Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Joint Modeling in Detecting Predictors of CD4 Cell Count and Status of Tuberculosis Among People Living with HIV/AIDS Under HAART at Felege Hiwot Teaching and Specialized Hospital, North-West Ethiopia
Received 18 February 2021
Accepted for publication 28 April 2021
Published 18 May 2021 Volume 2021:13 Pages 527—537
DOI https://doi.org/10.2147/HIV.S307069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Setegn Bayabil,1 Awoke Seyoum2
1Department of Statistics, Debre Tabor University, Debre Tabor, Ethiopia; 2Department of Statistics, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Setegn Bayabil Email [email protected]
Background: Globally, for individuals infected with HIV, the presence of other infections including TB tends to increase the rate of HIV replication. Of the 8.8 million TB cases worldwide, an estimated 1.1 million (13%) were found to be co-infected with HIV. This research was conducted with the objective to identify potential predictors for the status of TB and CD4 cell count under PLWHIV at Felege Hiwot Specialized Hospital, North-west Ethiopia.
Methods: A retrospective repeated measurement was taken from a sample of 226 HIV patients. Separate and joint models were conducted for data analysis of CD4 cell count and TB status of people living with HIV.
Results: The descriptive statistics indicated that among the HIV patients receiving HAART, 26.6% had additional TB. AIDS clinical stage, weight, and hemoglobin level had a significant positive association with CD4 cell count, but a negative association with TB status. Weight and CD4 cell count have a negative relationship with the event of HIV/TB co-infection. Hence, the expected number of CD4 cell count of HIV patients who were co-infected with TB was decreased by 2.34 as compared to people living with HIV without TB. As visiting times of patients to hospitals for treatment increased by one unit, the odds of being co-infected with TB was decreased by 0.05, and the expected number of CD4 cell count was increased by 0.2. As patients’ age increased by one year, the expected number of CD4 cell count was decreased by 0.025 cells per/mm3.
Conclusion: Having lower CD4 cell count, lower weight, late WHO clinical stage, being non-adherent, having opportunistic infection, having lower hemoglobin, being ambulatory and bedridden were associated with a higher risk of co-infection of HIV/TB and were indicators of progression of the disease.
Keywords: CD4 cell, TB status, separate model, joint model, HAART
Background
There were an estimated 1.2 million (range, 1.1–1.3 million) TB deaths among HIV-negative people in 2019 (a reduction from 1.7 million in 2000), and an additional 208,000 deaths (range, 177,000–242,000) 6 among HIV-positive people (a reduction from 678,000 in 2000). The number of TB patients who had been diagnosed with HIV status reached 2.1 million in 2010 which is equivalent to 34% of notified cases of TB in the world. About one third of 39.5 million HIV infected people worldwide were co-infected with TB1 and up to 50% of individuals living with HIV are expected to develop TB. For individuals infected with HIV, the presence of other infections including TB tends to increase the rate of HIV replication. This acceleration may result in higher viral replication along with a more rapid progression to AIDS.2 Of the 8.8 million TB cases worldwide, an estimated 1.1 million (13%) were found to be co-infected with HIV.3
East and Southern Africa is a region with high incidence of HIV. This region is home to 6.2% of the world’s population and about 19.4 million people are living with the virus.4
Ethiopia is one of the few African countries with the highest number of people living with HIV/AIDS. The country has 786,040 people living with HIV and 28,650 HIV/AIDS deaths.5 Tuberculosis was the cause of 76,000 deaths in Ethiopia, out of which 30% were among HIV+ patients.6 Besides the rate of mortality, the co-infection of HIV and TB has a negative impact on the quality of life and results in mental disorders.7 The country is one of the 22 high burden TB countries which accounts for an estimated annual incidence of 379 per 100,000 persons and prevalence of 643 cases per 100,000 persons.8 The prevalence of HIV among TB patients was estimated as high as 41%.9,10 CD4 cell count is one of the most repeated measured variables for people living with HIV and it is used as an indicator of disease progression.11
HIV and tuberculosis have been closely linked since the emergence of AIDS, and TB is the most common infectious disease affecting HIV positive individuals and causing their death.
