Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Keloid treatment: what about adjuvant radiotherapy?
Authors Petrou IG, Jugun K, Rüegg EM, Zilli T, Modarressi A, Pittet-Cuénod B
Received 25 January 2019
Accepted for publication 1 April 2019
Published 3 May 2019 Volume 2019:12 Pages 295—301
DOI https://doi.org/10.2147/CCID.S202884
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Ilias G Petrou,1 Kheeldass Jugun,2 Eva Meia Rüegg,1 Thomas Zilli,3 Ali Modarressi,1 Brigitte Pittet-Cuénod1
1Division of Plastic, Reconstructive and Aesthetic Surgery, Geneva University Hospitals, University of Geneva Faculty of Medicine, Geneva, Switzerland; 2Private Practice, Wellkin Hospital, Moka, Mauritius; 3Division of Radiation Oncology, Geneva University Hospitals, University of Geneva Faculty of Medicine, Geneva, Switzerland
Background: Keloids are debilitating fibrous skin proliferations with a high recurrence rate after surgical treatment. Postoperative radiotherapy (PORT) is a well-tolerated adjuvant treatment to reduce the risk of recurrence, but the optimal regimen for this combined treatment remains unknown. The aim of this study is to evaluate the efficacy of combining surgical excision and immediate PORT.
Methods: We retrospectively reviewed the records of patients with keloid lesions treated with adjuvant PORT in the period 2005–2014 at Geneva University Hospitals. Main outcomes were the rates of complications and recurrence in patients with a minimal follow-up of 1 year, including the Patient and Observer Scar Assessment Scale satisfaction scores.
Results: 10 patients with 16 keloids were eligible (mean follow-up, 37 months). Only one recurrence was reported (6%). In 12.5% of cases, mild erythema appeared in the early postoperative period. No major complications were observed. The overall patient and observer satisfaction rate was excellent.
Conclusion: Surgical excision combined with immediate PORT is an effective and easy treatment with good esthetic results and an acceptable recurrence rate. It should be considered for patients with persistent keloid formation after failure of other treatments and those at high risk of relapse.
Keywords: keloid, scars, radiotherapy, intralesional excision, scar correction, recurrence
Introduction
Keloids are benign fibrous skin proliferations, which develop after a skin injury. Similar to hypertrophic scars, keloids are the result of a fibroproliferative disorder in the reticular dermis.1 Contrary to hypertrophic scars, the main clinical characteristic of keloids is the tendency to invade the healthy skin and to extend beyond the initial wound limits. They form an indurate, raised firm mass with irregular margins, which may increase in size over the years. Their surface is glabrous, hypo- or hyperpigmented, smooth or bumpy, sometimes giving them a tumor-like appearance.2 They can also be responsible for itching and pain and may appear after several months or even years of the initial injury. The epidemiology of keloids varies greatly according to the Fitzpatrick skin type, with an incidence ranging from 4.5% to 16% in type VI Fitzpatrick skin type to only 0.09% in type I.3 The most frequently affected areas are the upper parts of the body, the pubic region and the ears (especially the lobules).2
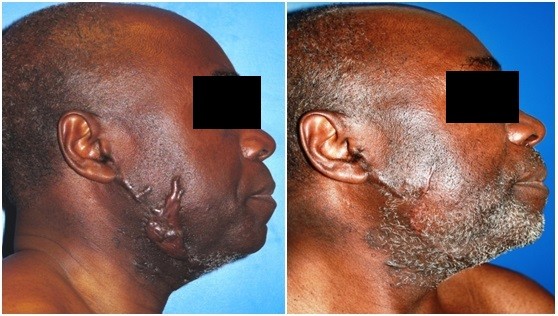
 | Figure 3 Jugal keloid. Before and after treatment result at 13 months’ follow-up. |
 | Figure 4 Auricular keloid. Before and after treatment result at 2-year follow-up. |
The pathophysiology of keloids and hypertrophic scars remains unknown. The activity of fibroblasts, extracellular matrix components, growth factors and cytokines, as well as immunological and genetic factors, have been studied in order to delineate the molecular basis of excessive fibrosis leading to the formation of these pathologic scars. In vitro, keloid fibroblasts react abnormally to stimulation and show a greater synthesis capacity of collagen (mainly type I), elastin, fibronectin and proteoglycans.4,5 By contrast, hypertrophic scar fibroblasts respond normally to growth factors and show only a slight increase in collagen production.6,7 Several studies have demonstrated the association of high levels of TGF-β), insulin-like growth factor-1 and platelet-derived growth factor with an increase in collagen synthesis by keloid fibroblasts.8,9 However, the hypothesis of the involvement of immunological and genetic factors in the formation of pathological scars remains very contradictory according to the literature.10,11
