Back to Journals » Clinical Ophthalmology » Volume 17
Kahook Dual Blade versus Trabectome (KVT): Comparing Outcomes in Combination with Cataract Surgery
Authors Fliney GD, Kim E , Sarwana M, Wong S, Tai TYT, Liu J, Sarrafpour S, Chadha N, Teng CC
Received 6 October 2022
Accepted for publication 19 December 2022
Published 10 January 2023 Volume 2023:17 Pages 145—154
DOI https://doi.org/10.2147/OPTH.S391527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Greg D Fliney,1,* Eliott Kim,2,* Miriam Sarwana,3 Sze Wong,2 Tak Yee Tania Tai,2 Ji Liu,1 Soshian Sarrafpour,1 Nisha Chadha,2 Christopher C Teng1
1Yale University School of Medicine, Department of Ophthalmology and Visual Science, New Haven, CT, USA; 2Icahn School of Medicine at Mount Sinai/New York Eye and Ear, Eye and Vision Research Institute, New York, NY, USA; 3BCT Partners, Newark, NJ, USA
*These authors contributed equally to this work
Correspondence: Christopher C Teng, Yale University School of Medicine, Department of Ophthalmology and Visual Science, 40 Temple Street Suite 3D, New Haven, CT, 06510, USA, Tel +1 203-785-2020, Fax +1 203-7856220, Email [email protected]
Purpose: To compare the safety and efficacy of Kahook Dual Blade (KDB) versus Trabectome with cataract surgery in reducing intraocular pressure (IOP) and medications used by patients with glaucoma.
Methods: Retrospective chart review comparing eyes after KDB or Trabectome with cataract surgery at 2 academic centers. Surgical success was defined as IOP < 21 mmHg with ≥ 20% IOP reduction at post-operative month 12 (POM12). Changes in IOP, number of glaucoma medications, and adverse events were assessed.
Results: Ninety eyes in the KDB group and 125 eyes in the Trabectome group were included. Mean changes in IOP at POM12 were − 1.9 ± 4.9 mmHg (11.2%, P = 0.002) in the KDB group and − 3.5 ± 5.5 mmHg (19.1%, P < 0.001) in the Trabectome group, without a significant difference between the groups (P = 0.20). Mean change in glaucoma medications at POM12 was − 0.8 ± 1.5 in the KDB group (58%, P < 0.001) and − 0.3 ± 1.3 (38%, P = 0.003) in the Trabectome group, with KDB having a greater decrease in medications (P = 0.02). The percentage of eyes achieving success was 30% for the KDB group and 54% for the Trabectome group (P = 0.01). Hyphema was the most common complication, with an incidence of 3% for the KDB group and 14% for the Trabectome group (P = 0.01).
Conclusion: KDB or Trabectome with cataract surgery is safe and effective at lowering IOP and medication burden, with KDB resulting in a greater reduction in medications and Trabectome more frequently achieving success with an increased incidence of hyphema. Considering the study’s limitations, the outcomes were similar.
Keywords: Kahook Dual Blade, Trabectome, goniotomy, minimally invasive glaucoma surgery
Plain Language Summary
- Kahook Dual Blade and Trabectome are two surgical devices used to perform a goniotomy that have been shown to be effective at reducing intraocular pressure both as standalone procedures and at the time of cataract surgery.
- There are no published studies to date that directly compare the outcomes from Kahook Dual Blade and Trabectome procedures.
- This study compares the efficacy and safety outcomes of Kahook Dual Blade and Trabectome and shows that there was a greater reduction of glaucoma medications in the Kahook Dual Blade group, while the patients in the Trabectome group had a higher rate of hyphema and achieved the success measure more frequently at 12 months.
- Taking the limitations of the study into consideration, the outcomes were relatively similar.
