Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Ketamine in Bipolar Disorder: A Review
Authors Wilkowska A , Szałach Ł , Cubała WJ
Received 17 September 2020
Accepted for publication 10 October 2020
Published 12 November 2020 Volume 2020:16 Pages 2707—2717
DOI https://doi.org/10.2147/NDT.S282208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Alina Wilkowska, Łukasz Szałach, Wiesław J Cubała
Department of Psychiatry, Faculty of Medicine, Medical University of Gdańsk, Gdańsk, Poland
Correspondence: Alina Wilkowska
Department of Psychiatry, Faculty of Medicine, Medical University of Gdańsk, Dębinki 7 Str, Gdańsk 80-211, Poland
Tel + 48 58 349 26 50
Fax + 48 349 27 48
Email [email protected]
Abstract: Bipolar disorder (BD) is a psychiatric illness associated with high morbidity, mortality and suicide rate. It has neuroprogressive course and a high rate of treatment resistance. Hence, there is an unquestionable need for new BD treatment strategies. Ketamine appears to have rapid antidepressive and antisuicidal effects. Since most of the available studies concern unipolar depression, here we present a novel insight arguing that ketamine might be a promising treatment for bipolar disorder.
Keywords: ketamine, bipolar disorder, staging, neuroprogression, treatment resistance
Introduction
Bipolar disorder (BD) is a burdensome and recurrent psychiatric condition that affects more than 1% of the population. It has the highest lifetime risk of suicide among all psychiatric disorders.1,2 Most patients with BD experience depression for a significant part of their lives.3,4 The treatment failure rates of bipolar depression are higher than those of major depressive disorder (MDD).5 A longer duration of bipolar depression leads to neuroprogression, which involves structural brain changes and neuropsychological deficits. Currently, there is an unmet need for both short- and long-term treatment options for patients with refractory bipolar depression.4,6 The robust antidepressant and antisuicidal effects of ketamine in treatment-resistant patients, as well as its unique mechanism of action, may point to ketamine as an interesting treatment option for BD. There are currently 140 trials of ketamine and its enantiomers registered in psychiatric disorders. Most of them include MDD patients, only 0.7% include bipolar patients, and 5.7% include both MDD and bipolar patients.7 Ketamine treatment in bipolar depression is clearly understudied; however, it is of great importance given the currently poor treatment outcomes for bipolar depression. This review describes current evidence regarding the potential benefits of ketamine for patients with BD and could serve to inform future clinical trials and clinicians on this subject.
Ketamine in Bipolar Disorder – Clinical Data
Single Administration
The rapid antidepressant effect of low-dose ketamine intravenous infusion was first reported by Berman et al in a placebo-controlled double-blind study conducted among patients with depression (including one bipolar patient); this effect was later confirmed by Zarate et al in a placebo-controlled, double-blind crossover study conducted among 18 patients with treatment-resistant MDD.8,9 Subsequent controlled studies have demonstrated the effectiveness of single low-dose ketamine in treatment-resistant cases of bipolar depression.10–12
The first randomized, placebo-controlled, double-blind, crossover study which administered ketamine to patients with bipolar depression reported an antidepressant effect when a single infusion was used as an adjunct to mood stabilizers.10 The results were replicated in a double-blind, randomized, crossover, placebo-controlled study with 15 bipolar patients.11 Similar results were obtained in another study.13 In an open-label study of 42 patients with bipolar depression, Permoda-Osip et al found that 52% responded to a single infusion of ketamine;14 the rapid antidepressant effect was replicated in a subsequent study conducted among 53 patients with bipolar depression.15 In another study, 23 and 4 patients with unipolar and bipolar depression, respectively, were administered esketamine (0.25mg/kg); almost half of the patients (48.1%) showed a response within 1 week of treatment. Ten patients (37.0%) experienced remission in the same period.16 Two meta-analyses including the above studies have also supported the effectiveness of a single ketamine infusion in the treatment of unipolar and bipolar depression.17,18 Predictors of positive response to a single ketamine infusion in unipolar and bipolar patients are high BMI (body mass index) and lower baseline levels of adiponectin suggesting that metabolic imbalance might be related to ketamine’s antidepressant effect.19,20
Multiple Administration
Data on multiple administration of ketamine in patients with bipolar depression are scarce. A very low dose of sublingual ketamine (administered every 2–3 days or weekly) was reported to produce rapid and consistent effects, which included the improvement of mood level and stability, cognition, and sleep in most patients (77%) with unipolar or bipolar depression; mild side effects were observed in 26 patients.21 Another study administered multiple ketamine intravenous infusions (six 0.5mg/kg infusions) in patients with unipolar (n=77) and bipolar (n=20) depression, and reported response and remission rates of 68% and 50.5%, respectively; however, the results of the bipolar group were not analyzed separately.22 A recent study on 6 low-dose ketamine infusions in 16 bipolar patients reported response and remission rate 73.7% and 63.2%, respectively, at 24 h after the 6th infusion.23 The most recent study by McIntyre included 213 TRD patients including 30 patients with bipolar disorder, but this subgroup was not analyzed separately. The authors observed rapid antidepressive and antisuicidal effects.24 The authors of the mentioned studies did not observe any significant side effects. It remains unclear as to how repeated ketamine infusions may affect the severity of subsequent episodes of bipolar depression. It is possible that this treatment regimen could decrease the severity of depressive symptoms, as well as improve quality of life and daily functioning.25 A recent retrospective study on IV ketamine in unipolar and bipolar depression suggests that using ketamine to treat resistant depression has a rapid effect in reducing agitation, irritability, anxiety, and suicidal ideation. Therefore, the authors suggest that this treatment should be considered in depression with mixed features, which is particularly common in bipolar disorders (2.2 in MDD vs 14.3% in BD) and related to high suicidality.26,27