Histologically, hypertrophic scars and keloids are both mono- and multinodular dermal formations composed of bundles of collagen in which no elastic fibers are found. This nodular architecture makes it possible to distinguish them from non-pathological scars, which are characterized by a horizontalization of the collagenic dermal bundles without nodular formation. Moreover, in pathological scars, there is an excess of dermal collagen compared to healthy skin and normal scars. Simple histological criteria exist to differentiate hypertrophic scars from keloid scars.12 Keloid scars have indurated hyalinized and homogeneous collagen bundles. These beams are not organized parallel to the epidermis, but in clusters. Hyalinization is a pathognomonic sign that distinguishes them from hypertrophic scars.13
Keloids present a physical and esthetic discomfort with significant psychological and social repercussions and patients are often highly motivated to undergo even aggressive therapies. The goals of treating keloids are the elimination of symptoms and improvement of the esthetic aspect of the scar without any recurrence. Non-invasive and invasive treatments have been extensively described, such as medical ointments,14 compression15,16 and occupational therapy,17 intralesional injections of corticosteroids,18 non-steroidal products,19,20 surgical excision,21 laser treatment,22,23 intra-lesional cryotherapy,24,25 and postoperative radiotherapy (PORT).26
Recurrence is a common clinical feature. Surgical excision is the most radical therapeutic option, but the recurrence rate after simple excision is generally over 50% at 1-year follow-up.27 The combination of surgical excision with corticosteroid injections has been proposed, but still leads to an important recurrence rate of 30–50%.28 Treatment combining surgical excision and adjuvant PORT has emerged as the method of choice, with a relatively low recurrence rate ranging from 6% to 27% according to the literature.29,30
Nevertheless, there is a considerable variation in radiotherapy protocols that can be used postoperatively with different fractionation schedules, radiotherapy techniques and interval times between excision and PORT. In addition, in some PORT series, patients received additional injections of corticosteroids, thus adding another bias to the analysis of the final outcome. Destruction of fibroblasts by radiation without replacement by bloodborne cells from distant tissues has been proposed as a possible mechanism explaining the efficacy of PORT. The most favorable treatments are clearly related to a short time interval between surgical excision and PORT (reduction of an accelerated repopulation of fibroblast and induction of apoptosis).31 Moreover, a clear dose–effect relationship has been described,30 with higher biologically effective doses associated with a lower risk of recurrence.32
In this retrospective study, we analyzed the intra-institutional results of the surgical excision of keloid scars combined with a standardized adjuvant radiotherapy protocol delivered in the immediate postoperative setting among patients at high risk of recurrence. We describe the outcomes of this combined treatment, together with patient and observer satisfaction rates. Complications and the recurrence rate are also compared with the available literature.
Materials and methods
Study design and patients
From 2005 to 2014, a total of 30 patients with 36 keloid lesions benefited from intralesional excision of a keloid scar and adjuvant PORT at the Division of Plastic, Reconstructive and Esthetic Surgery, Geneva University Hospitals (Geneva, Switzerland). Inclusion criteria were the presence of a keloid lesion confirmed by histopathological examination, the absence of concomitant local or systematic treatments that could interfere with the healing process, age >18 years, an absence of serious health conditions and a follow-up of >1 year. Data collected from plastic surgery and radiation-oncology medical charts included demographics, location of keloids, previous treatments, description of initial scars, and patient complaints. Each patient underwent a preoperative consultation with the surgeon and the radio-oncologist where the risks and benefits of the combined treatment were clearly explained. The protocol was reviewed and approved by Geneva University Hospitals institutional review board for scientific research (#GE14-158). Written informed consent for the use of the data collected as well as their images was obtained from all participants.