Introduction
Glaucoma is a progressive optic neuropathy with characteristic visual field loss and is the second leading cause of global blindness. Approximately 2% of Americans have primary open-angle glaucoma (POAG), the most common form.1,2 Intraocular pressure (IOP) is the only modifiable risk factor to decrease vision loss from glaucoma and can be controlled with IOP lowering medications, laser, and surgery. Surgical options include filtering surgeries, tube shunts and a growing selection of microinvasive glaucoma surgery (MIGS) devices. MIGS increases aqueous humor outflow via trabecular, suprachoroidal, or subconjunctival routes, or reduces aqueous humor production.3,4 As the surgical options offered by MIGS continue to expand and their use continues to increase in comparison to traditional glaucoma surgeries, comparative analyses are critical in properly guiding surgeons in their choice of procedure.5
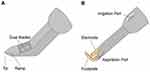
The trabecular meshwork (TM) is the site of greatest resistance to aqueous humor outflow in the eye, and removing this tissue via goniotomy or trabeculotomy reduces IOP by increasing aqueous outflow.6 The Kahook Dual Blade (KDB; New World Medical, Rancho Cucamonga, CA) and Trabectome (Microsurgical Technology, Redmond, WA) are two MIGS devices that excise or ablate TM and have been shown to be effective at lowering IOP via this similar mechanism, both as standalone procedures and at the time of cataract surgery.7–10 Schematic diagrams of the KDB and Trabectome can be found in Figure 1.
Although the KDB and Trabectome procedures are similar, there are no published manuscripts that directly compare them.11 The purpose of this study is to compare the efficacy and safety of KDB versus Trabectome, both in combination with cataract surgery. Given both devices similarly remove TM in the nasal angle, the authors hypothesize that KDB and Trabectome will have comparable levels of success and efficacy. Furthermore, since post-operative bleeding commonly occurs from blood reflux through collector channels, the authors hypothesize that KDB and Trabectome will have comparable rates of post-operative hyphema.12 In comparing these two devices, we hope that our findings will assist clinicians in selecting optimal MIGS treatments for their patients.
Materials and Methods
This was a retrospective chart review comparing all patients undergoing KDB goniotomy between January 2016 and March 2020 at New York Eye and Ear Infirmary of Mount Sinai and Trabectome between January 2013 and December 2019 at Yale Eye Center, both in combination with cataract surgery. This study was approved by the Mount Sinai Institutional Review Board (IRB) (IRB-20-03241) and the Yale IRB (IRB-2000026321). Patient consent to review medical records was not required by the Mount Sinai IRB and the Yale IRB, as the use or disclosure of patient health information involved no more than a minimal risk to the privacy of individuals. All efforts were made to maintain patient data confidentiality including use of an encrypted local database and an adequate plan to destroy patient identifiers at the earliest opportunity consistent with the conduct of research. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data were collected from subjects aged 18 years or older with at least 1 month of follow-up after surgery. All glaucoma subtypes and severities were included, along with patients with history of selective laser trabeculoplasty (SLT) or argon laser trabeculoplasty (ALT). Exclusion criteria included age less than 18 years, having prior intraocular surgery, and having additional glaucoma surgery performed at the time of KDB or Trabectome with cataract surgery or within 12 months of the initial surgery.
Patient demographics and pre-operative characteristics were gathered. Visual acuity was evaluated via Snellen chart, intraocular pressure (IOP) via Goldmann applanation tonometry, visual fields via 24–2 Humphrey visual field, anterior segment via slit-lamp examination, and posterior segment via dilated fundus examination. Medication regimens were determined at the discretion of each surgeon but included the following medications: bimatoprost, brimonidine, brinzolamide, dorzolamide, latanoprost, methazolamide, netarsudil, pilocarpine, tafluprost, timolol, travoprost, and unoprostone. IOP and number of medications were collected from the visits closest to post-operative month 1 (POM1), month 6 (POM6), and month 12 (POM12) following surgery. Complications including hyphema, IOP elevation greater than 10 mmHg above pre-operative value, ciliary body detachment, hypotony, aqueous misdirection, wound leak, and postoperative infection were recorded. Hyphema was defined as either dispersed or layering and was recorded on post-operative day 1 (POD1). All grades of hyphemas including microhyphema were recorded. Anticoagulation use was recorded categorically in patients taking acetylsalicylic acid, clopidogrel, direct oral anticoagulants, or warfarin. Chart documentation was completed by each individual surgeon. The Enhanced Glaucoma Severity Staging system was used to grade severity.13 Success was defined as an IOP <21 mmHg and at least 20% reduction in IOP at POM12.
Surgical Techniques
KDB and Trabectome were both performed at the time of cataract surgery prior to phacoemulsification. All surgeons were fellowship-trained glaucoma specialists and had prior experience performing the respective surgeries. Patients were selected for surgery based on inability to control IOP with topical medication alone or to reduce medication burden. The ultimate decision for the MIGS procedure was at the discretion of the surgeon. All patients had visually significant cataracts with best corrected visual acuity (BCVA) <20/40 or reduced vision by at least 2 lines on the Snellen chart with glare testing. Surgical techniques were consistent across glaucoma subtypes.