Rapid Antisuicidal Effect of Ketamine
Suicidality accounts for 15–20% of deaths in BD patients.28–30 Risk of suicidal act in BD patients with mixed or psychotic symptoms is one of the highest of all psychiatric disorders.31 Currently, there are no approved pharmacological interventions for suicidality in BD. Two open-label studies found that a single intravenous ketamine infusion significantly reduced suicidal ideation in subjects with treatment-resistant depression.32,33
A systematic review examining the effects of a single dose of ketamine on suicidal ideation concluded that it was able to rapidly reduce suicidal thoughts within 1 day.18 While the majority of the included studies were conducted among patients with MDD, some of the studies also enrolled patients with BD.34 Another meta-analysis revealed a significant and sustained decrease of suicidality within 4 h of a single ketamine infusion.35 Such a rapid antisuicidal effect is in stark contrast to the delayed onset of currently available pharmacological treatment A recent open-label clinical trial that administered six intravenous doses of 0.5 mg/kg ketamine three times a week in patients with unipolar and bipolar depression reported a significant reduction in suicidal ideation 24 h after the first infusion in 57% of patients and in 65% after the sixth infusion.36
The antisuicidal mechanism of ketamine remains speculative. Plasma kynurenine levels have been implicated in the pathophysiology of suicidal behavior,37 and it has been suggested that ketamine may play a role in their modification.38 On the other hand, a post-mortem study of suicidal patients with BD, MDD, and schizophrenia suggested a possible role for microglia in the pathophysiology of these conditions.39 This is notable, considering the evidence on the anti-inflammatory effect of ketamine on microglia.40 As suggested by studies investigating the antisuicidal effect of clozapine in patients with schizophrenia, suicidality may constitute a separate symptom dimension. It is, therefore, possible that ketamine also has an antisuicidal effect which is independent of its antidepressive effect.41,42
Risk of Affective Switch
Due to a lack of data, it is unclear whether ketamine induces an affective switch in treated patients. Current evidence suggests that the risk for affective switch is increased in patients treated with antidepressants, as well as in patients with a history of substance abuse, especially opioid use disorder.41 Data from three studies, conducted among patients with treatment-resistant unipolar and bipolar depression, reported transient mood elevation in 7% and 10% of subjects administered placebo and subanesthetic doses of ketamine, respectively. As mood levels returned to baseline by the next day, the authors concluded that their results did not reflect a persistent substance-induced syndrome.43 While the neurobiological basis of affective switch has not been fully elucidated, limited evidence suggests that brain-derived neurotrophic factor (BDNF) may play a major role. One study has reported that individuals with bipolar depression who also have the val/val BDNF genotype may be at a greater risk for either a spontaneous or antidepressant-related switch to mania.42 Nevertheless, future studies are needed to confirm the risk factors for affective switch.
Ketamine in Bipolar Disorder – Molecular Data
Antidepressant Effect
Although the robust antidepressant effect of ketamine is known, the precise molecular and cellular mechanisms involved are unclear.44 The blockade of N-methyl-D-aspartate receptors (NMDARs) at inhibitory interneurons causes the disinhibition of pyramidal cells; this leads to the activation of glutamatergic transmission.44 As non-ketamine NMDAR antagonists do not exhibit such an effect in patients with depression, this suggests that mechanisms other than NMDAR inhibition play a key role in this disease. Maeng et al reported that α-amino-3-hydroxy-5-methyl-4 isoxazole propionic acid receptor (AMPAR) antagonists blocked the antidepressant effects of ketamine in rodents, implicating a role for AMPAR activation in mediating the antidepressant effect of ketamine.45 In addition to NMDA and AMPA receptors, other pathways that may be involved in the antidepressant effect of ketamine include mechanistic target of rapamycin (mTOR) and BDNF-tyrosine kinase receptor B (TrkB).
Animal models suggest that long-lasting activation of the BDNF-TrkB cascade in the prefrontal cortex (PFC) and hippocampus may be responsible for the long-term antidepressant effects of ketamine.44 Animal studies have also suggested that ketamine can increase the levels of BDNF, cyclic adenosine 3ʹ, 5ʹ-monophosphate response element binding (CREB), protein kinase C (PKC), and protein kinase A (PKA) in brain areas known to be involved in MDD, such as the PFC, hippocampus, amygdala, and nucleus accumbens.46 Wei et al47 suggested that increased CREB Ser133 phosphorylation, as well as expression of CREB and glutamate receptor 1 (GluR1), are crucial for the antidepressant effect of ketamine.
A study in mice showed that the metabolites of ketamine have rapid antidepressant effects, and that they do not act through NMDARs. Specifically, it was found that (2R, 6R)-hydroxynorketamine activated AMPARs and rapidly upregulated BDNF expression.48 Additional studies are needed to confirm the effect of (2R, 6R) HNK on BDNF, and its potential to act as an antidepressant.
Neuroplasticity and BDNF
Neurotrophins are essential for the sprouting of neurites, differentiation of neurons, and synaptogenesis.49 One of the neurotrophins involved in the pathophysiology of BD is BDNF.50 In addition to its role in neuronal maturation, differentiation, and survival, BDNF is also involved in synaptic plasticity.51 Preclinical evidence suggests that BDNF regulates the release of serotonin, glutamate, and gamma-aminobutyric acid (GABA).52,53 A BDNF gene polymorphism (66 Val/Met) may be associated with a higher risk for early-onset BD, as well as rapid cycling, suicidality, and treatment response. Apart from its role in neuroplasticity, BDNF is also involved in intracellular signal transduction.50 Several clinical studies have demonstrated decreased levels of BDNF in bipolar patients during depressive and manic episodes.51 Other studies have shown that low levels of BDNF are associated with an increased severity of bipolar episodes.54,55 This suggests the use of ketamine as a viable treatment option for bipolar patients, given that ketamine increases BDNF expression although the exact mechanism of BDNF effect in bipolar disorder needs to be elucidated.
