Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Joint and Separate Analysis for Longitudinal and Survival Data on Mother-to-Child Transmission of HIV Among Infected Mothers on Option B+ at Health Centers in North Shewa Zone, Ethiopia, 2017
Authors Mekuria AD , Sisay AL, Hailegiorgies KK , Abebe AM
Received 30 July 2020
Accepted for publication 24 September 2020
Published 20 October 2020 Volume 2020:13 Pages 1179—1189
DOI https://doi.org/10.2147/JMDH.S269558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Abinet Dagnaw Mekuria,1 Assefa Legesse Sisay,2 Kassa Ketsela Hailegiorgies,3 Ayele Mamo Abebe4
1Department of Public Health, College of Health Science, Debre Berhan University, Debre Berhan, Ethiopia; 2Department of Epidemiology and Bio-Statistics, Jimma University, Jimma, Ethiopia; 3Department of Nursing, College of Health Science, Debre Berhan University, Debre Berhan, Ethiopia; 4Pediatrics nursing Department, College of Health Science, Debre Berhan University, Debre Berhan, Ethiopia
Correspondence: Ayele Mamo Abebe
Pediatrics nursing Department, Debre Berhan University, College of Health Science, PO Box 445, Debre Berhan, Ethiopia
Email [email protected]
Background: Prevention of mother-to-child transmission of HIV (PMTCT) is a frequently used word for programs and intervention methods to decrease the risk of mother-to-child transmission of HIV. The aim of this study was to identify determinants of the reduction of CD4 count through time and the maternal transmission of HIV to their child on the PMTCT program at health centers in North Shewa Zone, Ethiopia.
Methods: The cohort study design was conducted by using secondary data collected from the cohort register of PMTCT starting from September 1, 2014 to November 30, 2017. In this study, a longitudinal study was conducted for two types of result; these were longitudinal response measurements of HIV infected women CD4 count and the time to maternal transmission of HIV taken from 203 patients.
Results: The prevalence rate of HIV infection among exposed infants was 5.58%. Baseline CD4 count, visiting times, weight, and interaction between visiting time and baseline CD4 count had a statistically significant effect on the longitudinal biomarker. From the Weibull AFT model, ART start, partner test, clinical stage, educational status, place of delivery, and MUAC were statistically significant. Hence, as a measurement unit decreased in square root CD4 cell count by 1.18 elevates the risk of maternal transmission of HIV.
Conclusion: In this study, the determinant of mother-to-child transmission of HIV including loss of weight, ART start (ANC), place of delivery at home, illiterate and mother with severe malnutrition, had a significant effect. The longitudinal biomarker also had a strong association with baseline CD4 and the risk of maternal transmission of HIV. Health education should be given about balanced diet, weight control, and take medication for HIV positive patients by the responsible bodies.
Keywords: longitudinal data, mixed effect model, option B+ program, parametric AFT model
Introduction
Each year, over half a million newborns are infected with HIV in sub-Saharan Africa through mother-to-child transmission (MTCT). Of all health crises in the African region, HIV/AIDS has attracted the most political support and resources. Prevention of HIV/AIDS mother-to-child transmission (PMTCT) is one of the efforts to reduce new HIV infections. The PMTCT program has encountered difficulties and confusion since the first isolation of HIV in an 18-month-old child in 1982.1 In the following few years, it became evident that 25% of pregnant HIV-infected mothers will transmit the infection to their children. Even though a clear risk of acquisition of HIV from an infected mother was established, few attempts were made to prevent transmission, such as avoiding pregnancy in infected mothers or avoiding breast feeding.2 The earliest management proxy of PMTCT was treating the mother herself, to help as a preventive strategy.3
Prevention of mother-to-child transmission of HIV (PMTCT) is a regularly used word for programs and interventions to decrease the risk of mother-to-child transmission (MTCT) of HIV.2,3 About 77% of all HIV-infected pregnant women globally used Antiretroviral treatment (ART) during pregnancy in 2015.1,3 Life-long ART was offered for pregnant women living with HIV, regardless of their CD4+ count.4,5 ART has been used during and after pregnancy and it is an important public health strategy to decrease vertical HIV transmission as well as to provide clinical benefits to the mother. But resources, health system factors, and personal factors like lack of awareness were reasons patients for did not take the drug.6 Due to this, limited resources and awareness provide challenges for universal implementation and retention in ART programs.7,8