A clear corneal incision was used for the procedures. For the KDB cases, ophthalmic viscosurgical device (OVD) was injected into the anterior chamber, whereas chamber stability for Trabectome cases was primarily via the Trabectome’s infusion port. The patients’ heads were then rotated nasally 30 to 45 degrees away from the surgeon and the microscope 30 to 45 degrees temporally toward the surgeon. A Swan-Jacobs gonio prism was placed on the cornea to directly visualize the TM.
The KDB or Trabectome was inserted into the anterior chamber through the temporal corneal wound to access the nasal TM. For the KDB surgeries, the tip of the blade was used to pierce the TM, and the heel was placed in Schlemm’s canal. The blade was advanced to create parallel incisions along the TM resulting in 3 to 4 clock hours of a TM strip that was then removed from the eye. For the Trabectome surgeries, the footplate was used to penetrate the TM and position the device into Schlemm’s canal. The electrode was activated with foot control to ablate 3 to 4 clock hours of TM with continuous aspiration of the ablated material.
Statistical Analysis
Mean change in IOP and number of medications were calculated for each time point following surgery, and standard deviations with 95% confidence intervals were included. Surgical success was calculated as a percentage. Wilcoxon rank sum tests and independent sample t-tests were used to compare continuous variables, and ANOVA and Chi Square tests were used to compare categorical variables. Multiple linear regression was used to identify demographic and baseline eye characteristics to control for. As both eyes from single patients were included in the study, multilevel modeling was used to correct for intereye effects. Multilevel modeling was also used to control for baseline IOP, number of medications, and self-reported race and ethnicity when comparing the main outcomes of KDB and Trabectome. A P value of <0.05 was considered statistically significant. All analyses were performed with SPSS version 23.0.0.0 (IBM Corp, Released 2015. Armonk, NY, USA).
Results
Demographics
Ninety eyes from 72 patients in the KDB group and 125 eyes from 88 patients in the Trabectome group were included in the analysis. IOP and medication measurements at 12 months included 71 eyes in the KDB group and 115 eyes in the Trabectome group.
There was no significant difference in demographic and baseline characteristics between the two groups (Table 1). In the KDB and Trabectome groups, respectively, the mean ages were 72 and 73 years (P = 0.68), 60% and 55% were females (P = 0.48), and most patients were African American (31% and 40%) and Caucasian (26% and 44%). POAG was the most common diagnosis in both groups (57% and 60%). More patients in the KDB group were on anticoagulation compared with those in the Trabectome group (33% vs. 12%, P < 0.001).
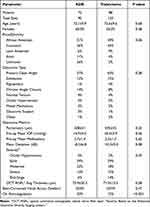 |
Table 1 Baseline Demographics and Pre-Operative Characteristics |
At the pre-operative visit, mean IOP was 16.9 ± 4.5 mmHg and 18.3 ± 5.9 mmHg (P = 0.06) for the KDB and Trabectome groups, respectively. Mean number of medications was 2.7 ± 1.4 and 2.3 ± 1.3 (P = 0.6) for the KDB and Trabectome groups, respectively. Visual field mean deviations were −8.2 ± 6.8 and −10.3 ± 9.0 dB (P = 0.08), and optical coherence tomography retinal nerve fiber layer (OCT RNFL) average thicknesses were 73.9 ± 20.3 and 71.3 ± 15.5 microns (P = 0.38) for the KDB and Trabectome groups, respectively.
Evaluation of Confounding Factors
The impact of demographic and baseline ocular characteristics on change in IOP, change in medication number, and success rate was evaluated at 12 months following surgery. Multiple regression showed no significant impact of age, gender, glaucoma subtype, or glaucoma severity based on visual field on these main outcomes (Table 2).
 |
Table 2 Multiple Regression Analysis to Assess for Significance of Interaction Between Baseline Characteristics and Change in IOP, Change in Medication Number, and Success Rate at 12 Months |
In the KDB group, Caucasian patients had an IOP reduction of 3.95 mmHg (22%) compared with 0.98 (6.6%) in non-Caucasian patients (P = 0.03) and a decrease in medications of 1.63 (55%) versus 0.69 (20%), respectively (P = 0.01). In the Trabectome group, Caucasian patients had no significant difference in IOP change compared with non-Caucasian patients; however, there was a greater decrease in medications of 0.7 (28%) versus 0.09 (4%) in the KDB group (P = 0.009). There was no significant difference between racial or ethnic groups regarding the success measure. Given these findings, race/ethnicity was controlled for in the multilevel modeling of the main outcomes.