Synaptogenesis
Evidence from preclinical studies has shown that ketamine rapidly induces synaptogenesis and reverses the synaptic changes caused by chronic stress, and that these actions are associated with its antidepressant effect.56 Ketamine has also been reported to improve spine density in the medial PFC of chronic social defeat stress-susceptible mice 1 week after the administration of a single dose.56,57 One recent animal study focusing on processes underlying acute and sustained effects of ketamine found that a single injection of ketamine caused in vivo restoration of spines in the PFC lost due to stress. The authors used the new technology of two-photon imaging to observe how chronic stress and ketamine effect dendritic spine modelling in PFC of living mice. Interestingly, behavioral improvement was observed prior to changes in spine formation. The authors suggest that maintaining the restored spines is necessary for maintaining behavioral remission and that dendritic spine formation in the PFC leads to long term, as opposed to acute, antidepressant effects of ketamine.58 This is supported by in vitro studies which have shown that ketamine treatment is correlated with increased differentiation and maturation of new neurons in the dentate gyrus.59,60 Furthermore, Yamada and Jinno have reported that ketamine increases the density of neuronal progenitors and newborn granule cells, and accelerates their maturation in the ventral hippocampus.61 However, some studies have shown contrary evidence and do not support the beneficial effect of ketamine on synaptic plasticity and the proliferation of neurons in rats.62,63 Taken together, it is likely that neurogenesis could have a role in the sustained actions of ketamine; nevertheless, this remains to be confirmed by future studies.
Epigenetics
Epigenetic changes probably play a role in the various phases of bipolar illness although this subject needs further investigation.64,65 Evidence suggests differential gene expression in depression, euthymia, and mania. In depression expression of the circadian gene cryptochrome 2 (CRY2) and neural cell adhesion molecule gene (NCAM‐140) was decreased compared to control. In euthymic bipolar patients, decreased expression of histone deacetylase (HDAC) genes was found compared to control. The elevation of the mitogen‐activated protein kinase 6 gene (MAPK6) was reported in mania compared to patients in euthymia.66,67 Manic episodes seem to cause oxidative damage to DNA, interfering with future DNA methylation.68 For example, hypomethylation of the COMT gene has been reported in patients with BD.69 One study found that ketamine produced an antidepressant effect by decreasing histone deacetylase activity in the nucleus accumbens of deprived rats; this suggested that ketamine may act via an epigenetic mechanism.70
Immunological Effects
Patients with BD present with several immunological alterations. Multiple proinflammatory cytokines, especially those responsible for innate immune hyperactivity (eg, Interleukin [IL] -1β, IL-4, IL-10, tumor necrosis factor alpha [TNF- α]) are elevated in bipolar patients.71 Increased levels of IL-1β and kynurenic acid have been reported in the cerebrospinal fluid of BD patients.72 In a post-mortem examination of brain tissue in patients with BD, PFC levels of IL-1β, IL-1R, myeloid differentiation factor 88, nuclear factor-kappa B subunits (IL-1 pathway), and astroglial and microglial neuroinflammatory markers were increased.73 Patients with BD also have a high prevalence (48.1%) of autoimmune diseases.74
In general, data pertaining to the immunological effects of ketamine in bipolar patients are limited. Studies have indicated that proinflammatory cytokines (mainly IL-6, G-CSF, and IL-1α) are reduced 4 h after a single dose of intravenous ketamine.75 One study has shown rapid decreases in levels of IL-6 and TNF-α, as well as a correlation between the decrease in TNF-α and a reduction in the Montgomery Asberg Depression Rating Scale (MADRS) score.76 Another study found that ketamine influences the kynurenine pathway by increasing the level of kynurenine, kynurenic acid and decreases the level of quinolinic acid acting as a rapid anti-inflammatory agent in patients with bipolar depression.77 No significant correlation has been found between the levels of inflammatory markers (C-reactive protein and intracellular adhesion molecule-1), before or after ketamine infusion in BD patients.14
In terms of the autoimmune effects of ketamine, in vitro studies have shown that it suppresses T-cell differentiation into Th17 cells. Th17 cells play a significant role in the autoimmunization of mice subjected to experimental autoimmune encephalomyelitis.78 This effect may indicate an immunomodulatory role for ketamine in BD patients, who have a predisposition to autoimmune disease. Further studies are needed to elucidate a potential immunomodulatory effect of ketamine in this patient population.
Microbiota
The brain-gut-microbiota axis has a role in the pathophysiology of depression79–81 through its involvement in the immune system, endocrine system, and vagus nerve.82,83 Moreover, it has been demonstrated that the gut microbiome modulates the level of central BDNF.84,85 The composition of the gut microbiota is altered in BD patients86 Negative correlations have been found between Lactobacillus counts and sleep, as well as between Bifidobacterium counts and serum cortisol levels, which are both altered in patients with depression compared to that of healthy controls.87 According to Lu et al, the antidepressive effect of quetiapine in bipolar depression can be attributed to its effects on the gut microbiota and immune activation.86
Existing evidence also suggests that the antidepressant action of ketamine can be attributed to its effects on the microbiome. For instance, randomized placebo-controlled study examining chronic ketamine administration in rats reported significant increases in the levels of low-abundance bacterial genera (eg, Lactobacillus, Turicibacter, and Sarcina), and decreases in the numbers of opportunistic pathogens.81 A study involving RNA sequencing of Chronic Social Defeat Stress (CSDS)-susceptible mice feces reported that (R)-ketamine, altered amounts of Bacteroidales, Clostridiales and Ruminococcaceae (R)-ketamine also reduced the stress-induced increases of Clostridium levels compared to control.88 Another placebo-controlled study conducted with the use of the same animal model of depression reported that (R)-ketamine administration inhibited the reduction in bacteria levels from Mollicutes class. Furthermore, both (R)- and (S)- ketamine enantiomers diminished the decrease in Butyricimonas bacteria genus.89 Furthermore, ketamine has been shown to positively affect the abnormal composition of gut microbiota in rodents with a depression-like phenotype.90 Thus, current evidence indicates that ketamine treatment improves gut microbiota composition, and mediates antidepressant effects through this mechanism.