Currently, HIV and TB treatments are common in many societies and the use of drugs has altered the joint dynamics of both diseases. Recent studies suggest that the risk of developing TB among the 38 million people living with HIV was 18 (range, 15–21) times higher than in the rest of the global population.11
In many medical cases, more than one clinical outcome is measured longitudinally at the same time in the same subject where such outcomes are correlated with each other. In such cases the univariate longitudinal analysis does not take into account correlation between observations on different response variables at each time point.
While joint models of longitudinal and time to event data are widely presented in different literature, research conducted jointly for two longitudinal outcomes of similar or different nature is scarce. This study was undertaken with the objective to identify common socio-demographic, clinical, and economic variables affecting the CD4 cell count and status of TB among HIV/TB co-infected patients receiving HAART at Felege Hiwot Teaching and specialized Hospital, North-West Ethiopia.
Methods
Population Eligibility
Data from 226 HIV-positive adults who initiated their treatment in Amhara region, North-west Ethiopia (Felege Hiwot Teaching and Specialized Hospital) were collected. The study was conducted in Amhara region, North-west Ethiopia (Felege Hiwot Teaching and Specialized Hospital). The data analyzed in the current investigation consisted of a retrospective evaluation of patients’ records in the hospital whose follow-ups were between September 2012 and August 2017.
Study Design
The data used under the current investigation consisted of secondary data from Felege Hiwot referral and teaching hospital, and a prospective longitudinal study design was employed. Cochran’s formula was applied for determining sample size in the current investigation. A total of 1574 PLWHIV were receiving treatment in the hospital and of these, 956 (60.7%) were receiving HAART. Random samples of 226 PLWHIV were included under the current investigation. The samples were selected using the stratified random sampling technique, considering their residence as strata. In sample size calculation, the possible missing records/values (for patients who did not have full information) were considered, and to compensate for such data, 5% of the total size “n” was added.
Variables Under Investigation
Response Variables
The two outcome variables considered for this study were the longitudinal measure of CD4 cell count and status of tuberculosis (TB) among PLWHIV.
Explanatory Variables
Covariates associated with CD4 count and status of tuberculosis were categorized into demographic, socioeconomic status, and clinical variable: demographic variables were sex (male, female), residence area (urban, rural), marital status (living with partner, living without partner), level of education (no education, primary, secondary, tertiary), age in years. Socioeconomic variables were level of income (low, middle, high), social support (yes, no), and ownership of cell phone (yes, no). And clinical variables were WHO stages for HIV/AIDS (stage 1, stage 2, stage 3, stage 4), whether or not the patient disclosed the disease (yes, no), and baseline CD4 cells count in cells/mm3, HIV medication adherence (adherent, not adherent), body mass index (overweight, normal, underweight), functional status (ambulatory, bedridden, other), hemoglobin level, opportunistic infection (yes, no).
Inclusion Criteria
Patients aged above 18 years and who had attended a minimum of two follow-up visits for HAART treatment in ART clinic for refilling their prescription, and who initiated their HAART from 1st January 2015 to 31st December 2018 at Felege Hiwot Teaching and Specialized Hospital were included in the study.
Statistical Models
In this study, the authors used the linear mixed model to investigate the determinant factors that can affect CD4 cell count and univariate longitudinal model analysis had been used to recognize determinant factors that affect the longitudinal CD4 cell count and status of tuberculosis progression separately. And also, statistical joint longitudinal analysis was used to assess the impact of longitudinal tuberculosis and CD4 cell count progression on people living with HIV/AIDS. Based on the complexity of the data and the desired objectives of the study, the authors considered the following three types of different statistical data models:
- a linear mixed-effects model (LMM) was used for continuous response variable for the longitudinal data like CD4 cell count.
- A generalized linear mixed-effects model (LMM) was used for binary response variable for the longitudinal data like status of tuberculosis.
- Statistical Joint longitudinal model for both status of tuberculosis and CD4 cell count.
Parameter Estimation
In this study, the authors used the pseudo likelihood estimation technique in which pseudo data are created based on a linearization of the mean. More specifically, the pseudo likelihood approach can be used to estimate parameters in marginal model and random effects with or without serial correlation, whilst quadrature or Laplace approximation can only estimate parameters in the conditional independent random effect models.