Efficacy Measures
Mean IOP and number of glaucoma medications at the pre-operative, POM1, POM6, and POM12 visits for the KDB and Trabectome groups are shown in Figure 2. IOP and medication number were significantly lower at each follow-up visit compared with the pre-operative visit for both groups (P < 0.05), and there was no significant difference between the treatment groups at each time point. Change in IOP and number of medications between the two groups from the pre-operative visit to POM12 are shown in Figure 3.
The average IOP changed by −1.9 ± 4.9 mmHg (11.2% reduction, P = 0.002) for the KDB group and −3.5 ± 5.5 mmHg for the Trabectome group (19.1% reduction, P < 0.001). There was a between-group difference of Trabectome having a greater change in IOP of −1.6 mmHg (CI −3.19 to −0.53, P = 0.04) that was not significant after controlling for baseline IOP, intereye effects, and race/ethnicity (P = 0.20). The average number of medications changed by −0.8 ± 1.5 (58% reduction, P < 0.001) for the KDB group and −0.3 ± 1.3 for the Trabectome group (38% reduction; P = 0.003). There was a between-group difference of KDB having a greater change in medication number by −0.5 medications (CI −1.0 to −0.2, P = 0.002) that remained significant after controlling for baseline number of medications, intereye effects, and race/ethnicity (P = 0.02).
In the KDB group, 58% of patient had a decrease in number of medications, 32% had no change, and 10% had an increase in medication compared with 38%, 45%, and 17%, respectively, for the Trabectome group shown in Figure 4. There was a significantly greater number of eyes with a decrease of at least 1 medication (P = 0.04) in the KDB group after controlling for baseline number of medications, intereye effects, and race/ethnicity.
Surgical success at 12 months was defined as an IOP <21 mmHg and IOP decrease of at least 20% without an increase in number of glaucoma medications. The Trabectome group had a greater rate of success at 12 months compared with the KDB group (54% vs. 30%, P = 0.01) after controlling for baseline IOP, intereye effects, and race/ethnicity.
Safety Measures
The incidence of hyphema following surgery on POD1 was 3% for the KDB group and 14% for the Trabectome group (P = 0.01). All cases of hyphema resolved spontaneously without the need for additional procedures, and there was no correlation between the incidence of post-operative hyphema and the use of anticoagulation (P = 0.86). During the 12-month follow-up period, 3% of patients in each group had IOP elevation greater than 10 mmHg. There were no incidences of hypotony, ciliary body detachment, wound leak, choroidal hemorrhage, or postoperative infection. A small number of patients in both groups required additional glaucoma surgery during the 12-month follow-up period. In the KDB group, 6.3% of eyes required filtration or shunting procedures compared with 3.4% in the Trabectome group (P = 0.76).
Discussion
In this retrospective comparative analysis of KDB and Trabectome combined with cataract surgery, KDB showed a greater reduction in medications, while Trabectome had a higher success rate despite no statistically significant difference in IOP change between these groups. KDB had a greater decrease in medications at 12 months of −0.5 medications with 58% of patients having a decrease in medications compared with 38% of Trabectome patients. Although there was no significant difference in IOP change at each time point between the two groups, 54% of eyes in the Trabectome group met the definition of success versus 30% of eyes in the KDB group at 12 months. Both surgeries had excellent safety profiles with no sight-threatening complications. There was a higher rate of hyphema in the Trabectome group which was self-limited and not associated with anticoagulation use.
To our knowledge, this is the first manuscript to directly compare KDB and Trabectome outcomes. While retrospective in nature, the strengths of the study include that the procedures were performed by fellowship-trained glaucoma specialists across two institutions and that the patient population was diverse. Analyzing results from two institutions allowed for capturing broader clinical data, and while the patient population was diverse, the procedure groups were similar in terms of age, demographics, and baseline ocular characteristics.