Lithium and Ketamine
Few mechanisms of ketamine's action are shared with one of the most efficient and widely used pharmacological agents for treating BD – lithium. One of the best known is glycogen synthase kinase-3 (GSK-3) inhibition, the enzyme which is involved in a wide range of signal transduction pathways.91 GSK-3 has mainly proapoptotic effects, though its inhibition leads to neuroprotection due to the disinhibition of BDNF synthesis, long-term neuroplasticity and mood stabilization.92 Studies with knocked-in mouse models of GSK-3beta S9A showed a lack of ketamine’s antidepressant effect, although it is still unknown if ketamine inhibits GSK-3 directly,93 The final effect of GSK-3 inhibition, which is increased synthesis of BDNF, is also similar for lithium and ketamine. Ketamine’s NMDA antagonism causes activation of mTOR pathway, which increases the BDNF synthesis due to increased eukaryotic elongation factor 2 (eEF2) levels.94 Lithium can also prolong the activation of mTOR/BDNF-TrkB pathways and maintain restoration of spine density induced by a single injection of ketamine, and as a consequence, increase the antidepressant-like effects of ketamine in mice;95 this observation is particularly notable, considering the possibility of simultaneous ketamine and lithium use in BD.
Both ketamine and lithium modulate the glutamatergic system in the brain. Chronic administration of lithium causes an increase of glutamate reuptake, thus diminishes the concentration of glutamate in the synaptic cleft, preventing the excitotoxic effect of glutamate.96 Another common mechanism of action of ketamine and lithium might be immunomodulation. Lithium’s immunomodulatory effect in patients suffering from BD has been shown in several studies, both in vitro and ex vivo, and some of them seem to be similar to those found in ketamine studies. A study performed by Leu et al showed that the presence of lithium-reduced production of IL-6, TNF-α and increased secretion of IL-10 by cultivated and activated human monocyte-derived dendritic cells.97 Lithium treatment increased IL-1β production and decreased IL-6 production by activated monocytes from BD patients, causing normalization of IL1b/IL-6 ratio, similar to healthy control.98
Staging and Neuroprogression in BD
BD may present with manic and depressive episodes, often with mixed features; its variable clinical manifestations can be described in stages.99 These stages include cognitive deterioration and functional decline, changes in inflammatory and neuroanatomical biomarkers, lowered response to treatment, and a worsened self-reported quality of life, all of which have been linked to disorder progression.100 To date, there has been a lack of staging models based on psychopathology.101 Kapczinski et al.102,103 suggested a model that accounted for functioning, cognitive performance, and blood biomarkers. Cosci and Fava subsequently proposed an integrative model emphasizing the lack of evidence for stage 0 (at-risk).104 Duffy, in turn, suggested a staging model that took into consideration the natural history of BD and the heterogeneity of the different subtypes.105
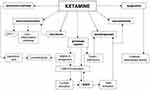
Neuroprogression is a concept that describes the pathological processes in the brain as a consequence of the toxicity-associated mood episodes.106 A large body of evidence supports the presence of structural brain abnormalities and reduced connectivity in bipolar patients.107,108 The process underlying neuroprogressive changes in BD patients is still poorly understood. Fries et al suggested that the epigenetic modifications in individuals with BD appear earlier in life compared to healthy individuals.109 Other correlates of aging and neuroprogression in mood disorders include reduced BDNF levels and oxidative stress imbalances.103,110–113 A recent imaging study found that bipolar patients with extensive blood-brain barrier (BBB) leakage had more severe and chronic symptoms. Furthermore, the authors also found insulin resistance in this group of patients and suggest that it may increase BBB dysfunction in BD.114 The role of insulin resistance in neuroprogression in BD has been previously described.115 Moreover, dysregulated glucose metabolism (insulin resistance or type 2 diabetes mellitus) is present in more than half of patients with BD and appears more often in patients with poor treatment response, cognitive impairment, and poor functioning. Hence, the proposed mechanisms connecting insulin resistance and neuroprogression are oxidative stress, lipid peroxidation, and endothelial dysfunction.115 The chronic and refractory nature of bipolar illness along with aging has a synergistic impact on the decline of neurotrophic signaling and an increase in inflammation.113,116,117 Indeed, an imbalance between inflammatory cytokines (especially TNF-α), mediators of oxidative stress, and BDNF is associated with the progression of structural brain changes and neurocognitive decline.117,118 Although this possibility requires further studies, ketamine could hypothetically influence the progression of BD by increasing BDNF levels through an epigenetic mechanism effecting histone acetylation and the induction of neuroplasticity. Furthermore, as mentioned previously, ketamine has demonstrated to have a better antidepressive effect in patients with high BMI and low adipokine levels,19,20 suggesting that ketamine may interfere with the metabolic mechanisms of neuroprogression. Moreover, one aspect of neuroprogression is cognitive dysfunction; currently, some evidence show that single and multiple ketamine infusions in subanaesthetic dose improve cognitive functions in patients with unipolar and bipolar depression.119,120 Possible mechanisms of ketamine's action in bipolar disorder are presented in Figure 1.