Even though different efforts were being made, about 330,000 children were infected with HIV in 2011 globally, with over 90% of these infections occurring in sub-Saharan Africa, mainly through MTCT.9,10 Twenty-two countries account for more than 90% of the global burden, and Ethiopia is one of these priority countries, where one of every three children born to women living with HIV was infected with HIV.10–13
MTCT of HIV remains one of the main sources of HIV infection among young children.6,14 In 2013, 3.2 million children under the age of 15 were living with HIV globally, 91% of whom were in Sub-saharan Africa.1,15 In the lack of careful interventions, the risk of MTCT of HIV is 15–45%.7,16
In Ethiopia, the prevalence was somewhat higher among women (1.9%) than men (1.0%).8,17 The number of HIV positive children was more than 160,000 in this country.18 Moreover, 800,000 orphaned children were died due to HIV/AIDS.19 But, ART coverage among HIV positive children was very low (12%) in a similar year.9,20
Abolition of this MTCT of HIV from HIV positive mothers to their children is possible through prevention, taking antiretroviral medications and HIV testing during pregnancy.21,31 But, small numbers of HIV positive pregnant women (24%) have received ART in Amhara region, Ethiopia.10,–22–28 But by using ARV drugs and appropriate preventive mechanisms, the risk can be decreased to less than 5% in under-resourced settings like Ethiopia.7,23,29 Few studies have described the determinants of HIV infection among children born to mothers on PMTCT in Ethiopia. In addition to this, these few studies had not shown consistent potential risk factors.16,21,29 Therefore, the aim of this study was to identify determinants of maternal transmission of HIV to their child on the PMTCT program at selected Health centers in North Shewa Zone.
Methods
Study Design
A retrospective cohort study design was conducted.
Study Period
The study was conducted from April 27 to May 10, 2019 from a PMTCT cohort who completed the standardized staying time to decide the HIV exposed infants/child final status. Here, we used secondary data from the cohort register of PMTCT starting from September 1, 2014 to November 30, 2017. Out of the total 412 registered patients, only 203 of them satisfied the inclusion criteria.
Study Setting
The study was carried out at North Shoa Zone, Amhara Regional State. The Zone is located in the Eastern part of Amhara region, 130 km from Addis Ababa, the capital city of Ethiopia. Regarding the health facilities, there were 97 health centers. From these Health Centers, Debre Berhan kebele 04, Shewa Robit, and Ataye Health Centers were selected as they are hot spot areas of HIV/AIDS. In these Health Centers, there were a total of 412 infected mothers initiated on the PMTCT program.
Inclusion Criterion
From this study population, all patients who initiated option B+ and measured their CD4+ cell counts at least twice, including the baseline, and those who completed the program started first line ART regimen class were included in the study population. All HIV infected mothers who started ART and finished their follow-up time from September 1, 2014 to November 30, 2017.
Exclusion Criterion
Study participant cards which did not have complete information were excluded.
Sample Size and Sampling Procedure
In this study, we used the simple random sampling method. For this study, 71 samples were determined from each site (Health Centers) with a total of 213 on the Web-based RMASS program https://www.Rmass.org/,11,12 Finally, out of 412, 203 subjects were selected.
Study Variables
The study used two response variables: one for the longitudinal outcome variable and the other for the time-to-event outcome variable. When repeated measurements were taken from the same subject at different time points, observations were assumed to be correlated. For survival analysis, the data had the nature of time-to-event (ie, the time of transmission of HIV) from the time started medication to the occurrence of an event.
Dependent Variables
Longitudinal Outcome Variable
CD4+ T-cells count for each infected mother measured approximately in every 6-month interval.
Time-to-Event Outcome Variable
Survival-time was the number of days from the date of enrollment to PMTCT program till one of the events of “HIV positive infant”, while “exposed infant for HIV” “lost to follow-up”, “dropped out”, “transferred out to other health care centers” occurred are censored.
