Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Keloid Scar Resistance to Intralesional Steroid Injections: Should We Look for Foreign Bodies? A Case Report
Authors Alfurayh N , Alqahtani R, AlFada M
Received 11 November 2023
Accepted for publication 13 December 2023
Published 21 December 2023 Volume 2023:16 Pages 3693—3697
DOI https://doi.org/10.2147/CCID.S443813
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Nuha Alfurayh,1 Reem Alqahtani,2 Mohammed AlFada1
1Department of Dermatology, College of Medicine, King Saud University, Riyadh, Saudi Arabia; 2College of Medicine, King Khalid University, Abha, Saudi Arabia
Correspondence: Nuha Alfurayh, Department of Dermatology, College of Medicine, King Saud University, Riyadh, Saudi Arabia, Email [email protected]
Abstract: Keloid is a challenging pathological condition characterized by abnormal scarring that extends beyond the boundaries of the original wound. Despite the available treatment options, keloid scars remain difficult to manage. This case report discusses a 10-year-old boy with a keloid scar on his neck following branchial anomaly repair surgery. The patient underwent multiple treatments, including triamcinolone injections, cryotherapy, and a pulsed dye laser session, with limited improvement. Subsequently, an X-ray and ultrasound were performed revealing the presence of metallic clips over the surgical site. To our knowledge, the effect of the presence of foreign bodies within the keloid scar on its response to treatment is not well understood yet. The findings of this case encourage performing imaging studies on post-surgical keloid scars resistant to treatment to rule out the presence of any foreign material. However, more trials to investigate the effect of the presence of foreign bodies on the treatment of keloid scar are required before it can be set as a mandatory investigation.
Keywords: keloid, scar, resistance, steroid, cryotherapy, foreign bodies
Introduction
Keloid is a pathological condition characterized by distinct clinical symptoms, with approximately 11 million cases reported annually in developed countries.1,2 Around 70% of cases occur in children.2,3 Its earliest documented case dates to 17th-century Egypt, as described in The Smith Papyrus, and the first modern medical study mentioning keloid scarring appeared in 1806.1 Clinically, keloid scars present as rough, firm papules and plaques with an irregular surface. They display a wide range of colors, including red, purple, and brown pigments. Unlike hypertrophic scars, keloids extend beyond the boundaries of the original wound and do not regress over time.3,4
The pathogenesis of keloids is initiated by a confluence of systemic and local variables, including hormonal imbalances, mechanical tension, metabolic perturbations, and inflammatory processes, as demonstrated by several studies.4,5 These abnormal scars are mostly composed of disorganized collagen bundles, especially type one and three collagen. The mechanism of keloid formation is complex and not fully understood yet.4 Fibroblasts, epithelial cells, and inflammatory cells are drawn to the wound site in the early stages of wound healing and aid in scar remodeling.3 Collagen containing extracellular matrix is primarily formed by fibroblasts and myofibroblasts. Disruptions in the synthesis of collagen and the breakdown of extracellular matrix result in scar development.1,3 Dysregulation of Tissue growth factor Beta (TGF-β) isoforms including TGF-β1, TGF-β2, and TGF-β3, as well as Th2 immune response has been linked to the formation of keloid scar.1,3 Keloids often cause symptoms such as itching, pain, and discomfort due to their disruptive nature and can develop months to years after the initial injury.3 Patients with keloids frequently experience psychological distress related to a negative self-image, side effects, and body dysmorphic disorder.6
Keloid treatment can be classified into topical therapies, intralesional injections, surgical interventions, radiation, and laser-based therapies. Topical agents include potent topical steroids, imiquimod 5%, mitomycin C, vitamin E, silicon sheets, and onion extracts. All topical therapies have limited use as monotherapy for keloid due to their low efficacy.7 Intralesional injections are the mainstay of treatment. The most commonly used intralesional agent is Triamcinolone acetonide (TCA) which is considered the first choice and the gold standard treatment for keloid scarring.3,7 Keloid regression following TAC injections showed 50% to 100% regression of keloid. Cryotherapy alone or in combination with intralesional TAC showed significant improvement in keloid scars.3,8 Surgical excision of keloid often requires an adjuvant therapy to decrease the rate of recurrence. Radiation therapy following keloid excision is an effective option for recalcitrant lesions.9 Different lasers have been proposed for the treatment of keloid including ablative lasers such as CO2 and erbium lasers, and non-ablative lasers such as pulsed dye laser (PDL), diode laser, and ND:YAG laser.3,7,8 Despite the variety of treatment options available, keloid scars remain a challenge to manage.2,3,7
Case Report
A 10-year-old Saudi boy presented to the dermatology department of King Khalid University Hospital in Riyadh. He had been referred from the otolaryngology department due to a keloid scar on his anterior neck following branchial anomaly repair surgery two years ago. On clinical examination, the patient had a horizontal, linear, raised, firm, erythematous scar over his left lower neck measured 3.7 by 1 centimeters (Figure 1). The scar was accompanied by persistent itchiness and discomfort.