Discussion
We hypothesize that the use of ketamine as an adjunctive treatment can have beneficial short-term and long-term effects on the course of BD. Previous studies, although still scarce have demonstrated the short-term antidepressant and antisuicidal effects of ketamine in patients with BD, and its administration also appears to carry a low risk of affective switch.7–36
Studies on the molecular mechanism of action of ketamine indicate that it has effects on glutamatergic transmission, BDNF levels, and intracellular signal transduction, which are all perturbed in patients with BD. There is mounting evidence for the beneficial effect of ketamine on synaptogenesis and neuroplasticity; such long-term effects are particularly interesting, considering the neuroprogressive nature of BD.38–55,121 There is some evidence for the modifying effects of ketamine on epigenetic processes present in BD,64–70 as well as its ability to regulate inflammation.62–69 Ketamine may also have favorable effects on gut microbiota, which have been shown to be disturbed in patients with BD.71–78 The majority of the above-mentioned effects, however, are based on preliminary evidence and engage unipolar depression model, and therefore require confirmation by studies in bipolar disorder.
Funding
This paper was funded by the Medical University of Gdańsk, Poland (Grant No. ST-02-0039/07/221).
Disclosure
Dr Alina Wilkowska has received research support from Angelini, Biogen, Eli Lilly and Company, Janssen- Cilag, Lundbeck, Polpharma, Sanofi and Valeant. Dr. Łukasz Szałach has received support from Angelini and +Pharma. Prof. Wiesław Jerzy Cubała has received research support from Acadia, Actavis, Alkermes, Allergan, Apodemus, Auspex, Biogen, Bristol-Myers Squibb, Cephalon, Celon, Cortexyme, Eli Lilly, Ferrier, Forest Laboratories, Gedeon Richter, GW Pharmaceuticals, Janssen, KCR, Lundbeck, NIH, NeuroCog, Orion, Otsuka, Sanofi, and Servier; he has served on speakers bureaus for Adamed, Angelini, AstraZeneca, Bristol-Myers Squibb, Celon, GlaxoSmithKline, Janssen, Krka, Lekam, Lundbeck, Novartis, Orion, Pfizer, Polfa Tarchomin, Sanofi, Servier, and Zentiva; and he has served as a consultant for GW Pharmaceuticals, Janssen, KCR, Quintiles, and Roche. The authors report no other potential conflicts of interest for this work.
References
1. Abreu LN, Lafer B, Baca-Garcia E, Oquendo MA. Suicidal ideation and suicide attempts in bipolar disorder type I: an update for the clinician. Rev Bras Psiquiatr. 2009;31:271–280. doi:10.1590/s1516-44462009005000003
2. Jamison KR. Suicide and bipolar disorder. J Clin Psychiatry. 2000;61:47–51.
3. Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9:531–535. doi:10.1111/j.1399-5618.2007.00467.x
4. Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214:27–35. doi:10.1192/bjp.2019.36
5. Li CT, Bai YM, Huang YL, et al. Association between antidepressant resistance in unipolar depression and subsequent bipolar disorder: cohort study. Br J Psychiatry. 2012;200:45–51. doi:10.1192/bjp.bp.110.086983
6. Tondo L, Vázquez GH, Baldessarini RJ. Options for pharmacological treatment of refractory bipolar depression. Curr Psychiatry Rep. 2014;16:431. doi:10.1007/s11920-013-0431-y
7. Peyrovian B, McIntyre RS, Phan L, et al. Registered clinical trials investigating ketamine for psychiatric disorders. J Psychiatr Res. 2020;127:1–12. doi:10.1016/j.jpsychires.2020.03.020
8. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi:10.1016/S0006-3223(99)00230-9
9. Zarate CA
10. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi:10.1001/archgenpsychiatry.2010.90
11. Zarate CA
12. Grunebaum MF, Ellis SP, Keip JG, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19(3):176–183. doi:10.1038/s41386-019-0317-8
13. Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CA
14. Permoda-Osip A, Skibinska M, Bartkowska-Sniatkowska A, Kliwicki S, Chlopocka-Wozniak M, Rybakowski JK. Factors connected with efficacy of single ketamine infusion in bipolar depression. Psychiatr Pol. 2014;48:35–47. doi:10.12740/PP/21175
15. Rybakowski JK, Permoda-Osip A, Bartkowska-Sniatkowska A. Ketamine augmentation rapidly improves depression scores in inpatients with treatment-resistant bipolar depression. Int J Psychiatry Clin Pract. 2017;21:99–103. doi:10.1080/13651501.2017.1297834
16. Correia-Melo FS, Argolo FC, Araujo-de-Freitas L, et al. Rapid infusion of esketamine for unipolar and bipolar depression: a retrospective chart review. Neuropsychiatr Dis Treat. 2017;13:1627–1632. doi:10.2147/NDT.S135623
17. Kishimoto T, Chawia JM, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–1472. doi:10.1017/S0033291716000064
18. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;175:150–158. doi:10.1176/appi.ajp.2017.17040472
19. Niciu MJ, Luckenbaugh DA, Ionescu DF, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75:e417–e423. doi:10.4088/JCP.13m08698
20. Machado-Vieira R, Gold PW, Luckenbaugh DA, et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry. 2017;22:127–133. doi:10.1038/mp.2016.36
21. Lara DR, Bisol LW, Munari LR. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol. 2013;16:2111–2117. doi:10.1017/S1461145713000485
22. Zheng W, Zhou YL, Liu WJ, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. Psychiatry Res. 2018;106:61–68. doi:10.1016/j.jpsychires.2018.09.013
23. Zheng W, Zhou YL, Liu WJ, et al. A preliminary study of adjunctive ketamine for treatment-resistant bipolar depression. J Affect Disord. 2020;275:38–43. doi:10.1016/j.jad.2020.06.020