Independent Variables
The independent variables for this study were socio-demographic factor, baseline CD4+ count, weight at the baseline in kg, time CD4+ count recorded, WHO clinical stage, regimen class, functional status, survival time, educational status, place of delivery, partner test results, residence, mid-upper arm circumference, time since ART initiation, and censoring status. ID uniquely identifies a patient. Time refers to the month when patient’s CD4+ count was recorded. It could be at the baseline, 1st, 2nd, or 3rd month.
Data Processing and Quality Assurance
The data were collected by the PMTCT trained professionals at each Health center from the cohort booklet and intake cards of the patient cards. The principal investigators supervised the whole data collection phases closely. The collected data were checked for its completeness using SPSS version 20. Then, the data were imported to R and SAS for analysis.
Method of Data Presentation and Analysis
SAS version 9.4 and R version 3.6 software were used for data analysis. The explanatory data analysis (EDA) was done for each variable. Linear mixed model and Weibull AFT model were fitted for longitudinal biomarker (sqrtCD4+ count) and for maternal transmission of HIV, respectively. Finally, the two outcomes of interests were jointly analyzed through joint analysis of longitudinal and survival data.
Consent Procedure
Ethical approval letter was written for each health center by Debre Berhan University. The written letter was given for each health center. We got agreement from each health center to conduct this research by considering the privacy and confidentiality of each personal profile up to publication of the research findings.
Results
Descriptive results
The average age and weight of HIV positive pregnant and lactating mothers in this study was 27 and 51.31, respectively. Out of 203 HIV infected mothers, 146 were start ART at ANC and out of them 131 (89.7%) were censored, the remaining 57 patients were start ART at labor and delivery, out of 57 patients 49 (86.0%) were censored and the remaining eight (14.0%) were event occurred. Out of 203 HIV infected mothers, 148 were married, and out of married patients, 131 (88.5% were censored, and the remaining 17 (11.5%) patients were event occurred. On the other hand, out of unmarried patients, 49 (89.1%) were censored and six (10.9%) were event occurred. Regarding the delivery, 73 (36%) mothers were delivered at home and, of them, 16 (21.9%) were event occurred. While the remaining 130 patients were delivered at a Health facility and, out of them seven (5.4%) had an event. Similarly, out of 203 patients, 22 of them had severe mid-upper arm circumference and, of them, 13 (59.1%) had an event, the remaining nine (40.9%) were censored. The other covariates can be described in the same way (Table 1).
 |
Table 1 Frequencies and Percentages for Baseline Categorical Covariates |
Factors Associated with Longitudinal Biomarker
From the linear mixed effect model with both random intercept and slope model the coefficients in the fixed effect part, the main effects of the covariates time (Visit), baseline CD4, Weight and time by baseline CD4 interactions were found to be significant at 5% level of significance. But the other covariates were not significant including the interaction effects. The time effect (Visit) was found to be significant and the coefficient had a positive sign indicating that on average the square root CD4 count increases over time. Therefore, after elimination of the insignificant variables from the LMM, the final model was fitted by using REML estimation model. The reduced fixed effects models for square root of CD4 count were given by:
The intercept (6.071) was interpreted as the mean of the outcome (sqrtCD4) when all the predictors had a value of zero. A one unit increase in the predictor baseline CD4+ count corresponds to a 0.026 increase in the outcome sqrtCD4. Likewise, a one unit increase in weight corresponds to a 0.057 increases in sqrtCD4 (Table 2).