Model Adequacy Checking
For the longitudinal part of CD4 cell count, two frequently used types of residuals are the standardized marginal and standardized subject-specific residuals, which are defined as
where 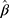 ,
, 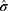 and
and 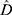 denote the maximum likelihood estimates under the model,
denote the maximum likelihood estimates under the model, 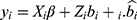 are the empirical Bayes estimates for the random effects, and
are the empirical Bayes estimates for the random effects, and 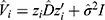 with I denoting the identity matrix of appropriate dimensions. The marginal residual
with I denoting the identity matrix of appropriate dimensions. The marginal residual 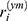 predict the marginal errors
predict the marginal errors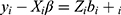 . The subject-specific residuals
. The subject-specific residuals 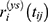 predict the conditional errors
predict the conditional errors 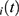 , and can be used for checking the homoscedasticity and normality assumptions.
, and can be used for checking the homoscedasticity and normality assumptions.
Exploring Individual Profile
To check for normality, the basic assumption of linear mixed effects model, histograms, boxplots and normal Q-Q plot of the CD4 cells count with corresponding Shapiro–Wilk test of normality were conducted.
Results
Descriptive statistics were used to summarize the baseline characteristics of participants in the study area. The baseline characteristics of respondents were summarized as indicated in Table 1.
 |
Table 1 Baseline Characteristics of Potential Predictors for HIV/AIDS Patients |
Table 1 indicated that out of a sample of 226 patients, 80.1% were females, 70.4% of the infected patients had working functional status, (ie, an individual able to perform usual work in and out of the house), and 6.6% were unable to perform activities (bedridden patients). Regarding the clinical stage of PLWHIV, 36.3% were at clinical stage I, 30.1% at clinical stage II, 23.0% at clinical stage III, and the rest (10.6%) were at clinical stage IV when they started HAART. Among the patients, 92.9% were adherent to the prescribed HAART medication and the rest were non-adherent, 60.6% were from a rural area, 59.7% of patients were free from opportunistic infectious diseases. The body mass index of patients revealed that 58% were at normal level, 28.3% underweight, and others were overweight, 28.8% of them were unemployed. Among the HIV-positive patients included in the current investigation, 26.6% also had TB. At enrollment, the average (standard deviation) baseline age of study participants was 33.15 (7.79) years. The average (standard deviation) baseline weight was 53.19 (9.55) kg and the average (standard deviation) baseline hemoglobin was 11.73 (2.46). Finally, the average (standard deviation) baseline CD4 cell count was 363.38 (227.68) cells per mm3.
Among the different covariance structures, unstructured (UN) had the smallest Akaike information criteria (AIC) and Bisayan information criteria (BIC) and was selected for data analysis in the current investigation.
The result in Table 2 shows that there was a significant effect of TB status on CD4 cell count. Hence, the expected number of CD4 cell count of HIV patients who were co-infected with TB was decreased by 2.34 as compared to people living with HIV/AIDS without TB.12 As age increased by one year, the expected number of CD4 cell count was decreased by 0.128, given that the other variables were constant. The expected number of CD4 cell count for ambulatory and bedridden HIV patients was decreased by 3.1 and 3.8 respectively as compared to those with working functional status. Similarly, the expected number of CD4 cell count for medication adherent and dietary adherent HIV patients were increased by 4.6 and 2.6 respectively as compared to non-adherent patients, given the other variables were constant.
 |
Table 2 Parameter Estimates of CD4 Cell Count, with Unstructured Covariance Structure |
Patients who had social support and those who had a cell phone had good improvements in their expected number of CD4 cell count as compared to their counterparts. Patients’ AIDS stages, hemoglobin levels, disclosure level of the disease, and residence area had a significantly positive effect on the expected number of CD4 cell count, but BMI and opportunistic infection had a significantly negative effect on the expected number of CD4 cell count.
