software involved in generating metabolic networks, molecular interaction networks, gene regulatory networks, and protein interaction networks.98–101 Matching the prediction results with clinical or experimental studies shortens the study duration and helps treat unknown diseases, but there are shortcomings. Network analysis is biased towards using old drugs with multiple uses and significant side effects. However, the data related to side effects are unbalanced and still need to rely on extracting clinical performance from cases, which is the right direction for future research.102–105
In this study, we conducted a bibliometric analysis of literature related to drug repositioning retrieved through the Web of Science database using Citespace, HistCite, and VOSviewer,106–108 and performed a quantitative and qualitative analysis of the research contributions of different countries, institutions, journals, and authors in the field in recent years. The strength of this paper lies in unearthing the development of the discipline through analysis of the literature in recent years and identification of the distinct periods of publication and critical authors and their articles. It also provides a clear picture of the current research trends and research foci in the field,109,110 thereby providing a background for future researchers to study drug repositioning. The drawback is that only the Web of Science database was searched, and no other databases were used. Some unpublished or ongoing publications have not been explored, which may lead to incomplete data statistics. Lastly, there are limitations as only the popular trends in the literature were analyzed, which need to be refined with clinical information. The development of drug repositioning will be continuously followed up.
Acknowledgments
The authors gratefully acknowledge the contribution of Gao Wenya (China Academy of Chinese Medical Sciences) in the study design and guidance.
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships construed as a potential conflict of interest.
References
1. Gazerani P. Identification of novel analgesics through a drug repurposing strategy. Pain Manag. 2019;9(4):399–415. doi:10.2217/pmt-2018-0091
2. Jin GX, Wong STC. Toward better drug repositioning: prioritizing and integrating existing methods into efficient pipelines. Drug Discov Today. 2014;19(5):637–644. doi:10.1016/j.drudis.2013.11.005
3. Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. doi:10.1038/nrd.2018.168
4. Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11(3):191–200. doi:10.1038/nrd3681
5. Haupt VJ, Daminelli S, Schroeder M. Drug promiscuity in PDB: protein binding site similarity is key. PLoS One. 2013;8(6):e65894. doi:10.1371/journal.pone.0065894
6. Adasme MF, Parisi D, Sveshnikova A, Schroeder M. Structure-based drug repositioning: potential and limits. Semin Cancer Biol. 2021;68:192–198. doi:10.1016/j.semcancer.2020.01.010
7. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673–683. doi:10.1038/nrd1468
8. Jourdan JP, Bureau R, Rochais C, Dallemagne P. Drug repositioning: a brief overview. J Pharm Pharmacol. 2020;72(9):1145–1151. doi:10.1111/jphp.13273
9. Fakhouri F, Hourmant M, Campistol JM, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68(1):84–93. doi:10.1053/j.ajkd.2015.12.034
10. Rutherford KD, Mazandu GK, Mulder NJ. A systems-level analysis of drug-target-disease associations for drug repositioning. Brief Funct Genomics. 2018;17(1):34–41. doi:10.1093/bfgp/elx015
11. Scherman D, Fetro C. Drug repositioning for rare diseases: knowledge-based success stories. Therapie. 2020;75(2):161–167. doi:10.1016/j.therap.2020.02.007
12. Gomolin A, Litvinov IV, Netchiporouk E. Oral minoxidil: a possible new therapy for androgenetic alopecia. J Cutan Med Surg. 2020;24(1):88–89. doi:10.1177/1203475419879887
13. Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–194. doi:10.1111/j.1365-2133.2004.05785.x
14. Pontremoli R, Robaudo C, Gaiter A, Massarino F, Deferrari G. Long-term minoxidil treatment in refractory hypertension and renal-failure. Clin Nephrol. 1991;35(1):39–43.