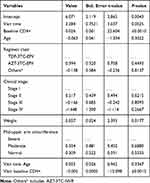 |
Table 2 Parameter Estimates for Fixed Effects |
Multiple Imputations for Longitudinal Biomarker
From the MI results the parameter estimates for visit (time), baseline CD4, and weight were found to be significant at the 5% level of significance. From this, when we see that weight and clinical stage II variables in the analysis of actual data were insignificant, but those variables were found to be significant after imputation, it indicates the imputation technique was found to be important (Table 3). The standard errors for the imputed data analysis were relatively small when compared with the corresponding standard error values for the actual data analysis while multiple imputations had efficient estimates for this data type and give unbiased parameter estimates (Table 4).
 |
Table 3 Parameter Estimates of 25 Imputations Using LMM |
 |
Table 4 Variance Information Table Using LMM |
Factors Associated with Maternal Transmission of HIV
From a Weibull AFT model the variables; ART start at ANC, partner test, clinical stage I and stage III, educational statues, place of delivery, and MUAC were found to have a statistically significant effect with maternal transmission (Table 5). The final fitted model can be written as:-
 |
Table 5 Parameter Estimation for Weibull AFT Model |
Results for Joint Analysis
The Joint analysis of longitudinal and time to event analysis revealed that most covariates were found to be statistically significant and a strong association b/n the longitudinal biomarker (sqrtCd4 count) and the risk of maternal transmission of HIV, (γ=-0.1655, “P≤0.001” meaning that a unit decreased in sqrtCD4 count corresponds to an 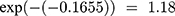 increase in the risk for maternal transmission of HIV. The longitudinal sub-model output (in the joint analysis) revealed that the covariates included in this analysis were found to be statistically significant. The time effect (Visit) was found to be significant and the coefficient had a positive sign indicating that on average the square root CD4 cell counts increased over time. Patients’ square roots of CD4 decreased by 4.68 were initiated in clinical stage IV when we compare with subjects in clinical stage I. A comparison between the standard Weibull AFT models with the joint model showed some interesting features. Most importantly, this study shows that age was found to be significant and this means a one unit increase in age was associated with a 2.712% increase in the expected time of maternal transmission of HIV, but age variables in separate analysis of the survival outcome were not significant. The weight of a patient was not significant in separate analysis of a Weibull AFT model, while it became significant in the survival sub-model as joint analysis output revealed that a one unit decrease in weight of a patient leads to an increase in the expected time-to-maternal transmission by
increase in the risk for maternal transmission of HIV. The longitudinal sub-model output (in the joint analysis) revealed that the covariates included in this analysis were found to be statistically significant. The time effect (Visit) was found to be significant and the coefficient had a positive sign indicating that on average the square root CD4 cell counts increased over time. Patients’ square roots of CD4 decreased by 4.68 were initiated in clinical stage IV when we compare with subjects in clinical stage I. A comparison between the standard Weibull AFT models with the joint model showed some interesting features. Most importantly, this study shows that age was found to be significant and this means a one unit increase in age was associated with a 2.712% increase in the expected time of maternal transmission of HIV, but age variables in separate analysis of the survival outcome were not significant. The weight of a patient was not significant in separate analysis of a Weibull AFT model, while it became significant in the survival sub-model as joint analysis output revealed that a one unit decrease in weight of a patient leads to an increase in the expected time-to-maternal transmission by 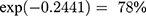 (Table 6).
(Table 6).
 |
Table 6 Parameter Estimates for Joint Analysis |
Discussion
In this retrospective study of mother-to-child transmission of HIV, 23 (11.3%) infants became HIV positive during the follow-up. The prevalence rate of HIV infection among exposed infants in this study was 5.58%, which was lower than the national prevalence rate of 17%.13 In similar studies in different countries with resource-poor settings, the prevalence rate reported was 11–21.8%.14 This finding was higher than the study done in Hawassa University Referral and Yirgalem general hospital, SNNPR (4.16%).15 However, this finding was lower than the studies done in Dire Dawa city administration (15.7%),13 in East and West Gojjam zones, Amhara region (5.9%),2 in Gondar University (10%),16 in South Gondar zone (10.1%),17 and meta-analysis in Ethiopia (9.93%).18 This might be due to the study participants having different information and health education levels in different areas. This may be the cause for different results in the research.
According to this study, in the final model of LMM, time (visit), baseline CD4, weight and time by baseline Cd4 showed a significant association at 5% significance level. This study was supported by different studies.19–21 As the weight of patients increased, the sqrtCD4 cell count increased.
Patients with a high initial CD4 count adhere with the prescribed medication more and these results in high rate of change of CD4 count through time. Different studies showed that CD4 count at the time of ART initiation were critical determinants of the progression while under ART.20,21,23 This may be due to the immune system being damaged and the risk of illness will increase.