Radiotherapy protocol
All patients were treated with PORT as the only adjuvant treatment following keloid excision. A 6-MeV electron beam technique was used in all but one patient who was treated with 100 kV X-rays. The delivered dose was 15 Gy in three daily fractions of 5 Gy (biological equivalent dose = 23.8 Gy) applied with a 1–1.5 cm margin around the surgical scar. A 0.5 cm tissue-equivalent bolus material was used in patients treated with electrons to achieve a homogeneous and precise depth dose distribution. The first PORT fraction was delivered within 2 hrs after surgical excision, followed by two other daily fractions for an overall treatment time of 3 days. Customized tailored blocks were prepared to shield the surrounding skin and spare organs at risk in proximity of the keloid scar. The region underneath the earlobes was shielded to avoid irradiation of the mastoid. All patients were treated on an outpatient basis.
Outcome evaluation
Patients were reviewed at 2- and 6-week follow-up and then every 3 months. Patients and the surgeon evaluated each scar using the Patient and Observer Scar Assessment Scale (POSAS),33,34 a well-established scar assessment tool that incorporates the subjective symptoms of pain and pruritus reported by patients, as well as the objective description of the lesion by the observer. The percentage of complications due to surgery or radiotherapy, the rate of pain and pruritus relief and the recurrence incidence were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 grading scale.
Results
Patient characteristics
Among the 30 patients, only 10 met the inclusion criteria and accepted to participate in the study. Sixteen keloids were treated (6 men; 4 women; average age, 34.5 years [range, 19–56]). Eight patients (80%) had already undergone one or more treatments; six patients with cortisone infiltrations and two with an excision of their keloids, as well as cortisone infiltrations. Two patients presented Fitzpatrick skin type II, 1 patient, type III and 7 patients, type VI. Fifty percent of treated keloids were cervical, 25% auricular, 12% in the trunk region including the upper limbs and 13% pubic (Figure 1). Median surface size was 5.14 cm2 (range, 1.2–25). Twenty-five percent of lesions were not symptomatic and they were removed for esthetic reasons. Among the symptomatic lesions, the main symptom was pain in 37.5% of cases and pruritus in 62.5%.
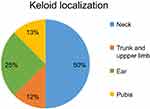 | Figure 1 Keloid localization. |
Treatment efficacy
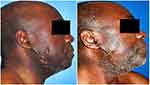
Mean follow-up was 37 months. According to the POSAS, the mean overall patient satisfaction rate was 1.7/10 (score of 10 being the worst), similar to the observer mean (1.7/10) (Table 1). Figures 2–5 illustrate some cases with an excellent POSAS score after a mean follow-up of 18 months. Only one recurrence was observed among the 16 lesions treated (6%). This recurrence appeared 6 months after treatment in the pubic zone of a 37-year-old woman, probably due to repetitive mechanical traumatic hair removal performed by the patient.
 | Table 1 Patient and observer evaluation using the Patient and Observer Scar Assessment Scale (POSAS) |
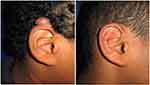 | Figure 2 Auricular keloid. Before and after treatment result at 2-year follow-up. |
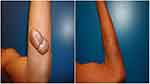 | Figure 5 Forearm keloid. Before and after treatment result at 2-year follow-up. |
Complications: adverse events
Erythema as an early adverse event of radiotherapy was observed in 12.5% of lesions treated, with spontaneous disappearance at 1–2 weeks after radiation (Grade I according to the CTCAE v3.0 grading scale). No major complications were observed.