15. Low ZY, Farouk IA, Lal SK. Drug repositioning: new approaches and future prospects for life-debilitating diseases and the COVID-19 pandemic outbreak. Viruses-Basel. 2020;12(9):1058. doi:10.3390/v12091058
16. Martiney JA, Cerami A, Slater AFG. Verapamil reversal of chloroquine resistance in the malaria parasite plasmodium-falciparum is specific for resistant parasites and independent of the weak base effect. J Biol Chem. 1995;270(38):22393–22398. doi:10.1074/jbc.270.38.22393
17. Paciorkowski A, Dai WW, Cerami A, Berger BJ. Synergism of cimetidine with anti-malarial agents. J Parasitol. 1997;83(5):960–963. doi:10.2307/3284300
18. Liu RF, Singh N, Tawa GJ, Wallqvist A, Reifman J. Exploiting large-scale drug-protein interaction information for computational drug repurposing. BMC Bioinform. 2014;15210. doi:10.1186/1471-2105-15-210
19. Sun W, Sanderson PE, Zheng W. Drug combination therapy increases successful drug repositioning. Drug Discov Today. 2016;21(7):1189–1195. doi:10.1016/j.drudis.2016.05.015
20. Matsuki E, Tsukada Y, Nakaya A, Yokoyama K, Okamoto S. Successful treatment of adult onset Langerhans cell histiocytosis with multi-drug combination therapy. Intern Med. 2011;50(8):909–914. doi:10.2169/internalmedicine.50.4808
21. Ilmer M, Westphalen CB, Niess H, et al. Repurposed drugs in pancreatic ductal adenocarcinoma an update. Cancer J. 2019;25(2):134–138. doi:10.1097/ppo.0000000000000372
22. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178104787. doi:10.1016/j.antiviral.2020.104787
23. Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38(4):379–381. doi:10.1038/d41587-020-00003-1
24. Liu J, Cao RY, Xu MY, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):16. doi:10.1038/s41421-020-0156-0
25. Gautret P, Lagier JC, Honore S, Hoang VT, Colson P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open label non-randomized clinical trial revisited. Int J Antimicrob Agents. 2021;57(1):106243. doi:10.1016/j.ijantimicag.2020.106243
26. Chen CM, CiteSpace II. Detecting and visualizing emerging trends and transient patterns in scientific literature. J Assoc Inf Sci Technol. 2006;57(3):359–377. doi:10.1002/asi.20317
27. Wu JF, Tsai HL. An explication of histCite (TM): updates, modifications, and a variety of applications. Ser Rev. 2022;48(1–2):41–48. doi:10.1080/00987913.2022.2101821
28. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
29. Andrews KT, Fisher G, Skinner-Adams TS. Drug repurposing and human parasitic protozoan diseases. Int J Parasitol Drugs Drug Resist. 2014;4(2):95–111. doi:10.1016/j.ijpddr.2014.02.002
30. Aronson JK. Old drugs - new uses. Br J Clin Pharmacol. 2007;64(5):563–565. doi:10.1111/j.1365-2125.2007.03058.x
31. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets-update. Nucleic Acids Res. 2013;41(D1):D991–D995. doi:10.1093/nar/gks1193
32. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Eng J Med. 2020;382(19):1787–1799. doi:10.1056/NEJMoa2001282
33. Cha Y, Erez T, Reynolds IJ, et al. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol. 2018;175(2):168–180. doi:10.1111/bph.13798
34. Cheng FX, Liu C, Jiang J, et al. Prediction of drug-target interactions and drug repositioning via network-based inference. PLoS Comput Biol. 2012;8(5):e1002503. doi:10.1371/journal.pcbi.1002503
35. Chong CR, Sullivan DJ. New uses for old drugs. Nature. 2007;448(7154):645–646. doi:10.1038/448645a
36. Corsello SM, Bittker JA, Liu ZH, et al. The drug repurposing hub: a next-generation drug library and information resource. Nat Med. 2017;23(4):405. doi:10.1038/nm.4306
37. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459. doi:10.1038/s41586-020-2286-9
38. Gottlieb A, Stein GY, Ruppin E, Sharan R. PREDICT: a method for inferring novel drug indications with application to personalized medicine. Mol Syst Biol. 2011;7496. doi:10.1038/msb.2011.26
39. Harder E, Damm W, Maple J, et al. OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput. 2016;12(1):281–296. doi:10.1021/acs.jctc.5b00864
40. Huang CL, Wang YM, Li XW, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/s0140-6736(20)30183-5
41. Jin ZM, Zhao Y, Sun Y, et al. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat Struct Mol Biol. 2020;27(6):529. doi:10.1038/s41594-020-0440-6
42. Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25(2):197–206. doi:10.1038/nbt1284
43. Keiser MJ, Setola V, Irwin JJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462(7270):175–U48. doi:10.1038/nature08506
44. Knox C, Law V, Jewison T, et al. DrugBank 3.0: a comprehensive resource for ‘Omics’ research on drugs. Nucleic Acids Res. 2011;39:D1035–D1041. doi:10.1093/nar/gkq1126
45. Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90–W97. doi:10.1093/nar/gkw377
46. Langedijk J, Mantel-Teeuwisse AK, Slijkerman DS, Schutjens M. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov Today. 2015;20(8):1027–1034. doi:10.1016/j.drudis.2015.05.001
47. Law V, Knox C, Djoumbou Y, et al. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 2014;42(D1):D1091–D1097. doi:10.1093/nar/gkt1068
48. Li J, Zheng S, Chen B, Butte AJ, Swamidass SJ, Lu ZY. A survey of current trends in computational drug repositioning. Brief Bioinform. 2016;17(1):2–12. doi:10.1093/bib/bbv020
49. Lounkine E, Keiser MJ, Whitebread S, et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486(7403):361. doi:10.1038/nature11159
50. Musa A, Ghoraie LS, Zhang SD, et al. A review of connectivity map and computational approaches in pharmacogenomics. Brief Bioinform. 2018;19(3):506–523. doi:10.1093/bib/bbw112
51. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi:10.1093/nar/gkv007
52. Sirota M, Dudley JT, Kim J, et al. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci Transl Med. 2011;3(96):96ra77. doi:10.1126/scitranslmed.3001318
53. Sleire L, Forde-Tislevoll HE, Netland IA, Leiss L, Skeie BS, Enger PO. Drug repurposing in cancer. Pharmacol Res. 2017;124:74–91. doi:10.1016/j.phrs.2017.07.013
54. Wang ML, Zhang JS, Ye D, et al. Time-dependent changes in the clinical characteristics and prognosis of hospitalized COVID-19 patients in Wuhan, China: a retrospective study. Clin Chim Acta. 2020;510:220–227. doi:10.1016/j.cca.2020.06.051
55. Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi:10.1093/nar/gkx1037
56. Wishart DS, Knox C, Guo AC, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34:D668–D672. doi:10.1093/nar/gkj067
57. Wu CR, Liu Y, Yang YY, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766–788. doi:10.1016/j.apsb.2020.02.008
58. Xu M, Lee EM, Wen ZX, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22(10):1101–1107. doi:10.1038/nm.4184
59. Xue HQ, Li J, Xie HZ, Wang YD. Review of drug repositioning approaches and resources. Int J Biol Sci. 2018;14(10):1232–1244. doi:10.7150/ijbs.24612
60. Yildirim MA, Goh KI, Cusick ME, Barabasi AL, Vidal M. Drug-target network. Nat Biotechnol. 2007;25(10):1119–1126. doi:10.1038/nbt1338
61. Zhang LL, Lin DZ, Kusov Y, et al. Alpha-ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020;63(9):4562–4578. doi:10.1021/acs.jmedchem.9b01828
62. Zheng W, Sun W, Simeonov A. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br J Pharmacol. 2018;175(2):181–191. doi:10.1111/bph.13895
63. Zhou YD, Hou Y, Shen JY, et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 2020;18(11):e3000970. doi:10.1371/journal.pbio.3000970
64. Zhu N, Wang WL, Liu ZD, et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun. 2020;11(1):3910. doi:10.1038/s41467-020-17796-z
65. Arend RC, Londono-Joshi AI, Gangrade A, et al. Niclosamide and its analogs are potent inhibitors of Wnt/beta-catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget. 2016;7(52):86803–86815. doi:10.18632/oncotarget.13466
66. Cheng FX, Hong HX, Yang SY, Wei YQ. Individualized network-based drug repositioning infrastructure for precision oncology in the panomics era. Brief Bioinform. 2017;18(4):682–697. doi:10.1093/bib/bbw051
67. Cheng YS, Williamson PR, Zheng W. Improving therapy of severe infections through drug repurposing of synergistic combinations. Curr Opin Pharmacol. 2019;48:92–98. doi:10.1016/j.coph.2019.07.006
68. de Almeida SMV, Soares JCS, Dos Santos KL, et al. COVID-19 therapy: what weapons do we bring into battle? Bioorg Med Chem. 2020;28(23):115757. doi:10.1016/j.bmc.2020.115757
69. Dubus E, Ijjaali I, Barberan O, Petitet F. Drug repositioning using in silico compound profiling. Future Med Chem. 2009;1(9):1723–1736. doi:10.4155/fmc.09.123
70. Fiscon G, Conte F, Farina L, Paci P. SAveRUNNER: a network-based algorithm for drug repurposing and its application to COVID-19. PLoS Comput Biol. 2021;17(2):e1008686. doi:10.1371/journal.pcbi.1008686
