Back to Journals » OncoTargets and Therapy » Volume 13
Knockdown of FAM83D Enhances Radiosensitivity in Coordination with Irradiation by Inhibiting EMT via the Akt/GSK-3β/Snail Signaling Pathway in Human Esophageal Cancer Cells
Authors Yang XX, Ma M, Sang M , Zhang X, Zou N, Zhu S
Received 11 January 2020
Accepted for publication 1 May 2020
Published 26 May 2020 Volume 2020:13 Pages 4665—4678
DOI https://doi.org/10.2147/OTT.S245681
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tohru Yamada
Xing-Xiao Yang,1 Ming Ma,2 Mei-xiang Sang,3 Xue-yuan Zhang,1 Nai-yi Zou,1 Shu-chai Zhu1
1Department of Radiation Oncology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei 050011, People’s Republic of China; 2Department of Clinical Laboratory, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei 050011, People’s Republic of China; 3Research Center, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei 050011, People’s Republic of China
Correspondence: Shu-chai Zhu
Department of Radiation Oncology, The Fourth Hospital of Hebei Medical University, 12 Jiankang Road, Shijiazhuang, Hebei 050011, People’s Republic of China
Tel +86311-86095673
Email [email protected]
Purpose: To explore the effects of FAM83D on the proliferation, invasion and radiosensitivity of human esophageal cancer cells and to elucidate the mechanism involved in the regulation of the growth and metastasis of esophageal cancer cells.
Methods and Materials: This study included sixty-nine patients with esophageal cancer. The expression levels of FAM83D in the esophageal cancer tissues and paracarcinoma tissues of the sixty-nine patients were measured. We also examined FAM83D expression in five cell lines. We analyzed the effects of FAM83D on the proliferation, invasion and radiosensitivity of human esophageal cancer cells via MTS, Transwell, and colony formation assays. The effect of FAM83D knockdown on cell apoptosis was assayed by flow cytometry. In addition, we also examined changes in the expression of metastasis-related molecules at the protein and mRNA levels by qRT-PCR and Western blotting after silencing FAM83D expression, and we detected the expression of PI3K/Akt signaling-related proteins by Western blotting.
Results: The results demonstrated that the expression of FAM83D was obviously higher in esophageal cancer tissues and cell lines than that in human adjacent normal tissues and normal esophageal epithelial cell lines. FAM83D overexpression was positively associated with tumor size, tumor-node-metastasis (TNM) stage, T classification, N classification, distant metastasis and relapse and was negatively associated with patient survival rates. FAM83D shRNA transfection suppressed its expression. Compared to that in the control group, the proliferation of tumor cells in the FAM83D shRNA group was hindered after exposure to radiation in vitro and in vivo; in addition, FAM83D knockdown inhibited cell invasion, induced apoptosis and regulated apoptosis-related protein expression. Moreover, the radiosensitivity of esophageal cancer cells was increased after depletion of FAM83D. In addition, FAM83D silencing was associated with the reversion of EMT, as reflected by an increase in the epithelial marker E-cadherin and a decrease in the mesenchymal markers N-cadherin and vimentin. Further study showed that FAM83D depletion suppressed the signaling pathway involving p-Akt, p-GSK-3β and Snail.
Conclusion: The results reveal that FAM83D may be a potential therapeutic target for esophageal squamous cell carcinoma (ESCC) and that lower expression of FAM83D in coordination with irradiation promotes the radiosensitization of ESCC by inducing EMT through the Akt/GSK-3β/Snail signaling pathway.
Keywords: FAM83D, ESCC, radiosensitivity, EMT, Akt/GSK-3β/Snail
Introduction
Esophagus carcinoma (EC) is one of the most prevalent malignant cancers; EC is reported to have the third highest morbidity rate and the fourth highest cancer-associated mortality rate in China.1 The most prevalent histologic subtype of EC is esophageal squamous cell carcinoma (ESCC),2 accounting for more than 90% of cases. Currently, radiotherapy is one of the prevailing therapies for ESCC and has obviously improved esophageal carcinoma outcomes, but the effect of radiotherapy alone is very poor due to the rapid proliferation of tumor cells; it is estimated that approximately 50~60% of patients with advanced ESCC present with local uncontrolled or regional recurrence of the lesion after radiotherapy.3 In addition, identification of early-stage ESCC is difficult due to a lack of symptoms or misdiagnosis.4 At present, the detailed pathogenesis of ESCC remains poorly understood. Therefore, it is urgently needed to identify the exact molecular mechanism of ESCC, search for its new biomarker and improve its prognosis.