The generalized linear mixed effect model (Binary logistic regression model) was implemented for the analysis of the levels of TB status as indicated in Table 3. Table 3 revealed that the odds of rural HIV patients being infected with TB were 6.3 times higher than those of urban patients. The odds of ambulatory HIV patients being co-infected with TB were greater by 14.6% as compared to patients with working functional status, given the other variables were constant.
 |
Table 3 Parameter Estimates of TB Status, Binary Logistic Regression Models Data Analysis |
In Table 3, it was observed that TB infection status was lower for patients whose AIDS stages were 1, 2, and 3 compared to stage 4, and patients whose religion was orthodox compared to others. Weight and CD4 cell count have a negative relationship with the event of HIV/TB co-infection. In Table 3 it is indicated that TB infection was higher for those who were bedridden compared to those with working functional status, for patients whose marital status was separated compared to widowed status. In this separate longitudinal data analysis, intra-class correlation was not investigated. To conduct such a correlation between the two responses, joint data analysis was employed. According to the results of longitudinal sub-models under separate analysis in Table 3, the random effect estimates depicted that intercepts and slopes vary significantly, which suggests that there was considerable variation among HIV/AIDS patients from visit to visit.
When the number of CD4 cell count increased, its average effect for odds of a patient being co-infected with TB was around 0.004 times lower for ART treatment [adjusted odds ratio (AOR)= 0.996; 95% confidence interval (CI): 0.994, 0.998]. And the amount of variability among patients due to the effect of time per month in each visit was 0.011. And the correlation was −0.176 which indicates that there was a negative correlation between intercept and slope (when a patient’s intercept increased by one unit of standard deviation, that patient’s slope decreased by 0.176 standard deviations). Table 3 also shows the covariance parameter estimates for separate longitudinal analyses for TB status.
To have an appropriate joint model that represents the longitudinally measured CD4 count and TB status of the HIV patients, different candidate joint models with a different random effect for the joint modeling were considered. The AIC and BIC were used as a guideline in selecting covariates for the model. Smaller AIC and BIC values generally indicated a better model.
In joint modeling of longitudinal outcomes, we have two types of modeling techniques: marginal model and conditional independency random-effects model. Marginal model was not functional in this study because it did not consider the random parts where individual effects on the variable of interests can be identified. Table 4 revealed that the joint model with random intercept effects with the inclusion of random linear slopes was the best model as compared to random intercept only.
 |
Table 4 Parameter Estimates of CD4 Cell Count and TB Status Using Joint Model |
Therefore, the joint model with random intercepts and the linear slope was considered as an appropriate model. In this study, both separate and joint models were fitted because in separate analysis one response was considered as a linear predictor for the other. Moreover, when we compared the two models (separate and joint), the joint model fit the data better as compared to the separate models. The result of selected appropriate joint models for the longitudinally measured CD4 count and TB status of HIV infected patients is indicated in Table 4.
The result in Table 4 shows that time of follow-up visits, residence area, functional status, adherence to HIV medication, AIDS clinical stages, weight, hemoglobin, and social support were significant variables for both CD4 cell count and TB status. AIDS clinical stages, weight, and hemoglobin level were significantly positively associated with CD4 cell count but had a negative association with TB status. In addition, one-way interaction terms (time * educational level) were also associated with both outcomes. Unstructured covariance structure was also applied for joint longitudinal data analysis, as was done in separate model analyses.
The random effects for the two outcomes were significantly and negatively associated (Table 4). This translates into a negative correlation between HAART CD4 cell and TB status. This means that increasing the average CD4 cell count per mm3 tends to decrease the chance of being co-infected with TB.13
According to the results of the longitudinal model under joint analysis in Table 4, the random effect estimates indicated that intercepts and slopes vary significantly, which suggests that there was considerable variation among PLWHIV from visit to visit.
As visiting times of patients to hospitals for treatment increased by one unit, the odds of being co-infected with TB was decreased by 0.05 and the expected number of CD4 cell count was increased by 0.2 given the other variables were constant.
As the weight of patients increased by one unit, the odds of being co-infected with TB was decreased by 1.3 and the expected number of CD4 cell count was increased by 0.03 given the other variables were constant. As the patient’s age increased by one year the expected number of CD4 cell count was decreased by 0.099 and the odds of being co-infected with TB was increased by 0.025 given the other variables were constant.
The expected number of CD4 cell count for patients whose functional status was ambulatory was decreased by 3.9 as compared to those patients who had working functional status. But, the odds of ambulatory patients being co-infected with TB was increased by 0.6 as compared to those patients who had working functional status. Similarly, the expected number of CD4 cell count for bedridden patients was decreased by 4.6 as compared to patients with working functional status and the odds of bedridden patients being co-infected with TB was increased by 3 as compared to patients with working functional status.