71. Haupt VJ, Schroeder M. Old friends in new guise: repositioning of known drugs with structural bioinformatics. Brief Bioinform. 2011;12(4):312–326. doi:10.1093/bib/bbr011
72. Zhou YD, Hou Y, Shen JY, Huang Y, Martin W, Cheng FX. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6(1):14. doi:10.1038/s41421-020-0153-3
73. Mokhtari RB, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi:10.18632/oncotarget.16723
74. Klaeger S, Heinzlmeir S, Wilhelm M, et al. The target landscape of clinical kinase drugs. Science. 2017;358(6367):eaan4368. doi:10.1126/science.aan4368
75. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623. doi:10.1038/nature25979
76. Dudley JT, Sirota M, Shenoy M, et al. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med. 2011;3(96):96ra76. doi:10.1126/scitranslmed.3002648
77. Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. The challenge of new drug discovery for tuberculosis. Nature. 2011;469(7331):483–490. doi:10.1038/nature09657
78. Khosla S, Hopbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907. doi:10.1016/s2213-8587(17)30188-2
79. Chaturvedi M, Kaczmarek L. MMP-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol. 2014;49(1):563–573. doi:10.1007/s12035-013-8538-z
80. Bachurin SO, Bovina EV, Ustyugov AA. Drugs in clinical trials for alzheimer’s disease: the major trends. Med Res Rev. 2017;37(5):1186–1225. doi:10.1002/med.21434
81. Mullins N, Forstner AJ, O’Connell KS, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53(6):817. doi:10.1038/s41588-021-00857-4
82. Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231(25):232–235. doi:10.1038/newbio231232a0
83. Nosengo N. New tricks for old drugs. News Item. Nature. 2016;534(7607):314–316. doi:10.1038/534314a
84. Ji -R-R, Xu -Z-Z, Gao Y-J. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–548. doi:10.1038/nrd4334
85. Marcus DA. Duloxetine use in painful conditions. Expert Opin Pharmacother. 2011;12(8):1333–1340. doi:10.1517/14656566.2011.580739
86. Schuessler B. What do we know about duloxetine’s mode of action? Evidence from animals to humans. BJOG. 2006;113:5–9. doi:10.1111/j.1471-0528.2006.00877.x
87. Aggarwal S, Verma SS, Aggarwal S, Gupta SC. Drug repurposing for breast cancer therapy: old weapon for new battle. Semin Cancer Biol. 2021;68:8–20. doi:10.1016/j.semcancer.2019.09.012
88. Dudley JT, Deshpande T, Butte AJ. Exploiting drug-disease relationships for computational drug repositioning. Brief Bioinform. 2011;12(4):303–311. doi:10.1093/bib/bbr013
89. Melnik T, Abdo CHN, de Moraes JF, Riera R. Satisfaction with the treatment, confidence and ‘naturalness’ in engaging in sexual activity in men with psychogenic erectile dysfunction: preliminary results of a randomized controlled trial of three therapeutic approaches. BJU Int. 2012;109(8):1213–1219. doi:10.1111/j.1464-410X.2011.10516.x
90. Andronis C, Sharma A, Virvilis V, Deftereos S, Persidis A. Literature mining, ontologies and information visualization for drug repurposing. Brief Bioinform. 2011;12(4):357–368. doi:10.1093/bib/bbr005
91. Ursu O, Holmes J, Knockel J, et al. DrugCentral: online drug compendium. Nucleic Acids Res. 2017;45(D1):D932–D939. doi:10.1093/nar/gkw993
92. Sternitzke C. Drug repurposing and the prior art patents of competitors. Drug Discov Today. 2014;19(12):1841–1847. doi:10.1016/j.drudis.2014.09.016
93. Novac N. Challenges and opportunities of drug repositioning. Trends Pharmacol Sci. 2013;34(5):267–272. doi:10.1016/j.tips.2013.03.004
94. Sardana D, Zhu C, Zhang M, Gudivada RC, Yang L, Jegga AG. Drug repositioning for orphan diseases. Brief Bioinform. 2011;12(4):346–356. doi:10.1093/bib/bbr021
95. AlQuraishi M. ProteinNet: a standardized data set for machine learning of protein structure. BMC Bioinform. 2019;20311. doi:10.1186/s12859-019-2932-0
96. Grammer AC, Lipsky PE. Drug repositioning strategies for the identification of novel therapies for rheumatic autoimmune inflammatory diseases. Rheum Dis Clin North Am. 2017;43(3):467. doi:10.1016/j.rdc.2017.04.010
97. Wallach I, Dzamba M, Heifets A. AtomNet: a deep, convolutional neural network for bioactivity prediction in structure-based drug discovery. Abstr Pap Am Chem Soc. 2016;2016:251.