Family with sequence similarity 83, member D (FAM83D) is located on chromosome 20q, and this family shares a highly conserved DUF1669 domain in the N terminus.5 More importantly, it was recently demonstrated that FAM83D exhibits oncogenic properties and acts as a novel oncogene in various human tumors, including gynecological, gastrointestinal and respiratory cancers.6–8 A recent study indicated that FAM83D participates in the development of colorectal cancer by downregulating the tumor suppressor gene FBXW7 and has prognostic value for patients with colorectal cancer.9 According to a previous report, FAM83D silencing by shRNA inhibits the proliferation, migration and invasion of hepatocellular carcinoma cells.10 However, the biological role of FAM83D and its molecular mechanism in regulating radiosensitization is still unknown in human ESCC.
To clarify the function of FAM83D in ESCC, we conducted a systematic analysis. At present, few studies have focused on its role in modulating the growth, metastasis, DNA damage and radiosensitivity of esophageal carcinoma cells. Given the crucial role of FAM83D in ionizing radiation-induced DNA damage response (DDR), we assume that knockdown of FAM83D may bring about DNA damage pathway defects and thus increase radiosensitivity. Thus, we explored the impact of FAM83D on the proliferation, apoptosis and invasion of ESCC cells and on potentially related signaling pathways. In this research, we attempted to verify the above hypothesis by using different types of cells in vitro to explore the regulatory mechanisms of FAM83D-induced carcinogenesis and tumor progression.
Methods and Materials
Tissue Specimens and Immunohistochemical Analysis
ESCC and para-carcinoma tissue samples were collected from sixty-nine patients with ESCC who came to the Department of Thoracic Surgery, the Fourth Hospital of Hebei Medical University (Hebei, China) from January 2010 to December 2012. All the patients signed informed consent forms, and all protocols in this study were approved by the ethics committee of the Fourth Hospital of Hebei Medical University. A standard immunohistochemical analysis protocol was applied using the streptavidin-peroxidase method as previously described.11 Briefly, after antigen retrieval, the slides were incubated with an anti-FAM83D monoclonal antibody (1:200; ab236882, Abcam, USA) followed by incubation with a horseradish peroxidase-conjugated anti-mouse secondary antibody (1:200; Dako, Denmark). Subsequently, antibody binding was visualized after diaminobenzidine coloring reaction and hematoxylin counterstaining. PBS acted as a negative control instead of the primary antibody. The immunostained results were independently evaluated by two clinical pathologists according to the scoring standard of previous studies.12
Construction of shRNA Plasmids
For the FAM83D knockdown analyses, the following shRNA sequences were used: for the targeting shRNA, 5ʹ-GCAGUAACUUGGUAAUUCUTT-3ʹ (sense) and 5ʹ-AGAAUUACCAAGUUACUGCTT-3ʹ (antisense); and for the negative control scrambled shRNA (NC-shRNA), 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ (sense); 5ʹ-ACGUGACAGGUUCGGAGAATT-3ʹ (antisense); these shRNAs were designed by Invitrogen. All of the sequences were subjected to the basic local alignment search tool (BLAST) to confirm the absence of homology to any additional known coding sequences in the human genome.
Cell Culture, Cell Transfection and X-Ray Irradiation
The human normal esophageal cell line HEEC was purchased from the Cellular Biology Institute of the Shanghai Academy of Sciences (Shanghai, China). The human ESCC cell lines ECA109 and TE1 were purchased from Procell Life Science & Technology Co., Ltd. (Wuhan, China). EC9706, KYSE30 and KYSE180 cell lines were purchased from Otwo Biotech Inc. (Shenzhen, China). The above cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 37°C in 5% CO2. Plasmid containing the short hairpin RNA (shRNA) of FAM83D and the negative control (shNC) shRNA were purchased from GeneChem. Cells were seeded in 6-well plates and incubated for 24 h and transfected using Lipofectamine RNAiMAX (Invitrogen, USA) according to the manufacturer’s protocol. To obtain cells stably expressing FAM83D shRNA, G418 was added twenty-four hours after transfection, and stable transfectants were obtained after several weeks. Cells were irradiated at room temperature with a 6-MV Siemens linear accelerator (Siemens, Concord, CA) at a dose rate of 5 Gy/min after stable transfection and allowed to recover in an incubator for the indicated time until harvesting.
Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Life Technologies Inc., Carlsbad, CA, USA). qRT-PCR was performed to determine the expression level of the target genes in the ECA109 and KYSE30 cells. Briefly, single-stranded cDNA templates were obtained by reverse transcription using RevertAid First Strand cDNA Synthesis kits, and PCR amplification was then performed by using Go Taq qPCR Master Mix (Promega, USA). The relative level of expression of the target genes was assessed by the 2−ΔΔCt method, according to the method described in our previous report.2
Western Blotting
The cellular total protein of the ECA109 and KYSE30 cells was solubilized in RIPA lysis buffer containing 10% protease inhibitor cocktail. Furthermore, a standard BCA assay was performed to detect the protein concentration of the lysate supernatant. Moreover, the proteins were subjected to Western blotting analyses according to the method described in article.13 Anti-human FAM83D (1:1000), Bid (1:1000), Mcl-1 (1:1000), caspase 3 (1:2000), E-cadherin (1:2000), N-cadherin (1:2000), vimentin (1:2000), Snail (1:1000), Akt (1:1000), GSK-3β (1:1000), p-Akt (1:1000), p-GSK-3β (1:1000) and β-actin (1:5000) primary antibodies were used. The expression level of those proteins was calculated as the ratio of intensity of the target protein to that of β-actin. Experiments were carried out in triplicate wells and were repeated three times.
Cell Proliferation Assay
Briefly, approximately 2×103 ECA109 and KYSE30 cells were cultured in 96-well culture plates with RPMI 1640 supplemented with 10% FBS until they reached 50% confluence; the cells were then transfected with FAM83D- or NC- shRNA. After transfection, 20 μL MTS solution (Sigma, USA) was added to each well and incubated for an additional 4 h. Finally, the OD value was evaluated at 492 nm using a microplate reader.
Colony Formation Assay
To investigate the inhibitory effect of radiation on the viability of ESCC cells, a standard colony formation assay was performed to generate cell survival curves.14 The tumor cells in the control, NC, and FAM83D shRNA groups were irradiated by graded single doses (0–8 Gy) and seeded in petri dishes with complete RPMI-1640 medium. Fourteen days later, the cells were fixed and stained with crystal violet (0.6%). Colonies of more than 50 cells were scored as viable. The experiments were repeated at least twice.
Invasion Assay
For the invasion assay, an aliquot of 5×104 irradiated ESCC cells was placed in 24-well Transwell chambers with polycarbonate filters precoated with Matrixgel (Corning, NY, USA); 200 μL medium without FBS was added to the upper chamber, and 500 μL medium with 10% FBS was added to the lower chamber and served as the chemoattractant. Chambers were incubated at 37°C in a humid atmosphere with 5% CO2 for 24 h. Then, the nonmigrating cells on the upper surface of each filter were removed with a cotton swab, and the inserts were fixed with 4% formaldehyde, stained with Wright-Giemsa and imaged. For each filter, the number of cells migrating through the membrane in five fields was observed and counted (magnification: ×200).
Analysis of Cell Apoptosis
FCM was performed to assess cell apoptosis events using a flow cytometer (Beckman Coulter, USA) and Kaluza analysis software (Beckman Coulter, USA). Briefly, after staining with annexin V-PE and 7-AAD at room temperature for 30 min, the cells were resuspended in PBS and analyzed using a flow cytometer.
Xenograft Tumor Models
To study the regulatory effect of FAM83D on the radiosensitivity of ESCC cells in vivo, 4- to 6-week-old Balb-c/null mice were used to construct a tumor xenograft model. In short, 4×106 FAM83D-shRNA or NC tumor cells with stable transfectant were implanted into the left hind paw of Balb/c nude mice, and then a dose of 15 Gy was administered with a collimator container to protect the normal tissue for three weeks after injection. All experiments with animals were performed with the approval of the Animal Care and Use Committee from the Fourth Hospital of Hebei Medical University and followed the laboratory animal-guideline for ethical review of animal welfare. The tumor diameter (mm) was evaluated using calipers twice a week, and the tumor volume (TV) was calculated with the following formula: TV= ab2/2 (a represents the long diameter and b represents the short diameter).
Statistical Analysis
All data are presented as the mean ± standard deviation (SD), and statistical analysis was performed using the SPSS software package version 21 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to determine the significance of differences between groups. Tukey’s method was used for multiple comparisons. A chi-square test was used to analyze the clinical significance of the expression of FAM83D. Kaplan–Meier survival analysis and a Log rank test were used to evaluate the survival curve of the ESCC patients. P-value less than 0.05 and 0.01 were considered to be statistically significant and are indicated by asterisks in the figures. Data were obtained from at least three independent experiments with a similar pattern.