Discussion
At present, there is neither a satisfactory therapeutic solution nor a real consensus for the management of keloids. The relapse rate has been significantly associated with the failure of previous treatments, low growth rate of the lesion, high body mass index, as well as low patient compliance with treatment.35 Intralesional excision of keloids in combination with adjuvant radiotherapy appears to be a promising treatment with a recurrence rate of 6–27%. However, published reports show a substantial heterogeneity in the inclusion criteria, the therapeutic protocol and the analysis of results. In our study, recurrence is defined as the reappearance of the keloid lesion without objective or subjective improvement, whereas there is no clear definition proposed in the literature.36 Moreover, we confirmed the diagnosis of a keloid lesion histopathologically, unlike other reports.37
By contrast to previous studies, we used the same radiotherapy protocol for all lesions, with particular attention to start the PORT within 2 hrs following surgical excision and with radiation fields strictly confined to the keloid region. The rapid application of PORT after surgery may explain the low recurrence rate observed in our series. Apoptosis induction and the prevention of an accelerated repopulation of fibroblasts may be considered as the pathophysiological mechanisms linked to the efficacy of PORT.
Another theory is that since the endothelial cells are more sensitive to radiation than the fibroblasts, PORT acts by suppressing angiogenesis and the formation of dysfunctional blood vessels in decreasing the local chronic inflammation,38 as well as interfering with the pathological expression of local inflammatory mediators like lymphocytes and macrophages.39
Moreover, our PORT schedule represents a good compromise to ensure good local control and low toxicity rates. Overall, very good cosmetic results were observed in our series with only mild acute side-effects.
The risk of carcinogenesis due to radiotherapy is the main reason for current disagreement regarding its use as an adjuvant treatment of keloids. Indeed, there does exist a low stochastic risk of radiation-induced secondary cancers, even if only sporadic cases have been reported in the treatment of keloids with PORT.40 Although Ragoowansi et al estimated a crude risk of one case for 1,300 treated keloids,41 a clear quantification of this risk is difficult. Similarly, a clear association between PORT and cancer induction remains difficult to prove in individual cases. Although it may seem a disproportionate measure, PORT is well accepted as a modern treatment option with minimal risks.42 Moreover, the frustration and desperation of patients who present with recurrent lesions, together with the significant physical and psychological impact in their everyday activities, must be taken into serious consideration. In addition, the use of customized treatment fields, the relatively low PORT doses, and a particular attention to protect surrounding tissues, such as in our series, should theoretically narrow the risk of carcinogenesis and complications in the short- and long-term period.
Our results present strong evidence that this specific protocol of a combined therapy is efficient and may offer the lowest recurrence rates among other treatment modalities. The main limitations are the low number of participants in a single center and the relatively “short” long-term follow-up that can be explained by a problematic visiting compliance of the population in our region. Indeed, after a short stay in our area, most patients are relocated to other parts of the country, thus rendering the follow-up difficult. The absence of a control group is another limitation. For these reasons, a larger prospective study including a control group is needed to confirm our data.
Most patients opting for combined surgical excision and PORT either present with recurrent keloids after therapeutic failures or are at high risk of recurrence. Despite the study limitations, we showed that this combined approach is an effective treatment with a satisfactory esthetic result and an acceptable low rate of recurrence.
IRB approval status
Reviewed and approved by Geneva University Hospitals IRB; #GE14-158.
Acknowledgment
We would like to thank Ms Rosemary Sudan for her contribution in the editing process.
Disclosure
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper, ie, no competing interests or conflicts of interest in this work.
References
1. Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg Clin North Am. 1997;77(3):701–730. doi:10.1016/S0039-6109(05)70576-4
2. Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84(5):827–837. doi:10.1097/00006534-198911000-00021
3. Marneros AG, Norris JE, Olsen BR, Reichenberger E. Clinical genetics of familial keloids. Arch Dermatol. 2001;137:1429–1434.