Patients’ treatment adherence status was also another important variable for CD4 cell count and TB status and the result showed that the expected number of CD4 cell count for adherent patients was 4.4 times that of non-adherent patients and the odds of being co-infected with TB for adherent patients was decreased by 1.0 given the other variables were constant.
The expected number of CD4 cell count for patients who did not get social support was decreased by 1.1 as compared to those who got social support but the odds of being co-infected with TB for patients who did not get social support was increased by 0.5 given the other variables were constant.
As the hemoglobin level of patients increased by one unit, the expected number of CD4 cell count of HIV patients increased by 0.59 cell per/mm3 but the odds of being co-infected with TB for such patients decreased by 0.4 keeping the other variables constant.
The expected number of CD4 cell count for patients with WHO stage 1 was greater by 2.5 cells per mm3 as compared to stage 4 but the odds of being co-infected with TB for WHO stage 1 patients was decreased by 9.1 as compared to stage 4. Similarly, the expected number of CD4 cell count of AIDS stage 2 patients was greater by 1.1 and the odds of being co-infected with TB for WHO stage 2 patients was decreased by 8.9 as compared to those patients whose HIV stage was stage 4, given the other variables were constant.
In Table 4, it is also indicated that the expected number of CD4 cell count for rural patients was decreased by 0.05 as compared to urban patients but the odds of being co-infected with TB for rural patients was increased by 1.3 as compared to urban patients given the other variables were constant.
The interaction effect in Table 4 indicates that, as visiting time of a patient increased by one unit, the expected number of CD4 cell count for non-educated patients was decreased by 0.2 but the odds of being co-infected with TB for such patients was increased by 0.15 as compared to tertiary-educated patients, keeping the other variables constant. Similarly, as visiting time of patients increased by one unit, the expected number of CD4 cell count for patients with primary education was decreased by 0.24 cells per mm3 and the odds of being co-infected with TB was increased by 0.14 as compared to patients with tertiary education, keeping the other variables constant.
Discussion
In this study, separate and joint models were explored. Joint models fitted the data better as compared to separate models. The two longitudinal responses are correlated, valid inferences can be made through the use of a joint modeling approach. This result was also consistent with one of the previous pieces of research.14
In the longitudinal sub-model of CD4 cell count, the predictors: age, functional status, HIV/AIDS stage, educational level, opportunistic infectious diseases, body mass index, adherence, weight, hemoglobin, and visiting times were statistically significant at 5% confidence level. The result was also consistent with the previous research.15
In joint analysis, AIDS clinical stage, weight, and hemoglobin level were significantly positively associated with CD4 cell count but had a negative association with TB status. In addition, one-way interaction terms (time * educational level) were also associated with both outcomes, and consistent with the previous study.16
Results from this study demonstrated that patients with first, second, and third HIV stages have higher expected number of CD4 cell count as compared to patients with the fourth stage but the odds of being co-infected with TB was decreased for patients with HIV stages 1, 2 and 3 as compared to stage 4.17 This indicates that the two responses are negatively correlated to each other. This result was supported by one of the previous investigations.18 The potential reason for this might be that among patients with these stages, the CD4 cells may not be destroyed by the disease as compared to stage 4, and such patients may not lose weight as compared to stage 4.19
Naturally, as age increased or being older and older, the expected number of CD4 cell count decreased from time to time. The odds of aged patients being co-infected increased and such result is consistent with another study.20
Patients who adhered to their prescribed HIV medication had higher CD4 cell count than those who did not adhere.21 And also, weight and hemoglobin level have a positive relationship with CD4 count, that is, for a unit change of weight or hemoglobin level, expected number of CD4 cell count also increased. This might be due to the fact that consuming low dietary diversity food is linked directly to weight and hemoglobin. This result was also consistent with the result obtained by previous research.22
Education significantly affected the level of CD4 cell count and the odds of being co-infected with TB. Hence, more educated patients have a better understanding of adherence to prescribed medication and this further leads to better CD4 cell count as compared to uneducated patients. Such educated patients with better CD4 cell count had smaller odds of being co-infected with TB. The result obtained in the current research is consistent with one of the previous investigations.23
HIV patients who got social support from the community around them had better CD4 cell count and such patients had low odds of being co-infected with TB. The potential reason for this may be the fact that such patients might have good HIV medication as well as food adherence, and this leads to a higher expected number of CD4 cell count and less probability of being co-infected with TB.24
Rural area patients are more likely to be co-infected with HIV/TB as compared to urban residents. This result is consistent with previously conducted research.25 This might be due to the fact that in rural areas, the patients may be faced with lack of balanced diet and proper adherence to medication because of limited accessibility of ART clinic settings or health facilities as compared to urban, as well as patients’ low level of awareness about the treatment. This indicates that the exposure status of TB among PLWHIV was high in a rural area.26
Conclusion
In conclusion, when the two longitudinal responses are correlated, valid inferences can be made through the use of a joint modeling approach. In the longitudinal sub-model of CD4 cell count, the predictors: age, functional status, HIV/AIDS stage, educational level, opportunistic infectious diseases, body mass index, treatment adherence, weight, hemoglobin, and visiting times were statistically significant at 5% confidence level.