In this retrospective study, the determinant factors for vertical transmission of HIV were weight, time of ART start of a patient, status of partner, clinical stage of patients, educational status, and place of delivery and MUAC of a patient. This study finding was supported with different studies.22,23,28
Based on this study, 72% of women were receiving ART at the time of their first antenatal care visit, as a result the time of ART initiation at ANC has a higher impact on maternal transmission. This finding was supported by studies done in Malawi and South Africa.24–27 This might be due to women who had no ANC follow-up ad usually delivered at home and no PMTCT intervention including ARV prophylaxis being at higher risk.
In our study findings on separate Weibull AFT model and joint analysis, ART initiation, education level of patients, place of delivery, and baseline CD4 had statistically significant effects on maternal transmission of HIV. From the study done on multivariate analysis using Poisson regression, the location of prenatal care, delivery type, CD4, viral load, time, and use of ARV were statistically significant. The findings of this study were similar with other studies.28–30
In our study findings delivered at home were the main determinant factors for vertical transmission of HIV to their infants. During the follow-up, 73 (35.96%) infants were delivered at home; among those mothers, 16 (21.9%) infants were HIV positive that delivered at health institutions. Our finding agreed with studies done in Ethiopia and in Brazil.13,22
During the follow-up, there was maternal-to-child HIV transmission in 60 (15.7%) HIV tested infants; most of them (55; 91.7%) were confirmed by DNA-PCR. Fifty percent of infants delivered at home were HIV positive, compared to 11.9% among infants delivered at health institutions. The transmission rate was higher among infants who did not receive ARV prophylaxis at birth (45.2%) compared to those who received it (7.4%). This finding was in line with the studies done in Dire Dawa city and Northwest Ethiopia.13,16
This study also showed that HIV positive women with severe malnutrition were the main determinant for vertical transmission of HIV. This study was supported by studies done in different areas.13,16,31 This may be due to the production of HIV virus being high in severe malnutrition HIV positive women. On the other hand, high production of HIV virus may increase the vertical transmission of HIV.
In summary, both the separate and joint analysis results showed that CD4 depletion was the determinant factor for MTCT and it confirms the importance of starting ART early. Our study was supported by different studies.14,30
Conclusion and Recommendations
The prevalence rate of HIV infection among exposed infants in this study was 5.58%. Separate analysis of survival data revealed that the covariates weight, ART start, partner test, clinical stage I and III, educational status (illiterate), place of delivery (at home), and MUAC were found to be statistically significant effects for time-to-maternal transmission of HIV.
Joint analysis revealed that sqrtCD4 count measured repeatedly over time was associated with the risk for maternal transmission of HIV. The covariates; Age, weight, partner test, Clinical Stage, and MUAC were found to be significantly associated with the risk of maternal transmission of HIV.
Therefore, HIV positive women should start ART prior to pregnancy, also governments and concerned bodies should provide awareness about the mode of maternal transmission of HIV and the importance of option B+ program and give support to the society to achieve the EMTCT goals.
Health education should be given about balanced diet, weight control, and medication use for HIV positive patients by the responsible bodies.
Abbreviations
AFT, accelerated failure time; AIC, Akaike Information Criterion; AIDS, acquired immunodeficiency syndrome; ANC, antenatal care; ART, antiretroviral therapy; BIC, Bayesian Information Criterion; CD4, cluster of differentiation 4; eMTCT, elimination of mother-to child transmission of HIV; HAART, highly active antiretroviral therapy; HIV, human immune deficiency virus; IATT, Interagency Task Team; ICC, intraclass correlation; KM, Kaplan Meier; L&D, labor and delivery; LMM, linear mixed model; LRT, likelihood ratio test; MI, multiple imputation; ML, maximum likelihood; MUAC, mid upper arm circumference; PHR, proportional hazard regression; PMTCT, prevention of mother-to-child transmission of HIV; PNC, postnatal care; REML, restricted maximum likelihood; UN, unstructured; WHO, World Health Organization.