Results
FAM83D Expression Is Upregulated in Esophageal Cancer Specimens and Indicates an Adverse Prognosis
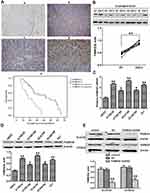
Compared to that in adjacent normal tissues, FAM83D expression in ESCC samples (Figure 1A) was higher by immunohistochemical staining; FAM83D was mainly expressed in cell nuclei, occasionally in the neoplastic epithelial cytoplasm and plasmalemma (Figure 1A-b, -c and -d). In addition, no staining or only weak staining was observed in normal epithelial cells (Figure 1A-a). Western blotting showed similar results: FAM83D expression in ESCC samples was higher than that in adjacent normal tissues (Figure 1B). The association between FAM83D expression and clinical parameters of patients with esophageal cancer was analyzed, including sex, age, tumor size, lesion location, TNM stage, T classification, N classification, distant metastasis, and relapse. As summarized in Table 1, the accumulation of FAM83D in esophageal cancer was significantly associated with tumor size (P=0.029), TNM stage (P=0.034), T classification (P=0.020), N classification (P=0.048), distant metastasis (P=0.024), and relapse (P=0.011), indicating a correlation between FAM83D expression and esophageal cancer invasion, and metastasis. However, no significant differences were observed between FAM83D expression and other clinical parameters such as patient age, sex and lesion location (P>0.05 for all comparisons). Furthermore, Kaplan–Meier analysis indicated that patients with lower FAM83D protein levels had a significantly longer overall survival than patients with higher levels of FAM83D expression (P<0.001, Log rank test; Figure 1A-e). Collectively, the above observations indicate that FAM83D could help to evaluate the prognosis of patients with ESCC.
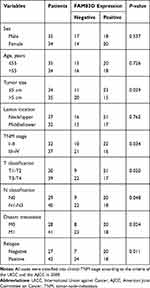 |
Table 1 Correlation Between FAM83D Expression and Clinicopathological Variables in Patients with ESCC |
FAM83D Expression Is Elevated in ESCC Cells
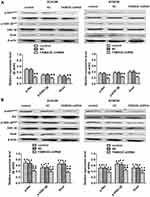
We determined FAM83D expression at the protein and mRNA levels in five ESCC cell lines (KYSE30, KYSE180, ECA109, EC9706 and TE1) and a normal esophageal epithelial cell line (HEEC) by Western blotting and qRT-PCR assays, respectively. The expression of FAM83D at the protein and mRNA levels in the ESCC cell lines was obviously higher than that in the HEEC cells (Figure 1C and D), particularly in ECA109 and KYSE30 cells. Therefore, we chose these two cell lines for further experiments.
Knockdown of FAM83D Enhances the Radiosensitivity of ESCC Cells After IR in vitro and in vivo
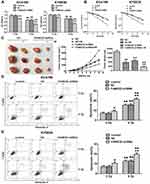
First, to evaluate the novel function of FAM83D in ESCC cells, FAM83D-specific shRNA was employed to deplete FAM83D expression in ECA109 and KYSE30 cells, with NC shRNA as a control. The expression of FAM83D at the protein level in cells was subsequently determined by Western blotting. The results showed that FAM83D-shRNA effectively suppressed the expression of FAM83D in both ECA109 and KYSE30 cells (P<0.01, Figure 1E). Meanwhile, the empty vector had no significant effect on the expression of FAM83D in the above cells. To assess the impact of FAM83D knockdown on the proliferation level of ESCC cells, an MTS assay was conducted. The results showed that cell growth was significantly inhibited in the FAM83D shRNA group compared with the control and NC groups at 48 h after IR (Figure 2A, P<0.01). Moreover, after exposure to IR, the cell viability in each group was obviously lower than that in the corresponding unirradiated groups, suggesting that FAM83D depletion might enhance the sensitivity of ESCC cells to radiation (Figure 2B). To further explore the radiosensitizing effect of FAM83D depletion in ESCC cells in vivo, ECA109 cells transfected with FAM83D shRNA and NC shRNA were inoculated into nude mice. The results revealed that the volume and weight (Figure 2C) of tumors in the FAM83D depletion group were significantly smaller than those in the NC group before and after irradiation (P<0.01). Taken together, the findings revealed that knockdown of FAM83D after irradiation markedly suppressed cell viability and increased radiosensitivity.