24. McIntyre RS, Rodrigues NB, Lee Y, et al. The effectiveness of repeated intravenous ketamine on depressive symptoms, suicidal ideation and functional disability in adults with major depressive disorder and bipolar disorder: results from the Canadian rapid treatment center of excellence. J Affect Disord. 2020;274:903–910. doi:10.1016/j.jad.2020.05.088
25. McAllister-Williams RH, Arango C, Blier P, et al. The identification, assessment and management of difficult-to-treat depression: an international consensus statement. J Affect Disord. 2020;15(267):264–282. doi:10.1016/j.jad.2020.02.023
26. McIntyre RS, Lipsitz O, Rodrigues NB, et al. The effectiveness of ketamine on anxiety, irritability, and agitation: implications for treating mixed features in adults with major depressive or bipolar disorder. Bipolar Disord. 2020. doi:10.1111/bdi.12941
27. Shim IH, Lee J, Kim MD, et al. The prevalence and diagnostic classification of mixed features in patients with major depressive episodes: a multicenter study based on the DSM-5. Int J Methods Psychiatr Res. 2019;28(3):e1773. doi:10.1002/mpr.1773
28. Schaffer A, Isometsä ET, Tondo L, et al. International society for bipolar disorders task force on suicide: metaanalyses and meta-regression of correlates of suicide attempts and suicide deaths in bipolar disorder. Bipolar Disord. 2015;17:1–16. doi:10.1111/bdi.12271
29. Carter TD, Mundo E, Parikh SV, Kennedy JL. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37:297–303. doi:10.1016/S0022-3956(03)00052-9
30. Baldessarini RJ, Pompili M, Tondo L. Suicide in bipolar disorder: risks and management. CNS Spectr. 2006;11(6):465–471. doi:10.1017/S1092852900014681
31. Baldessarini RJ, Tondo L, Vázquez GH. Pharmacological treatment of adult bipolar disorder. Mol Psychiatry. 2019;24(2):198–217. doi:10.1038/s41380-018-0044-2
32. Price RB, Nock MK, Charney DS, et al. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi:10.1016/j.biopsych.2009.04.029
33. DiazGranados N, Ibrahim LA, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi:10.4088/JCP.09m05327blu
34. Ballard ED, Ionescu DF, Vande Voort JL, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–166. doi:10.1016/j.jpsychires.2014.07.027
35. Bartoli F, Riboldi I, Crocamo C, Di Brita C, Clerici M, Carrà G. Ketamine as a rapid-acting agent for suicidal ideation: a meta-analysis. Neurosci Biobehav Rev. 2017;77:232–236. doi:10.1016/j.neubiorev.2017.03.010
36. Zhan Y, Zhang B, Zhou Y, et al. A preliminary study of anti-suicidal efficacy of repeated ketamine infusions in depression with suicidal ideation. J Affect Disord. 2019;15(251):205–212. doi:10.1016/j.jad.2019.03.071
37. Sublette ME, Galfalvy HC, Fuchs D, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25:1272–1278. doi:10.1016/j.bbi.2011.05.002
38. Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–117. doi:10.1016/j.bbi.2014.07.012
39. Steiner J, Bielau H, Brisch R, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi:10.1016/j.jpsychires.2006.10.013
40. Verdonk F, Petit AC, Abdel-Ahad P, et al. Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav Immun. 2019;81:361–373. doi:10.1016/j.bbi.2019.06.033
41. Allen ND, Rodysill BR, Bostwick JM. A report of affective switching associated with ketamine: the case of ketamine-induced mania is not closed. Bipolar Disord. 2019;21(2):176–178. doi:10.1111/bdi.12728
42. Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA
43. Niciu MJ, Luckenbaugh DA, Ionescu DF, Mathews DC, Richards EM, Zarate CA
44. Yang C, Yang J, Luo A, Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry. 2019;9(1):280. doi:10.1038/s41398-019-0624-1
45. Maeng S, Zarate CA, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi:10.1016/j.biopsych.2007.05.028
46. Réus GZ, Stringari RB, Ribeiro KF, et al. Ketamine plus imipramine treatment induces antidepressant- like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res. 2011;221(1):166–171. doi:10.1016/j.bbr.2011.02.024
47. Wei Z, Zhang K, Zhou Q, et al. Differential mechanisms underlying antidepressant responses of ketamine and imipramine. CNS Neurol Disord Drug Targets. 2017;16(7):846–853. doi:10.2174/1871527316666170428123248
48. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi:10.1038/nature17998
49. Freund N, Juckel G. Bipolar disorder: its etiology and how to model in rodents. Methods Mol Biol. 2019;2011:61–77. doi:10.1007/978-1-4939-9554-7_4
50. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98. doi:10.3389/fpsyt.2014.00098
51. Grande I, Fries GR, Kunz M, Kapczinski F. The role of BDNF as a mediator of neuroplasticity in bipolar disorder. Psychiatry Investig. 2010;7:243–250. doi:10.4306/pi.2010.7.4.243
52. Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi:10.1523/JNEUROSCI.5510-07.2008
53. Shaltiel G, Chen G, Manji HK. Neurotrophic signalling cascades in the patho-physiology and treatment of bipolar disorder. CurrOpinPharmacol. 2007;7:22–26. doi:10.1016/j.coph.2006.07.005
54. Cunha AB, Frey BN, Andreazza AC, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. NeurosciLett. 2006;398:215–219. doi:10.1016/j.neulet.2005.12.085
55. Machado-Vieira R, Dietrich MO, Leke R, et al. Decreased plasma brain derived neurotrophic factor levels in unmedicated bipolar patients during manic episode. BiolPsychiatry. 2007;61:142–144. doi:10.1016/j.biopsych.2006.03.070
56. Yang C, Shirayama Y, Zhang J-C, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi:10.1038/tp.2015.136
57. Dong C, Zhang JC, Yao W, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int J Neuropsychopharmacol. 2017;20:228–236.