Data Sharing Statement
The raw materials that support the conclusion of this research will be available per request to whom in need of the data to use for non-commercial purposes through requesting Ayele Mamo Abebe.
Ethics Approval
A legal permission was obtained from Debre Berhan University. Ethical review board and official letters of cooperation were written by the department of Statistics to selected Health Centers. The study participants (mothers) gave informed consent to participate in this study and this study was performed in accordance with the Declaration of Helsinki.
Consent for Publication
We have got informed written consent from health centers from which the data have been collected for possible publication.
Acknowledgments
We feel pleasure to express our appreciation for those institutions in which the study has been conducted for their cooperation to avail the data. We are also delighted to thank our data collectors who produced this data set.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Bardi J. First pediatric cases of HIV: recalling 1981 UCSF AIDS 2012 blog. Available from: http://aids2012.ucsf.edu/2012/07/22/first-pediatric-cases-of-hivrecalling-1981.
2. Moges NA, Kassa GM, Boneya DJ. Rate of HIV transmission and associated factors among HIV-exposed infants in selected health facilities of East and West Gojjam Zones, Northwest Ethiopia; retrospective cohort study. BMC Infect Dis. 2017;17(1):475. doi:10.1186/s12879-017-2578-3
3. Hurst SA, Appelgren KE, Kourtis AP. Prevention of mother-to-child transmission of HIV type 1: the role of neonatal and infant prophylaxis. Expert Rev Anti Infect Ther. 2015;13(2):169–181. doi:10.1586/14787210.2015.999667
4. Gulaid LA, Kiragu K. Lessons learnt from promising practices in community engagement for the elimination of new HIV infections in children by 2015 and keeping their mothers alive: summary of a desk review. J Int AIDS Soc. 2012;15:17390. doi:10.7448/IAS.15.4.17390
5. Keehn E, Karfakis J. Current Practices to Increase Uptake, Retention and Adherence for Option B+ in Malawi. Lilongwe: mothers2mothers Malawi; 2014.
6. Organization, W.H. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach-2010 version. World Health Organization; 2010.
7. Ejara D, Mulualem D, Gebremedhin S. Inappropriate infant feeding practices of HIV-positive mothers attending PMTCT services in Oromia regional state, Ethiopia: a cross-sectional study. Int Breastfeed J. 2018;13(1):37.
8. Ababa A, Calverton E. Central statistical agency (Ethiopia) and ICF international. Ethiopia and Calverton:Ethiopia Demographic and Health Survey; 2011.
9. Pegurri E, Konings E, Crandall B, et al. The missed HIV-positive children of Ethiopia. PLoS One. 2015;10(4):e0124041. doi:10.1371/journal.pone.0124041
10. Berhan Z, Abebe F, Gedefaw M, et al. Prevalence of HIV and associated factors among infants born to HIV positive women in Amhara Region, Ethiopia. Int J Clin Med. 2014;2014.
11. WHO/UNAIDS. A progress report on the global plan towards the elimination of new HIV Infections among children by 2015 and keeping their mothers alive. Geneva: WHO, UNAIDS; 2012.
12. Roy A, Bhaumik DK, Aryal S, et al. Sample size determination for hierarchical longitudinal designs with differential attrition rates. Biometrics. 2007;63(3):699–707. doi:10.1111/j.1541-0420.2007.00769.x
13. Wudineh F, Damtew B. Mother-to-child transmission of HIV infection and its determinants among exposed infants on care and follow-up in Dire Dawa City, Eastern Ethiopia. AIDS research and treatment; 2016.