Downregulation of FAM83D Increases the Apoptosis of ESCC Cells in vitro
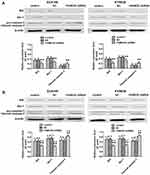
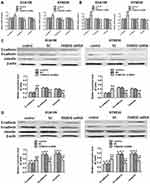
Cell apoptosis was quantified through flow cytometric analysis. The apoptosis rate in the FAM83D shRNA group was higher than that in the control and NC groups before IR, as evidenced by both annexin V and 7-AAD staining. Additionally, suppression of FAM83D induced more obvious apoptosis after IR than the control in ECA109 (Figure 2D) and KYSE30 cells (Figure 2E). However, there was no significant difference in apoptosis between the control group and NC group. To further explore the apoptosis mechanism, we detected the levels of Bid, Mcl-1 and caspase-3 protein expression before and after IR. Significant downregulation of Bid and Mcl-1 and upregulation of the active form of caspase-3, but not total caspase-3, were observed in the FAM83D shRNA group before IR (Figure 3A). Notably, there were more obvious changes among the three groups after IR (Figure 3B). These results demonstrated that knockdown of FAM83D induced the apoptosis of esophageal cancer cells by regulating apoptosis-related proteins in vitro.
Silencing of FAM83D Downregulates Cell Invasion
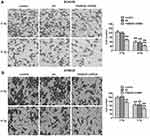
To determine the effect of FAM83D on the invasion potential of esophageal carcinoma cells, a Transwell assay was employed in ECA109 and KYSE30 cells. Our data showed that the number of invaded ECA109 cells in the FAM83D shRNA group was significantly lower than that in other groups (Figure 4A). Similar results were found in KYSE30 cells (Figure 4B), indicating that downregulation of FAM83D has a marked inhibitory effect on cell invasion. However, there was no significant difference in cell invasion between the control group and NC group.
Knockdown of FAM83D Inhibits the Expression Levels of Metastasis-Related Molecules in ESCC Cells
Epithelial-mesenchymal transition (EMT) is crucial in the metastatic progression of many malignant tumors.15 To confirm whether FAM83D depletion inhibits the metastatic potential of ESCC cells partially by impairing the process of EMT, we detected the expression of EMT-related proteins by qRT-PCR and Western blotting assays. Our data demonstrated that silencing FAM83D can upregulate the expression of an epithelial marker (E-cadherin) and downregulate mesenchymal markers (N-cadherin and vimentin) at the RNA and protein levels before IR in both ECA109 and KYSE30 cells (Figure 5A and C). Moreover, similar changes were also observed in ESCC cells after IR (Figure 5B and D).
Depletion of FAM83D Inhibits EMT by Suppressing the Akt/GSK-3β/Snail Signaling Pathway in ESCC Cells
Plenty of evidence has revealed that the Akt/GSK-3β/Snail signaling pathway may trigger EMT to induce tumorigenesis.16 To further elucidate the potential signaling pathways involved in FAM83D-mediated EMT, we explored the main effects of FAM83D depletion on the Akt/GSK-3β/Snail signaling pathway. Our results suggested that the protein levels of phosphorylated Akt (Ser473) and phosphorylated GSK-3β (Ser9), but not total Akt or total GSK-3β, were significantly decreased after silencing FAM83D. Additionally, the expression level of Snail was also downregulated by FAM83D depletion before (Figure 6A) and after IR (Figure 6B). These findings suggest that FAM83D knockdown acts through the Akt/GSK-3β/Snail signaling pathway to hinder the progression of EMT in ESCC.
Discussion
ESCC, a common malignant tumor of the digestive tract, is characterized by early local invasion and systemic metastasis.17 Although treatment strategies have been improved, the overall survival time of patients with ESCC is short owing to the presence of tumor metastasis and recurrence and the lack of response to therapy.18 It has been reported that more than 30% of ESCC patients have metastasis at sites including the lungs, bones, brain and liver when they are diagnosed. Radiotherapy is an effective treatment for advanced cases, but the five-year survival rate of esophagus carcinoma with radiotherapy is only 20%.3 Therefore, the identification of effective therapeutic targets, such as molecules and signaling pathways involved in the proliferation and metastasis of tumors, may enhance radiosensitivity and improve survival in patients with ESCC.