58. Moda-Sava RN, Murdock MH, Parekh PK, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:
59. Ma Z, Zang T, Birnbaum SG, et al. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun. 2017;8(1):1668. doi:10.1038/s41467-017-01709-8
60. Soumier A, Carter RM, Schoenfeld TJ, Cameron HA. New hippocampal neurons mature rapidly in response to ketamine but are not required for its acute antidepressant effects on neophagia in rats. eNeuro. 2016;3(2). doi:10.1523/ENEURO.0116-15.2016
61. Yamada J, Jinno S. Potential link between antidepressant-like effects of ketamine and promotion of adult neurogenesis in the ventral hippocampus of mice. Neuropharmacology. 2019;158:107710. doi:10.1016/j.neuropharm.2019.107710
62. Choi M, Lee SH, Chang HL, Son H. Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochim Biophys Acta. 2016;1862(7):1247–1254. doi:10.1016/j.bbadis.2016.04.001
63. Michaelsson H, Andersson M, Svensson J, et al. The novel antidepressant ketamine enhances dentate gyrus proliferation with no effects on synaptic plasticity or hippocampal function in depressive-like rats. Acta Physiol. 2019;225(4):e13211. doi:10.1111/apha.13211
64. Ludwig B, Dwivedi Y. Dissecting bipolar disorder complexity through epigenomic approach. Mol Psychiatry. 2016;21:1490–1498.
65. Machado-Vieira R, Ibrahim L, Zarate CA
66. Hobara T, Uchida S, Otsuki K, et al. Altered gene expression of histone deacetylases in mood disorder patients. J Psychiatr Res. 2010;44:263–270. doi:10.1016/j.jpsychires.2009.08.015
67. Munkholm K, Vinberg M, Berk M, Kessing LV. State-related alterations of gene expression in bipolar disorder: a systematic review. Bipolar Disord. 2012;14:684–696. doi:10.1111/bdi.12005
68. Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int J Neuropsychopharmacol. 2013;16:1505–1512. doi:10.1017/S1461145713000047
69. Abdolmaleky HM, Cheng KH, Faraone SV, et al. Hypomethylation of MB-COMT promoter is a major risk factor for Schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15:3132–3145. doi:10.1093/hmg/ddl253
70. Réus GZ, Abelaira HM, Dos Santos MA, et al. Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res. 2013;256:451–456. doi:10.1016/j.bbr.2013.08.041
71. Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74(1):15–25. doi:10.1016/j.biopsych.2013.01.007
72. Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. 2018;44(1):75–83. doi:10.1093/schbul/sbx035
73. Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15(4):384–392. doi:10.1038/mp.2009.47
74. Perugi G, Quaranta G, Belletti S, et al. General medical conditions in 347 bipolar disorder patients: clinical correlates of metabolic and autoimmune-allergic diseases. J Affect Disord. 2015;170:95–103. doi:10.1016/j.jad.2014.08.052
75. Kiraly DD, Horn SR, Van Dam NT, et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7:e1065. doi:10.1038/tp.2017.31
76. Chen MH, Li CT, Lin WC, et al. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: a randomized, double-blind control study. Psychiatry Res. 2018;269:207–211. doi:10.1016/j.psychres.2018.08.078
77. Kadriu B, Farmer CA, Yuan P, et al. The kynurenine pathway and bipolar disorder: intersection of the monoaminergic and glutamatergic systems and immune response. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0589-8
78. Lee JE, Lee JM, Park YJ, et al. Inhibition of autoimmune Th17 cell responses by pain killer ketamine. Oncotarget. 2017;8(52):89475–89485. doi:10.18632/oncotarget.18324
79. Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodelling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–796.
80. Macedo D, Filho AJ, de Sousa CNS, Quevedo J, Barichello T
81. Getachew B, Aubee JI, Schottenfeld RS, Csoka AB, Thompson KM, Tizabi Y. Ketamine interactions with gut-microbiota in rats: relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018;18:222.
82. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi:10.1038/nrgastro.2009.35
83. El Aidy S, Dinan TG, Cryan JF. Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin Ther. 2015;37:954–967. doi:10.1016/j.clinthera.2015.03.002
84. Bercik P, Collins SM, Verdu EF. Microbes and the gut–brain axis. Neurogastroenterol Motil. 2012;24(5):405–413.
85. Maqsood R, Stone TW. the gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem Res. 2016;41(11):2819–2835.
86. Lu Q, Lai J, Lu H, et al. Gut microbiota in bipolar depression and its relationship to brain function: an advanced exploration. Front Psychiatry. 2019;29;10:784. doi:10.3389/fpsyt.2019.00784
87. Aizawa E, Tsuji H, Asahara T, et al. Bifidobacterium and lactobacillus counts in the gut microbiota of patients with bipolar disorder and healthy controls. Front Psychiatry. 2018;9:730. doi:10.3389/fpsyt.2018.00730
88. Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci Rep. 2017;7:15725.
89. Yang C, Qu Y, Fujita Y, et al. Possible role of gut-microbiota in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. 2017;7:1294.