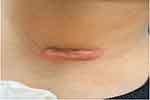 |
Figure 1 Keloid scar over the left anterior neck before treatment. |
The patient was diagnosed with keloid scar and the treatment was started immediately. He had undergone three sessions of triamcinolone injections (40 mg/cc) in combination with two sessions of cryotherapy. Around 0.8 to 1 mL was injected in each session. The intervals between treatment sessions were two to four weeks. However, all showed limited regression in keloid scar (Figure 2). A trial of a single session of Pulsed dye laser (PDL) was performed. Nevertheless, the response was deficient. Subsequently, an X-ray imaging was requested. It showed multiple metallic clips over the surgical site (Figure 3). Afterward, an ultrasound was performed to locate the clips accurately. The ultrasound result indicated that the clips were situated over the bottom part of the scar (Figure 4). The patient was referred back to the otolaryngology clinic to consider removing the metallic clips if no longer needed. However, the physician and the family decided to postpone the surgery for a couple of years. The informed consent for the case details and the images to be published was obtained from the patient’s mother.
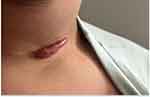 |
Figure 2 Keloid scar two weeks post-treatment with three sessions of intralesional steroid injections combined with two sessions of cryotherapy. |
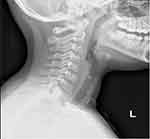 |
Figure 3 X-ray imaging showing multiple metallic clips over the anterior neck. |
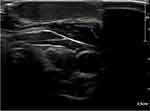 |
Figure 4 Ultrasound imaging of the keloid scar showing metallic clips over the bottommost part of the scar labeled by the blue arrow. |
Discussion
Keloid scarring is a complex condition that can cause significant physical and emotional distress to the affected individuals.3 The primary therapeutic approach for treating keloid scar involves the administration of intralesional triamcinolone. However, it has been hypothesized that roughly 50% of keloid cases exhibit resistance to intralesional steroid treatment.10 Moreover, keloid scarring is known to exhibit a high recurrence rate. Given the challenges associated with keloid treatment, primary prevention in the form of avoiding traumas and elective procedures whenever possible is recommended.9 In our case, surgery was deemed necessary for the child, and the presence of the clips was considered an important trigger for keloid formation and resistance to treatment. Although intralesional steroid injections are a common method of reducing the thickness of keloids.3,7 There is not enough evidence to suggest that the presence of certain types of foreign bodies, such as metallic clips, can interfere with the response of keloid scars to steroid injections. One study found that the use of staples for wound closure may increase the risk of adverse events, including keloid scar formation, compared with the use of sutures, but the reliability of evidence was low.11 There is currently no coherent, unifying hypothesis that explains keloid formation, and the wide range of treatments used for keloid treatment is indicative of our poor understanding of its pathogenesis. More trials to investigate the effect of the presence of foreign bodies on the treatment of keloid scars are required before certain imaging can be set as a mandatory investigation. The goal of this case report is to encourage further studies to determine the worthiness of performing imaging studies for resistant keloid scars and the impact of the presence of foreign materials in treating such challenging conditions.
Conclusion
Keloid scars are complex, challenging to manage, and often resistant to treatment. Imaging studies, such as X-ray and ultrasound, can be valuable tools in identifying the presence of foreign materials in keloids that show resistance to treatment. This case report has highlighted the importance of considering the presence of foreign bodies with keloids and their potential impact on the response to treatment.
Institutional Approval
The institutional approval was not required for case reports.
Funding
There is no funding to report.
Disclosure
The authors have no conflict of interest to declare.
References
1. Berman B, Bieley HC. Keloids. J Am Acad Dermatol. 1995;33(1):117–123. doi:10.1016/0190-9622(95)90035-7
2. Sund B. New Developments in Wound Care. London: PJB Publications; 2000:1–255.
3. Ekstein SF, Wyles SP, Moran SL, Meves A. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60(6):661–671. doi:10.1111/ijd.15159
4. Delaleu J, Charvet E, Petit A. Keloid disease: review with clinical atlas. Part I: definitions, history, epidemiology, clinics and diagnosis. In: Annales de Dermatologie et de Vénéréologie. Elsevier Masson; 2022.
5. Liu R, Xiao H, Wang R, et al. Risk factors associated with the progression from mild keloids to severe keloids. Chinese Med J. 2022;135(07):828–836. doi:10.1097/CM9.0000000000002093
6. Tomas-Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Dermato Venereologica. 2016;96(217):47–50. doi:10.2340/00015555-2368
7. Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12(1):1–33. doi:10.1186/s13643-023-02192-7
8. Jannati P, Aref S, Amin jannati A, Jannati F, Moravvej H. Comparison of therapeutic response of keloids to cryotherapy plus intralesional triamcinolone acetonide or verapamil hydrochloride. J Ski Stem Cell. 2015;2(1):e29284.
9. Zainib M, Amin NP. Radiation therapy in the treatment of keloids. In: StatPearls [Internet]. StatPearls Publishing; 2022.
10. Hietanen KE, Järvinen TA, Huhtala H, Tolonen TT, Kuokkanen HO, Kaartinen IS. Treatment of keloid scars with intralesional triamcinolone and 5- fluorouracil injections–a randomized controlled trial. J Plast Reconstruct Aesthetic Surg. 2019;72(1):4–11. doi:10.1016/j.bjps.2018.05.052
11. Mackeen AD, Sullivan MV, Schuster M, Berghella V. Suture compared with staples for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstetrics Gynecol. 2022;140(2):293–303. doi:10.1097/AOG.0000000000004872
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