FAM83D, one of the FAM83 family members, is elevated in various types of human cancers, such as malignant solid tumors.10 A recent study showed that FAM83D overexpression exerts oncogenic effects and promotes the interaction of FAM83D and FBXW7 in breast cancer cells. Moreover, FAM83D overexpression is significantly correlated with poor disease-free survival in patients with breast cancer.19 However, studies elucidating the role and mechanism of FAM83D in esophageal carcinoma have not yet been conducted. Here, we provide clinicopathological evidence and a mechanistic basis for the function of FAM83D in human esophageal carcinoma progression. Our results indicate that FAM83D expression is obviously elevated in esophageal carcinoma tissues and cell lines, which suggests that FAM83D may act as an oncogene. Similar to a previous study,20 our data demonstrated that overexpression of FAM83D is positively associated with tumor size, TNM stage, distant metastasis and relapse and negatively associated with a poor survival rate. As tumor size increased, so did TNM stage, lymph node metastasis, tumor development and FAM83D expression; these features are related to the proliferation and metastasis of tumor cells and therefore may bring about a poor prognosis by regulating the development of esophageal cancer, indicating that FAM83D may be used as an important indicator for the monitoring progression of esophageal cancer in the clinic and as a molecular marker in the diagnosis of esophageal cancer.
To analyze the significance of FAM83D overexpression in the progression of esophageal carcinoma, we designed and constructed a recombinant vector with an shRNA targeting FAM83D to inhibit FAM83D protein expression in ECA109 and KYSE30 cells and investigated the role of FAM83D knockdown in the proliferation and radiosensitivity of esophageal carcinoma cells. It has been reported that depletion of FAM83D inhibits the proliferation, and invasion of cancer cells.9 We found similar results: silencing FAM83D obviously inhibited the proliferation of tumor cells exposed to radiation or not in vitro and in vivo, and the effect of FAM83D knockdown combined with irradiation on their suppression was more obvious. Additionally, FAM83D depletion markedly increased radiosensitivity. The above results showed that radiotherapy combined with shRNA can effectively suppress the proliferation of tumor cells and promote radiosensitivity. In this research, our data further demonstrated that cell apoptosis was induced after downregulation of FAM83D and that the promotive effect was more significant after exposure to IR. The Bcl-2 family mediates a major apoptotic signal transduction cascade.21,22 In this study, we found that the induction of apoptosis in ECA109 and KYSE30 cells after IR was markedly correlated with a decrease in Bid and Mcl-1 expression and an increase in activated caspase-3, which have a crucial effect on the apoptotic program and apoptosis initiation, respectively. Therefore, the above data indicated that repression of FAM83D effectively enhanced IR-induced apoptosis and increased radiosensitivity.
In addition to proliferation and radiosensitization, FAM83D also plays a vital role in the invasion and migration of cells. Our data demonstrated that downregulation of FAM83D hindered the invasion and migration of esophageal cancer cells without irradiation, and the inhibitory effect was more significant after irradiation. Consistent with our results, a recent study demonstrated that FAM83D modulates the migration of hepatocellular carcinoma cells.7 Shi et al suggested that knockdown of FAM83D markedly suppressed the metastasis of lung adenocarcinoma cells.8 Our findings also revealed that FAM83D promoted the invasion and migration of ESCC cells.