4. Abergel RP, Pizzurro D, Meeker CA, et al. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985;84(5):384–390. doi:10.1111/1523-1747.ep12265471
5. Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98(5):827–833. doi:10.1097/00006534-199610000-00012
6. Luo S, Benathan M, Raffoul W, Panizzon RG, Egloff DV. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg. 2001;107(1):87–96. doi:10.1097/00006534-200101000-00014
7. Akasaka Y, Fujita K, Ishikawa Y, et al. Detection of apoptosis in keloids and a comparative study on apoptosis between keloids, hypertrophic scars, normal healed flat scars, and dermatofibroma. Wound Repair Regen. 2001;9:501–506. doi:10.1046/j.1524-475x.2001.00501.x
8. Younai S, Nichter LS, Wellisz T, et al. Modulation of collagen synthesis by transforming growth factor-(beta) in keloid and hypertrophic scar fibroblasts. Ann Plast Surg. 1994;33:148–154. doi:10.1097/00000637-199408000-00005
9. Calderon M, Lawrence WT, Banes AJ. Increased proliferation in keloid fibroblasts wounded in vitro. J Surg Res. 1996;61:343–347. doi:10.1006/jsre.1996.0127
10. Boyce DE, Ciampolini J, Ruge F, Murison MS, Harding KG. Inflammatory-cell subpopulations in keloid scars. Br J Plast Surg. 2001;54(6):511–516. doi:10.1054/bjps.2000.3509
11. Bernabei P, Rigamonti L, Ariotti S, Stella M, Castagnoli C, Novelli F. Functional analysis of T lymphocytes infiltrating the dermis and epidermis of post-burn hypertrophic scar tissues. Burns. 1999;25(1):43–48. doi:10.1016/S0305-4179(98)00128-4
12. Nakaoka H, Miyauchi S, Miki Y. Proliferating activity of dermal fibroblasts in keloids and hypertrophic scars. Acta Derm Venereol. 1995;75(2):102–104.
13. Eraud J, Gonnelli D, Carmassi M, et al. [Differential diagnosis between keloid and hypertrophic scars: a new approach by full-field optical coherence tomography]. Ann Chir Plast Esthet. 2014;59(4):253–260. doi:10.1016/j.anplas.2014.02.001
14. Zurada JM, Kriegel D, Davis IC. Topical treatments for hypertrophic scars. J Am Acad Dermatol. 2006;55:1024–1031. doi:10.1016/j.jaad.2006.03.021
15. Renò F, Grazianetti P, Cannas M. Effects of mechanical compression on hypertrophic scars: prostaglandin E2 release. Burns. 2001;27(3):215–218. doi:10.1016/S0305-4179(00)00101-7
16. Chan KY, Lau CL, Adeeb SM, Somasundaram S, Nasir-Zahari M, Randomized A. Placebo-controlled, double-blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plast Reconstr Surg. 2005;116(4):1013–1020. doi:10.1097/01.prs.0000178397.05852.ce
17. Richard R, Ward RS. Splinting strategies and controversies. J Burn Care Rehabil. 2005;26(5):392–396. doi:10.1097/01.bcr.0000176886.63559.8b
18. Ud-Din S, Bowring A, Derbyshire B, Morris J, Bayat A. Identification of steroid sensitive responders versus non-responders in the treatment of keloid disease. Arch Dermatol Res. 2013;305(5):423–432. doi:10.1007/s00403-013-1328-7
19. D’Andrea F, Brongo S, Ferraro G, Baroni A. Prevention and treatment of keloids with intralesional verapamil. Dermatology. 2002;204(1):60–62. doi:10.1159/000051812
20. Naeini FF, Najafian J, Ahmadpour K. Bleomycin tattooing as a promising therapeutic modality in large keloids and hypertrophic scars. Dermatol Surg. 2006;32(8):
21. Tan KT, Shah N, Pritchard SA, McGrouther DA, Bayat A. The influence of surgical excision margins on keloid prognosis. Ann Plast Surg. 2010;64(1):55–58. doi:10.1097/SAP.0b013e31819b6c3a
22. Cassuto DA, Scrimali L, Siragò P. Treatment of hypertrophic scars and keloids with an LBO laser (532 nm) and silicone gel sheeting. J Cosmet Laser Ther. 2010;12(1):32–37. doi:10.3109/14764170903453846