The outcomes from KDB with cataract surgery were similar to other studies. Our KDB group had an 11.2% reduction in IOP compared with 10.6–31% reduction in other studies and a 33% reduction in medications compared with 21–83% at 12 months.7,14–19 Based on our multiple regression analysis, the differences between this study and previously published results do not appear to be related to glaucoma subtypes or disease severity based on visual field although the lower average pre-operative IOP in this study could be a contributing factor. Notably, in a heterogeneous glaucoma cohort including patients with POAG, exfoliative glaucoma, and uveitic glaucoma, Murata et al found that KDB ab interno trabeculotomy was effective in reducing IOP regardless of glaucoma type.20 The greater decrease in IOP and medication number following KDB among Caucasians has not been shown in other studies to date. Race and ethnicity have not been associated with failure of KDB goniotomy, and analysis of Black, Afro-Latinx, and Latino patients had similar outcomes to those reported in other KDB studies that had a largely Caucasian demographic.21–23 Further investigation with larger studies including randomized control trials should be considered to investigate this.
Results from Trabectome with cataract surgery were similar to those previously reported at 12 months. The Trabectome group had a 19.1% reduction in IOP compared with 12.5–30% in other studies and a 15% reduction in medications compared with 14.4–56% at 12 months.24–28 Based on our multiple regression analysis, the differences between this study and these previously published results do not appear to be related to glaucoma subtypes or disease severity based on visual field although the lower average pre-operative IOP in this study could be a contributing factor. The smaller reduction in medications among non-Caucasian patients following Trabectome has not been found in prior studies that directly evaluated the impact of race and ethnicity on Trabectome outcomes.29,30
The efficacy measures comparing KDB to Trabectome had seemingly conflicting results. While KDB had a greater reduction in medications, Trabectome achieved success more frequently, and there was no significant difference in IOP reduction between the groups. These differing outcomes make it difficult to determine if one procedure is superior to the other. The differences in medication outcomes could be affected by a lack of a medication washout period or standardized protocol for post-operative glaucoma medications. The higher success rate of the Trabectome group could be related to the higher mean pre-operative IOP compared to the KDB group. Although this difference was not statistically significant, it may have impacted our success variable which was dependent on a 20% reduction in IOP. With the minor difference in efficacy measures between these two groups in the context of these limitations, the outcomes appear to be relatively similar.
Interestingly, this study found a higher incidence of hyphema after Trabectome (14%) compared with KDB (3%), which was not associated with anticoagulation use. Though Trabectome contains an electrosurgical unit, its higher incidence of hyphema suggests no apparent benefit of cauterizing vessels of the TM area. As reflux from episcleral veins due to decreased IOP after phacoemulsification can contribute to hyphema, one possible cause of a lower hyphema incidence in the KDB group could be retained viscoelastic decreasing the extent of blood reflux from collector channels in comparison to Trabectome which has an irrigation-aspiration function. Overall, the hyphemas in both groups spontaneously resolved, and there were no other adverse outcomes that were clinically significant.
An important factor to consider in selection of surgical modalities is cost. Medicare physician reimbursement for goniotomy in Connecticut and New York as a stand-alone procedure ranges from $900–1000, regardless of the modality used.31 This makes the profit margin of goniotomy based on the cost of performing the procedure. The KDB price ranges from $350–500 in the United States, whereas a Trabectome procedure pack costs $700–750, not accounting for the upfront cost of a Trabectome system (previously cited as ~$50,000 in 2014) or the cost of the disposable materials for either procedure, which is similar.32 While cost can be considered in choosing between these devices, this study nonetheless found that KDB and Trabectome are similar in their safety and efficacy profiles.
The limitations of this study include the retrospective nature, the procedures being performed at different institutions, the low number of subjects with subtypes of secondary glaucoma, and the absence of a medication washout period without pre-defined criteria for changing glaucoma medications post-operatively. Given that this study is retrospective, there was no randomization, and patients were selected based on being likely to respond well to the respective procedure. Furthermore, this study included various subtypes of glaucoma; however, there was no significant difference in distribution of subtypes between the KDB and Trabectome cohorts.
The procedures were performed at different institutions by surgeons with non-standardized indications for surgery between sites. This could result in a difference in patient selection, surgical technique, documentation of hyphema, pre-operative management of anticoagulation, and post-operative management. There was no washout period of medications prior to surgery or pre-defined criteria for changing glaucoma medications post-operatively, which confounds medication outcomes. Additionally, patients with concurrent ophthalmologic diseases were not excluded from the study. Furthermore, cataract surgery and intraocular lens implantation alone in ocular hypertensive patients can result in moderate but persistent reductions in IOP.33 While the reductions in IOP reported in this study are similar to those found in similar studies, it is unclear what degree of IOP reduction can be attributed to cataract surgery alone versus the MIGS procedure itself.