To verify this more directly, we examined the effects of FAM83D depletion on the expression levels of several metastasis-related molecules. EMT is a key event during tumor metastasis, which occurs when tumor cells undergo cytoskeletal remodeling and acquire an aggressive phenotype.23 At the molecular level, EMT involves a decrease in E-cadherin and an increase in N-cadherin and vimentin.24 A number of studies have shown that EMT is closely related to radioresistance; Liu et al showed that Akt was activated in nasopharyngeal carcinoma SUNE-1 cells after X-ray irradiation, which promoted the radioresistance of tumor cells by inducing EMT; after blocking the activation of Akt with gsk690693, the expression level of vimentin significantly decreased in cells, the invasive activity of tumor cells was significantly weakened, and the radiosensitivity was significantly improved.25 Similar to the above study, we found that FAM83D depletion in ESCC inhibited EMT by increasing of E-cadherin expression and reducing of N-cadherin and vimentin expression before IR; the alteration was more obvious after IR, implying that FAM83D may play an important role in regulating radiosensitivity by inducing EMT to mediate the invasion and migration of cells. In addition, EMT is mediated by a variety of transcription factors. It has been reported that Snail, a nuclear transcription factor involved in EMT, can effectively prevent the transcription of E-cadherin by binding to the E-box site of the promoter region of the E-cadherin gene and subsequently inducing EMT; Snail can also be activated by PI3K.26 The ability of Snail to activate EMT is dependent on the phosphorylation of Akt and GSK-3β, which in turn promotes the location and stabilization of Snail.16 Indeed, the activated Akt/GSK-3β/Snail signaling pathway has been demonstrated to trigger EMT and leads to an aggressive phenotype in pancreatic and colon cancer.27,28 Recently, published studies have demonstrated that overexpression of FAM83D enhances the proliferation, motility and invasiveness of non-small-cell lung cancer cells, facilitates concurrent EMT-like molecular changes and is associated with the activation of the Akt/mTOR signaling pathway; however, repression of FAM83D reverses the expression of EMT markers and inhibits the Akt/mTOR pathway.29 Consistent with the above report, our data showed that suppression of FAM83D led to an obvious decrease in p-AKT, p-GSK-3β and Snail expression before and after IR, especially after IR, compared to that in the control group indicating that FAM83D helps to trigger EMT through the Akt/GSK-3β/Snail signaling pathway, which leads to radioresistance in ESCC.
Conclusion
In conclusion, the present study determined the expression and prognostic significance of FAM83D in esophageal carcinoma. Our findings demonstrated that FAM83D is overexpressed in esophageal carcinoma tissues and cell lines and correlated with poor prognosis of patients with esophageal carcinoma. The findings reveal the important regulatory effect of FAM83D knockdown on growth, radiosensitivity and apoptosis in response to irradiation. In addition, we found that FAM83D and radiation coordinately affect the invasion of ESCC cells by inducing EMT at least partially through the Akt/GSK-3β/Snail signaling pathway. Moreover, the characterization of FAM83D may support its exploration as a valuable biomarker target for the diagnosis and treatment of patients with ESCC.
Ethics Approval and Consent to Participate
Approval from patients and the Ethics Committee of the Fourth Hospital of Hebei Medical University was obtained for the usage of the specimens for research. All patients signed informed consent forms.
Author Contributions
All authors contributed to data analysis, drafted or revised the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Ma M, Zhao LM, Yang XX, et al. p-Hydroxylcinnamaldehyde induces the differentiation of oesophageal carcinoma cells via the cAMP-RhoA-MAPK signalling pathway. Sci Rep. 2016;6:31315. doi:10.1038/srep31315
3. Xu C, Xi M, Moreno A, et al. Definitive chemoradiation therapy for esophageal cancer in the elderly: clinical outcomes for patients exceeding 80 year old. Int J Radiat Oncol Biol Phys. 2017;98(4):811–819. doi:10.1016/j.ijrobp.2017.02.097
4. Walter FM, Emery JD, Mendonca S, et al. Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: results from a prospective cohort study. Br J Cancer. 2016;115(5):533–541. doi:10.1038/bjc.2016.221
5. Cipriano R, Miskimen KL, Bryson BL, et al. Conserved oncogenic behavior of the FAM83 family regulates MAPK signalling in human cancer. Mol Cancer Res. 2014;12(8):1156–1165. doi:10.1158/1541-7786.MCR-13-0289
6. Walian PJ, Hang B, Mao JH. Prognostic significance of FAM83D gene expression across human cancer types. Oncotarget. 2016;7(3):3332–3340. doi:10.18632/oncotarget.6620
7. Liao W, Liu W, Liu X, et al. Upregulation of FAM83D affects the proliferation and invasion of hepatocellular carcinoma. Oncotarget. 2015;6(27):24132–24147. doi:10.18632/oncotarget.4432
8. Shi R, Sun J, Sun Q, et al. Upregulation of FAM83D promotes malignant phenotypes of lung adenocarcinoma by regulating cell cycle. Am J Cancer Res. 2016;6(11):2587–2598.