Having lower CD4 cell count, lower weight, late WHO clinical stage, being non-adherent to medication, having opportunistic infection, lower hemoglobin, and being bedridden were associated with a higher risk of co-infection of HIV/TB and are indicators of progression of the disease. Therefore, patients should be informed about the need for early diagnosis of HIV infection, and starting treatment early is very important as per the recent WHO “treat all” recommendation.
Integrated interventions for HAART program enhanced treatment access with improved health quality and food security. Supporting healthcare providers, development of social policies, cooperation between various agencies is required to facilitate ART and retention in care by HAART. It is also recommended that further studies that include viral load might provide additional information for the current investigation.
Limitation of the Study
The study did not cover HIV-positive individuals who take ART outside Bahir Dar Felege Hiwot Teaching and Specialized Hospital. Time to default events was not considered in this study, this could be a potential area for further investigation.
Abbreviation
HAART, highly active antiretroviral therapy; AOR, adjusted odds ratios; CI, confidence interval; BMI, body mass index; TB, tuberculosis; PLWHIV, people living with HIV; AIC, Akaike information criteria; BIC, Bayesian information criteria.
Data Sharing Statement
Authors confirmed that the data used for this research are available from the corresponding author.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and adhered to Good Clinical Practice guidelines. Approval for the protocol was obtained from the local ethics committee for each participating site. The data used in current investigation were collected previously by the health staff for treatment purpose/for diagnosis of HIV/AIDS and to start ART. To use this previously collected data, Ethical approval certificate had been obtained from two committees namely Bahir Dar University Ethical approval committee, Bahir Dar University, Ethiopia with reference number: RCS/1412/201.
Consent for Publication
This manuscript has not been published elsewhere and is not under consideration by any other journal. Both authors approved the final manuscript and agreed with its submission. We agreed about authorship and the order of authors for this manuscript.
Acknowledgment
The authors acknowledge the Amhara Region Health Research & Laboratory Center at Felege Hiwot Teaching and Specialized Hospital, Ethiopia, for the data they supplied for the success of this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no financial and non-financial competing interests in this work.
References
1. Initiative ST, World Health Organization. The Stop TB Partnership Annual Report 2006: A Portrait of Progress. Geneva: World Health Organization; 2007.
2. Sharma S, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis & management. Indian J Med Res. 2005;121(4):550–567.
3. World Health Organization. Global tuberculosis report; 2012.
4. Unaids A. by the numbers; 2016. 2017.
5. Wang H, Wolock TM, Carter A, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3(8):e361–e387. doi:10.1016/S2352-3018(16)30087-X
6. Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J. 2009;33(4):871–881. doi:10.1183/09031936.00168008
7. Deribew A, Tesfaye M, Hailmichael Y, et al. Tuberculosis and HIV co-infection: its impact on quality of life. Health Qual Life Outcomes. 2009;7(1):105. doi:10.1186/1477-7525-7-105
8. Organization WH. Global tuberculosis control: surveillance, planning, financing: WHO report 2008. Vol 393. World Health Organization; 2008.