Potential areas for future investigation include comparing these procedures in prospective, randomized, and controlled trials, comparing their safety and efficacy as stand-alone procedures, and evaluating the effectiveness of glaucoma medication type after goniotomy or trabeculotomy given the difference in IOP and medication outcomes between the KDB and Trabectome groups in this study.
Conclusion
While the KDB cohort showed greater reduction in number of glaucoma medications and the Trabectome cohort met our criteria for surgical success at a greater rate compared to KDB, both devices performing goniotomy in combination with cataract surgery were similarly safe and effective. Although there were statistically significant differences in medication reduction and success rates, the outcomes were comparable when considering the inherent limitations of the study. No metric analyzed in our study heavily favored one surgical device over the other. Additional studies including randomized control studies are needed to fully assess the differences in outcomes for different minimally invasive glaucoma surgeries.
Disclosure
The authors report no conflicts of interest in this work. The authors did not receive support from any organization for the submitted work. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Mount Sinai and Yale IRBs and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent was not required.
References
1. Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–888.
2. Eye Disease Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532. doi:10.1001/archopht.122.4.532
3. Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi:10.1371/journal.pone.0183142
4. Chou J, Turalba A, Hoguet A. Surgical innovations in glaucoma: the transition from trabeculectomy to MIGS. Int Ophthalmol Clin. 2017;57(4):39–55. doi:10.1097/IIO.0000000000000192
5. Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122(8):1615–1624. doi:10.1016/j.ophtha.2015.04.015
6. Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82(4):545–557. doi:10.1016/j.exer.2005.10.011
7. Sieck EG, Epstein RS, Kennedy JB, et al. Outcomes of Kahook Dual Blade Goniotomy with and without phacoemulsification cataract extraction. Ophthalmol Glaucoma. 2018;1(1):75–81. doi:10.1016/j.ogla.2018.06.006
8. Dang Y, Roy P, Bussel II, Loewen RT, Parikh H, Loewen NA. Combined analysis of trabectome and phaco-trabectome outcomes by glaucoma severity. F1000Res. 2016;5:762. doi:10.12688/f1000research.8448.2
9. Iwasaki K, Kakimoto H, Orii Y, Arimura S, Takamura Y, Inatani M. Long-Term Outcomes of a Kahook Dual Blade Procedure Combined with Phacoemulsification in Japanese Patients with Open-Angle Glaucoma. J Clin Med. 2022;11(5):1354. doi:10.3390/jcm11051354
10. Bravetti GE, Gillmann K, Salinas L, et al. Surgical outcomes of excisional goniotomy using the kahook dual blade in severe and refractory glaucoma: 12-month results. Eye. 2022. doi:10.1038/s41433-022-02196-y
11. Gillmann K, Mansouri K. Minimally invasive glaucoma surgery: where is the evidence? Asia Pac J Ophthalmol. 2020;9(3):203–214. doi:10.1097/APO.0000000000000294
12. Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci. 2010;51(3):1498–1504. doi:10.1167/iovs.09-4327
13. Brusini P, Filacorda S. Enhanced Glaucoma Staging System (GSS 2) for classifying functional damage in glaucoma. J Glaucoma. 2006;15(1):40–46. doi:10.1097/01.ijg.0000195932.48288.97
14. Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-Month outcomes of goniotomy performed using the kahook dual blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35(9):1460–1469. doi:10.1007/s12325-018-0755-4
15. ElMallah MK, Seibold LK, Kahook MY, et al. 12-Month retrospective comparison of Kahook Dual Blade excisional goniotomy with iStent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv Ther. 2019;36(9):2515–2527. doi:10.1007/s12325-019-01025-1