98. Alaimo S, Bonnici V, Cancemi D, Ferro A, Giugno R, Pulvirenti A. DT-Web: a web-based application for drug-target interaction and drug combination prediction through domain-tuned network-based inference. BMC Syst Biol. 2015;9S4. doi:10.1186/1752-0509-9-s3-s4
99. Alaimo S, Giugno R, Pulvirenti A. Recommendation techniques for drug-target interaction prediction and drug repositioning. In: Carugo O, Eisenhaber F, editors. Data Mining Techniques for the Life Sciences. Springer; 2016:441–462.
100. Brown AS, Patel CJ. A standard database for drug repositioning. Sci Data. 2017;4170029. doi:10.1038/sdata.2017.29
101. Shahreza ML, Ghadiri N, Mousavi SR, Varshosaz J, Green JR. A review of network-based approaches to drug repositioning. Brief Bioinform. 2018;19(5):878–892. doi:10.1093/bib/bbx017
102. Chen B, Greenside P, Paik H, Sirota M, Hadley D, Butte AJ. Relating chemical structure to cellular response: an integrative analysis of gene expression, bioactivity, and structural data across 11,000 compounds. CPT. 2015;4(10):576–584. doi:10.1002/psp4.12009
103. Iwata H, Sawada R, Mizutani S, Yamanishi Y. Systematic drug repositioning for a wide range of diseases with integrative analyses of phenotypic and molecular data. J Chem Inf Model. 2015;55(2):446–459. doi:10.1021/ci500670q
104. Li L, Ruau DJ, Patel CJ, et al. disease risk factors identified through shared genetic architecture and electronic medical records. Sci Transl Med. 2014;6(234):234ra57. doi:10.1126/scitranslmed.3007191
105. Paik H, Chen B, Sirota M, Hadley D, Butte AJ. Integrating clinical phenotype and gene expression data to prioritize novel drug uses.; research support, N.I.H, extramural; research support, non-U.S. Gov’t. CPT. 2016;5(11):599–607. doi:10.1002/psp4.12108
106. Guo Y, Hao Z, Zhao S, Gong J, Yang F. Artificial intelligence in health care: bibliometric analysis. J Med Internet Res. 2020;22(7):e18228. doi:10.2196/18228
107. Li B, Hu K, Lysenko V, et al. A scientometric analysis of agricultural pollution by using bibliometric software VoSViewer and Histcite (TM). Environ Sci Pollut Res. 2022;29(25):37882–37893. doi:10.1007/s11356-022-18491-w
108. Yu Y, Li Y, Zhang Z, et al. A bibliometric analysis using VOSviewer of publications on COVID-19. Ann Transl Med. 2020;8(13):816. doi:10.21037/atm-20-4235
109. Chen C. Visualizing and exploring scientific literature with Citespace: an introduction.
110. Xia D-M, Wang X-R, Zhou P-Y, Ou T-L, Su L, Xu S-G. Research progress of heat stroke during 1989–2019: a bibliometric analysis. Mil Med Res. 2021;8(1):5. doi:10.1186/s40779-021-00300-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