9. Demissie M, Lindtjørn B, Tegbaru B. Human Immunodeficiency virus (HIV) infection in tuberculosis patients in Addis Ababa. Ethiopian J Health Develop. 2000;14(3). doi:10.4314/ejhd.v14i3.9900
10. Ahmed Yassin M, Takele L, Gebresenbet S, et al. HIV and tuberculosis coinfection in the southern region of Ethiopia: a prospective epidemiological study. Scand J Infect Dis. 2004;36(9):670–673. doi:10.1080/00365540410020848
11. Adams M, Luguterah A. Longitudinal analysis of change in CD4+ cell counts of HIV-1 patients on antiretroviral therapy (ART) in the Builsa district hospital. Eur Scientific J. 2013;9(33).
12. Aabye MG, Ravn P, PrayGod G, et al. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS One. 2009;4(1):e4220. doi:10.1371/journal.pone.0004220
13. Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am J Respir Crit Care Med. 1993;148(5):1292–1297.
14. Fujiwara P, Clevenbergh P, Dlodlo R. Management of adults living with HIV/AIDS in low-income, high-burden settings, with special reference to persons with tuberculosis [State of the Art Series. HIV infection in low-income, high-burden settings, Edited by JF Murray. Number 5 in the series]. Int J Tuberculosis Lung Dis. 2005;9(9):946–958.
15. Dessie ZG, Zewotir T, Mwambi H, North D. Modelling of viral load dynamics and CD4 cell count progression in an antiretroviral naive cohort: using a joint linear mixed and multistate Markov model. BMC Infect Dis. 2020;20:1–14. doi:10.1186/s12879-020-04972-1
16. Temesgen A, Kebede T. Joint modeling of longitudinal CD4 count and weight measurements of HIV/tuberculosis co-infected patients at Jimma University specialized hospital. Ann Data Sci. 2016;3(3):321–338. doi:10.1007/s40745-016-0085-9
17. Kofoed K, Gerstoft J, Mathiesen LR, Benfield T. Syphilis and human immunodeficiency virus (HIV)-1 coinfection: influence on CD4 T-cell count, HIV-1 viral load, and treatment response. Sex Transm Dis. 2006;33(3):143–148. doi:10.1097/01.olq.0000187262.56820.c0
18. Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14(4):731–747. doi:10.1007/s10461-008-9489-7
19. Manyara J. Factors associated with delayed antiretroviral therapy initiation among Tuberculosis/Human Immunodeficiency Virus co-infected patients in Lupane district, 2015; 2016.
20. Bello EJM, Correia AF, Marins JRP, Merchan-Hamann E, Kanzaki LIB. Predictors of virologic failure in HIV/AIDS patients treated with highly active antiretroviral therapy in Brasília, Brazil during 2002–2008. Drug Target Insights. 2011;5(DTI):S7527. doi:10.4137/DTI.S7527
21. Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. JAIDS. 2004;35(3):261–268. doi:10.1097/00126334-200403010-00006
22. Katubulushi M, Zulu I, Yavwa F, Kelly P. Slow decline in CD4 cell count in a cohort of HIV-infected adults living in Lusaka, Zambia. Aids. 2005;19(1):102–103. doi:10.1097/00002030-200501030-00016
23. Katlama C, Esposito R, Gatell JM, et al. Efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients: 24-week results of POWER 1. Aids. 2007;21(4):395–402. doi:10.1097/QAD.0b013e328013d9d7
24. Worodria W, Massinga-Loembe M, Mazakpwe D, et al. Incidence and predictors of mortality and the effect of tuberculosis immune reconstitution inflammatory syndrome in a cohort of TB/HIV patients commencing antiretroviral therapy. JAIDS. 2011;58(1):32–37. doi:10.1097/QAI.0b013e3182255dc2
25. Montales MT, Chaudhury A, Beebe A, Patil S, Patil N. HIV-associated TB syndemic: a growing clinical challenge worldwide. Fron Public Health. 2015;3:281. doi:10.3389/fpubh.2015.00281
26. Silva DI, Braga Ceccato M, Rosa Silveira M, et al. Predictors of mortality among individuals with tuberculosis and human immunodeficiency virus coinfection at a reference center in southeastern Brazil: a Retrospective Cohort Study. J Young Pharmacists. 2018;10(4):476–480. doi:10.5530/jyp.2018.10.103
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.