16. Le C, Kazaryan S, Hubbell M, Zurakowski D, Ayyala RS. Surgical outcomes of phacoemulsification followed by iStent implantation versus goniotomy with the Kahook Dual Blade in patients with mild primary open-angle glaucoma with a minimum of 12-month follow-up. J Glaucoma. 2019;28(5):411–414. doi:10.1097/IJG.0000000000001143
17. Kornmann HL, Fellman RL, Feuer WJ, et al. Early results of goniotomy with the Kahook Dual Blade, a novel device for the treatment of glaucoma. Clin Ophthalmol. 2019;13:2369–2376. doi:10.2147/OPTH.S224643
18. Ventura-Abreu N, Garcia-Feijoo J, Pazos M, Biarnes M, Morales-Fernandez L, Martinez-de-la-Casa JM. Twelve-month results of ab interno trabeculectomy with Kahook Dual Blade: an interventional, randomized, controlled clinical study. Graefes Arch Clin Exp Ophthalmol. 2021;259(9):2771–2781. doi:10.1007/s00417-021-05213-0
19. Tan Q, Li J, Lin D, Zhao P. Risk factors of surgical failure in combined phacoemulsification and excisional goniotomy for angle-closure glaucoma. Graefe’s Arch Clin Exp Ophthalmol. 2022. doi:10.1007/s00417-022-05808-1
20. Murata N, Takahashi E, Saruwatari J, Kojima S, Inoue T. Outcomes and risk factors for ab interno trabeculotomy with a Kahook Dual Blade. Graefes Arch Clin Exp Ophthalmol. 2022. doi:10.1007/s00417-022-05799-z
21. Salinas L, Chaudhary A, Berdahl JP, et al. Goniotomy using the Kahook Dual Blade in severe and refractory glaucoma: 6-Month outcomes. J Glaucoma. 2018;27(10):849–855. doi:10.1097/IJG.0000000000001019
22. Porter M, Garza A, Gallardo M. Excisional goniotomy in Latino patients with open-angle glaucoma: outcomes through 24 months. Clin Ophthalmol. 2020;14:3619–3625. doi:10.2147/OPTH.S271923
23. Laroche D, Nkrumah G, Ugoh P, Ng C. Real world outcomes of Kahook Dual Blade goniotomy in Black and Afro-Latinx adult patients with glaucoma: a 6-Month retrospective study. J Natl Med Assoc. 2021;113(2):230–236. doi:10.1016/j.jnma.2020.09.147
24. Ahmed SF, Bhatt A, Schmutz M, Mosaed S. Trabectome outcomes across the spectrum of glaucoma disease severity. Graefes Arch Clin Exp Ophthalmol. 2018;256(9):1703–1710. doi:10.1007/s00417-018-4023-8
25. Gonnermann J, Bertelmann E, Pahlitzsch M, Maier-Wenzel AB, Torun N, Klamann MK. Contralateral eye comparison study in MICS & MIGS: trabectome vs. iStent inject. Graefes Arch Clin Exp Ophthalmol. 2017;255(2):359–365. doi:10.1007/s00417-016-3514-8
26. Kurji K, Rudnisky CJ, Rayat JS, et al. Phaco-trabectome versus phaco-iStent in patients with open-angle glaucoma. Can J Ophthalmol. 2017;52(1):99–106. doi:10.1016/j.jcjo.2016.06.018
27. Ting JLM, Rudnisky CJ, Damji KF. Prospective randomized controlled trial of phaco-trabectome versus phaco-trabeculectomy in patients with open angle glaucoma. Can J Ophthalmol. 2018;53(6):588–594. doi:10.1016/j.jcjo.2018.01.033
28. Bussel II, Kaplowitz K, Schuman JS, Loewen NA, Trabectome Study G. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br J Ophthalmol. 2015;99(7):914–919. doi:10.1136/bjophthalmol-2014-305577
29. Nazarali SA, Damji KF. Ab interno trabeculectomy with Trabectome: outcomes in African American versus Caucasian patients. Can J Ophthalmol. 2018;53(4):361–364. doi:10.1016/j.jcjo.2017.10.018
30. Okeke CO, Miller-Ellis E, Rojas M, Trabectome Study G. Trabectome success factors. Medicine. 2017;96(24):e7061. doi:10.1097/MD.0000000000007061
31. Centers For Medicare & Medicaid Services. Physician fee schedule search; 2021. Available from: https://www.cms.gov/medicare/physician-fee-schedule/search.
32. Iordanous Y, Kent JS, Hutnik CM, Malvankar-Mehta MS. Projected cost comparison of Trabectome, iStent, and endoscopic cyclophotocoagulation versus glaucoma medication in the Ontario Health Insurance Plan. J Glaucoma. 2014;23(2):e112–8. doi:10.1097/IJG.0b013e31829d9bc7
33. Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119(9):1826–1831. doi:10.1016/j.ophtha.2012.02.050
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.