14. Gumbo F, Duri K, Kandawasvika GQ, et al. Risk factors of HIV vertical transmission in a cohort of women under a PMTCT program at three peri-urban clinics in a resource-poor setting. J Perinatol. 2010;30(11):717–723. doi:10.1038/jp.2010.31
15. Tadele T, Tamiso A, Tadele T. Incidences and predictors of HIV positivity among infants who born from HIV positive mother who have follow up at two hospitals of southern Ethiopia, 2014. Sci J Public Health. 2014;2(5):431–9. doi:10.11648/j.sjph.20140205.19
16. Koye DN, Zeleke BM. Mother-to-child transmission of HIV and its predictors among HIV-exposed infants at a PMTCT clinic in northwest Ethiopia. BMC Public Health. 2013;13(1):398. doi:10.1186/1471-2458-13-398
17. Berhan Z, Abebe F, Gedefaw M, et al. Risk of HIV and associated factors among infants born to HIV positive women in Amhara region, Ethiopia: a facility based retrospective study. BMC Res Notes. 2014;7(1):876. doi:10.1186/1756-0500-7-876
18. Endalamaw A, Demsie A, Eshetie S, et al. A systematic review and meta-analysis of vertical transmission route of HIV in Ethiopia. BMC Infect Dis. 2018;18(1):283. doi:10.1186/s12879-018-3189-3
19. Yende Zuma N, Modelling CD4+ count over time in HIV positive patients initiated on HAART in South Africa using linear mixed models. 2009.
20. Awoke Ayele TA, Worku A, Kebede Y, et al. Model-based prediction of CD4 cells counts in HIV-infected adults on antiretroviral therapy in Northwest Ethiopia: A flexible mixed effects approach. PLoS One. 2019;14(7):e0218514.
21. Jacobson LP, Phair JP, Yamashita TE. Virologic and immunologic response to highly active antiretroviral therapy. Curr Infect Dis Rep. 2012;4(1):88–96.
22. Coelho AVC, Campos Coelho HF, Arraes LC, et al. HIV-1 mother-to-child transmission in Brazil (1994–2016): a time series modeling. Braz J Infect Dis. 2019;23(4):218–223. doi:10.1016/j.bjid.2019.06.012
23. Sibanda EL, Weller IVD, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. Aids. 2013;27(17):2787–2797. doi:10.1097/QAD.0000000000000027
24. Giuliano M, Andreotti M, Liotta G, et al. Maternal antiretroviral therapy for the prevention of mother-to-child transmission of HIV in Malawi: maternal and infant outcomes two years after delivery. PLoS One. 2013;8(7):e68950. doi:10.1371/journal.pone.0068950
25. Goga A, Jackson D, Lombard C, et al. Highest risk of mother to child transmission of HIV or death in the first 6 months postpartum: results from 18 month follow-up of an HIV-exposed national cohort, South Africa. J Int AIDS Soc. 2016.
26. Miller K, Muyindike W, Matthews LT, et al. Program implementation of option B+ at a President’s emergency plan for AIDS relief-supported HIV clinic improves clinical indicators but not retention in Care in Mbarara, Uganda. AIDS Patient Care STDS. 2017;31(8):335–341. doi:10.1089/apc.2017.0034
27. Damania KR, Tank PD, Lala MM. Recent trends in mother to child transmission of HIV in pregnancy. J Obstet Gynaecol India. 2010;60(5):395. doi:10.1007/s13224-010-0065-5
28. Barral MF, Oliveira GRD, Lobato RC, et al. Risk factors of HIV-1 vertical transmission (VT) and the influence of antiretroviral therapy (ART) in pregnancy outcome. Revista Do Instituto De Medicina Tropical De São Paulo. 2014;56(2):133–138. doi:10.1590/S0036-46652014000200008
29. Tsegaye D, Deribe L, Wodajo S. Levels of adherence and factors associated with adherence to option B+ prevention of mother-to-child transmission among pregnant and lactating mothers in selected government health facilities of South Wollo Zone, Amhara Region, northeast Ethiopia, 2016. Epidemiol Health. 2016;38:e2016043. doi:doi:10.4178/epih.e2016043
30. Izudi J, Apangu P, Bajunirwe F, et al. High baseline CD4 count and exclusive breastfeeding are associated with lower rates of mother to child HIV transmission in Northwestern Uganda: a two-year retrospective cohort study. Adv Public Health. 2018;2018:1–8. doi:10.1155/2018/4140254
31. Dreyfuss ML, Fawzi WW. Micronutrients and vertical transmission of HIV-1. Am J Clin Nutr. 2012;75(6):959–970. doi:10.1093/ajcn/75.6.959
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.