9. Mu Y, Zou H, Chen B, et al. FAM83D knockdown regulates proliferation, migration and invasion of colorectal cancer through inhibiting FBXW7/Notch-1 signalling pathway. Biomed Pharmacother. 2017;90:548–554. doi:10.1016/j.biopha.2017.03.073
10. Wang D, Han S, Peng R, et al. FAM83D activates the MEK/ERK signalling pathway and promotes cell proliferation in hepatocellular carcinoma. Biochem Biophys Res Commun. 2015;458(2):313–320. doi:10.1016/j.bbrc.2015.01.108
11. Zhang X, Yang X, Zhu S, et al. Radiosensitization of esophageal carcinoma cells by knockdown of HMGB1 expression. Oncol Rep. 2019;41(3):1960–1970. doi:10.3892/or.2018.6923
12. Kasajima A, Papotti M, Ito W, et al. High interlaboratory and interobserver agreement of somatostatin receptor immunohistochemical determination and correlation with response to somatostatin analogs. Hum Pathol. 2018;72:144–152. doi:10.1016/j.humpath.2017.11.008
13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi:10.1006/meth.2001.1262
14. Yang XX, Ma M, Sang MX, et al. Radiosensitization of esophageal carcinoma cells by knockdown of RNF2 expression. Int J Oncol. 2016;48(5):1985–1996. doi:10.3892/ijo.2016.3404
15. Wang Y, Shi J, Chai K, et al. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–972. doi:10.2174/15680096113136660102
16. Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9(4):317–324. doi:10.1080/19336918.2015.1016686
17. Jiang L, Zhao Z, Zheng L, et al. Downregulation of miR-503 promotes ESCC cell proliferation, migration, and invasion by targeting cyclin D1. Genom Proteom Bioinf. 2017;15(3):208–217. doi:10.1016/j.gpb.2017.04.003
18. Mohamed A, El Rayes B, Khuri FR, et al. Targeted therapies in metastatic esophageal cancer: advances over the past decade. Crit Rev Oncol Hematol. 2014;91(2):186–196. doi:10.1016/j.critrevonc.2014.01.010
19. Wang Z, Liu Y, Zhang P, et al. FAM83D promotes cell proliferation and motility by downregulating tumor suppressor gene FBXW7. Oncotarget. 2013;4(12):2476–2486. doi:10.18632/oncotarget.1581
20. Lin B, Chen T, Zhang Q, et al. FAM83D associates with high tumor recurrence after liver transplantation involving expansion of CD44+ carcinoma stem cells. Oncotarget. 2016;7(47):77495–7 7507. doi:10.18632/oncotarget.12715
21. Zhu P, Zhang J, Zhu J, et al. MiR-429 induces gastric carcinoma cell apoptosis through Bcl-2. Cell Physiol Biochem. 2015;37(4):1572–1580. doi:10.1159/000438524
22. Seo TB, Kim TW, Shin MS, et al. Aerobic exercise alleviates ischemia-induced memory impairment by enhancing cell proliferation and suppressing neuronal apoptosis in hippocampus. Int Neurourol J. 2014;18(4):187–197. doi:10.5213/inj.2014.18.4.187
23. Akrida I, Nikou S, Gyftopoulos K, et al. Expression of EMT inducers integrin-linked kinase (ILK) and ZEB1 in phyllodes breast tumors is associated with aggressive phenotype. Histol Histopathol. 2018;33(9):937–949. doi:10.14670/HH-11-987
24. Rataj F, Planel S, Denis J, et al. Targeting AU-rich element-mediated mRNA decay with a truncated active form of the zinc-finger protein TIS11b/BRF1 impairs major hallmarks of mammary tumorigenesis. Oncogene. 2019;38(26):5174–5190. doi:10.1038/s41388-019-0784-8
25. Liu Y, Chen LH, Yuan YW, et al. Activation of AKT is associated with metastasis of nasopharyngeal carcinoma. Tumour Biol. 2012;33(1):241–245. doi:10.1007/s13277-011-0272-4
26. Dong C, Wu Y, Yao J, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Invest. 2012;122(4):1469–1486. doi:10.1172/JCI57349
27. Liu A, Shao C, Jin G, et al. miR-208-induced epithelial to mesenchymal transition of pancreatic cancer cells promotes cell metastasis and invasion. Cell Biochem Biophys. 2014;69(2):341–346. doi:10.1007/s12013-013-9805-3
28. Li Q, Wu J, Wei P, et al. Overexpression of fork head Box C2 promotes tumor metastasis and indicates poor prognosis in colon cancer via regulating epithelial-mesenchymal transition. Am J Cancer Res. 2015;5(6):2022–2034.
29. Yin C, Lin X, Wang Y, et al. FAM83D promotes epithelial-mesenchymal transition, invasion and cisplatin resistance through regulating the AKT/mTOR pathway in non-small-cell lung cancer. Cell Oncol (Dordr). 2020;43(3):395–407. doi:10.1007/s13402-020-00494-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.