23. Yun J-S, Choi Y-J, Kim W-S, Lee G-Y. Prevention of thyroidectomy scars in Asian adults using a 532-nm potassium titanyl phosphate laser. Dermatol Surg. 2011;37(12):1747–1753. doi:10.1111/j.1524-4725.2011.02128.x
24. Har-Shai Y, Sabo E, Rohde E, Hyams M, Assaf C, Zouboulis CC. Intralesional cryosurgery enhances the involution of recalcitrant auricular keloids: a new clinical approach supported by experimental studies. Wound Repair Regen. 2006;14(1):18–27. doi:10.1111/wrr.2006.14.issue-1
25. Har-Shai Y, Mettanes I, Zilberstein Y, Genin O, Spector I, Pines M. Keloid histopathology after intralesional cryosurgery treatment. J Eur Acad Dermatol Venereol. 2011;25:1027–1036. doi:10.1111/j.1468-3083.2010.03911.x
26. Borok TL, Bray M, Sinclair I, Plafker J, LaBirth L, Rollins C. Role of ionizing irradiation for 393 keloids. Int J Radiat Oncol Biol Phys. 1988;15:865–870. doi:10.1016/0360-3016(88)90119-8
27. Furtado F, Hochman B, Ferreira LM. Evaluating keloid recurrence after surgical excision with prospective longitudinal scar assessment scales. J Plast Reconstr Aesthet Surg. 2012;65(7):e175–81. doi:10.1016/j.bjps.2012.02.005
28. Berman B, Bieley HC. Adjunct therapies to surgical management of keloids. Dermatol Surg. 1996;22(2):126–130. doi:10.1111/j.1524-4725.1996.tb00493.x
29. Bischof M, Krempien R, Debus J, Treiber M. Postoperative electron beam radiotherapy for keloids: objective findings and patient satisfaction in self-assessment. Int J Dermatol. 2007;46(9):971–975. doi:10.1111/ijd.2007.46.issue-9
30. Cheraghi N, Cognetta A, Goldberg D. Radiation therapy for the adjunctive treatment of surgically excised keloids: a review. J Clin Aesthet Dermatol. 2017;10(8):12–15.
31. Doornbos JF, Stoffel TJ, Hass AC, et al. The role of kilovoltage irradiation in the treatment of keloids. Int J Radiat Oncol Biol Phys. 1990;18(4):833–839.
32. Kal HB, Veen RE. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Dose-effect relationship. Strahlenther Onkol. 2005;181(11):717–723. doi:10.1007/s00066-005-1407-6
33. Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113:
34. Van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg. 2005;116:514–522.
35. Park TH, Seo SW, Kim JK, Chang CH. Outcomes of surgical excision with pressure therapy using magnets and identification of risk factors for recurrent keloids. Plast Reconstr Surg. 2011;128(2):431–439. doi:10.1097/PRS.0b013e31821e7006
36. Norris JE. Superficial X-ray therapy in keloid management: a retrospective study of 24 cases and literature review. Plast Reconstr Surg. 1995;95:1051–1055. doi:10.1097/00006534-199505000-00015
37. Ollstein RN, Siegel HW, Gillooley JF, Barsa JM. Treatment of keloids by combined surgical excision and immediate postoperative X-ray therapy. Ann Plast Surg. 1981;7:281–285. doi:10.1097/00000637-198110000-00005
38. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):E606. doi:10.3390/ijms18030606.
39. Dong X, Mao S, Wen H. Upregulation of proinflammatory genes in skin lesions may be the cause of keloid formation (Review). Biomed Rep. 2013;1(6):833–836. doi:10.3892/br.2013.169
40. Ogawa R, Yoshitatsu S, Yoshida K, Miyashita T. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124(4):1196–1201. doi:10.1097/PRS.0b013e3181b5a3ae
41. Ragoowansi R, Cornes PG, Moss AL, Glees JP. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg. 2003;111(6):1853–1859. doi:10.1097/01.PRS.0000056869.31142.DE
42. Leer JW, van Houtte P, Seegenschmiedt H. Radiotherapy of non-malignant disorders: where do we stand? Radiother Oncol. 2007;83(2):175–177. doi:10.1016/j.radonc.2007.04.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