90. Huang N, Hua D, Zhan G, et al. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav. 2018;176:93–100.
91. Cui H, Meng Y, Bulleit RF. Inhibition of glycogen synthase kinase 3beta activity regulates proliferation of cultured cerebellar granule cells. Brain Res Dev Brain Res. 1998;111(2):177–188.
92. Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29(1):32–38.
93. Polter A, Beurel E, Yang S, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35(8):1761–1774.
94. Costemale-Lacoste JF, Guilloux JP, Gaillard R. The role of GSK-3 in treatment-resistant depression and links with the pharmacological effects of lithium and ketamine: a review of the literature. Encephale. 2016;42(2):156–164.
95. Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol Psychiatry. 1999;4(2):117–128.
96. Chiu CT, Scheuing L, Liu G, et al. The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int J Neuropsychopharmacol. 2014;18:pyu102.
97. Leu SJ, Yang YY, Liu HC, et al. Valproic acid and lithium meditate anti-inflammatory effects by differentially modulating dendritic cell differentiation and function. J Cell Physiol. 2017;232(5):1176–1186.
98. Knijff EM, Breunis MN, Kupka RW, et al. An imbalance in the production of IL-1beta and IL-6 by monocytes of bipolar patients: restoration by lithium treatment. Bipolar Disord. 2007;9(7):743–753. doi:10.1111/j.1399-5618.2007.00444.x
99. Vieta E, Salagre E, Grande I, et al. Early intervention in bipolar disorder. Am J Psychiatry. 2018;175(5):411–426. doi:10.1176/appi.ajp.2017.17090972
100. de la Fuente-tomás L, Sierra P, Sanchez-Autet M, et al. A clinical staging model for bipolar disorder: longitudinal approach. Transl Psychiatry. 2020;10:45. doi:10.1038/s41398-020-0718-9
101. Berk M, Conus P, Lucas N, et al. Setting the stage: from prodrome to treatment resistance in bipolar disorder. Bipolar Disord. 2007;9(7):671–678. doi:10.1111/j.1399-5618.2007.00484
102. Kapczinski F, Dias VV, Kauer-Sant’Anna M, et al. Clinical implications of a staging model for bipolar disorders. Expert Rev Neurother. 2009;9:957–966. doi:10.1586/ern.09.31
103. Kapczinski F, Dias VV, Kauer-Sant’Anna M, et al. The potential use of biomarkers as an adjunctive tool for staging bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1366–1371. doi:10.1016/j.pnpbp.2009.07.027
104. Cosci F, Fava GA. Staging of mental disorders: systematic review. Psychother Psychosom. 2013;82(1):20–34. doi:10.1159/000342243
105. Duffy A. Toward a comprehensive clinical staging model for bipolar disorder: integrating the evidence. Can J Psychiatry. 2014;59(12):659–666. doi:10.1177/070674371405901208
106. Passos IC, Mwangi B, Vieta E, Berk M, Kapczinski F. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134:91–103. doi:10.1111/acps.12581
107. Suh JS, Schneider MA, Minuzzi L, et al. Cortical thickness in major depressive disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:287–302. doi:10.1016/j.pnpbp.2018.08.008
108. Syan SK, Smith M, Frey BN, et al. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J Psychiatry Neurosci. 2018;43:298–316. doi:10.1503/jpn.170175
109. Fries GR, Bauer IE, Scaini G, et al. Accelerated epigenetic aging and mitochondrial DNA copy number in bipolar disorder. Transl Psychiatry. 2017;7:1283. doi:10.1038/s41398-017-0048-8
110. Maurya PK, Noto C, Rizzo LB, et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:134–144. doi:10.1016/j.pnpbp.2015.08.016
111. Gama CS, Kunz M, Magalhães PV, Kapczinski F. Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Braz J Psychiatry. 2013;35:70–74. doi:10.1016/j.rbp.2012.09.001
112. Fries GR, Pfaffenseller B, Stertz L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14:667–675. doi:10.1007/s11920-012-0319-2
113. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–817. doi:10.1016/j.neubiorev.2010.10.001
114. Kamintsky L, Cairns KA, Veksler R, et al. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. Neuroimage Clin. 2020;26:102049. doi:10.1016/j.nicl.2019.102049
115. Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5–6):281–293. doi:10.1080/07853890.2019.1659511
116. Grande I, Magalhaes PV, Kunz M, Vieta E, Kapczinski F. Mediators of allostasis and systemic toxicity in bipolar disorder. Physiol Behav. 2012;106:46–50. doi:10.1016/j.physbeh.2011.10.029
117. Kapczinski F, Vieta E, Andreazza AC, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–692. doi:10.1016/j.neubiorev.2007.10.005
118. Yatham LN, Kapczinski F, Andreazza AC, Trevor Young L, Lam RW, Kauer-Sant’anna M. Accelerated age-related decrease in brain-derived neurotrophic factor levels in bipolar disorder. Int J Neuropsychopharmacol. 2009;12:137–139. doi:10.1017/S1461145708009449
119. Permoda-Osip A, Kisielewski J, Bartkowska-Sniatkowska A, Rybakowski JK. Single ketamine infusion and neurocognitive performance in bipolar depression. Pharmacopsychiatry. 2015;48(2):78–79. doi:10.1055/s-0034-1394399
120. Zhou Y, Zheng W, Liu W, et al. Neurocognitive effects of six ketamine infusions and the association with antidepressant response in patients with unipolar and bipolar depression. J Psychopharmacol. 2018;32(10):1118–1126. doi:10.1177/0269881118798614
121. Hennen J, Baldessarini RJ. Reduced suicidal risk during treatment with clozapine: meta-analysis. Schizophre Res. 2005;73:139–145. doi:10.1016/j.schres.2004.05.015
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.