Back to Journals » Vascular Health and Risk Management » Volume 18
Jetstream Atherectomy Followed by Paclitaxel-Coated Balloons versus Balloon Angioplasty Followed by Paclitaxel-Coated Balloons: Twelve-Month Exploratory Results of the Prospective Randomized JET-RANGER Study
Authors Shammas NW , Purushottam B, Shammas WJ, Christensen L, Shammas G, Weakley D, Jones-Miller S
Received 5 May 2022
Accepted for publication 7 July 2022
Published 2 August 2022 Volume 2022:18 Pages 603—615
DOI https://doi.org/10.2147/VHRM.S371177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Mirna N Chahine
Nicolas W Shammas,1 Bhaskar Purushottam,2 W John Shammas,1 Lori Christensen,1 Gail Shammas,1 Desyree Weakley,1 Sue Jones-Miller1 On behalf of the JET-RANGER Investigators
1Midwest Cardiovascular Research Foundation, Davenport, IA, USA; 2Regional Health CR, Cardiovascular Medicine, Monument Health, Rapid City, SD, USA
Correspondence: Nicolas W Shammas, Research Director, Midwest Cardiovascular Research Foundation, 630 East 4th Street, Suite A, Davenport, IA, USA, Email [email protected]
Background: It is unknown at this time whether Jetstream atherectomy (JET) and paclitaxel-coated balloon (PCB) provides a superior outcome to balloon angioplasty (PTA) followed by PCB in treating femoropopliteal (FP) arterial disease.
Methods: The JET-RANGER study was a multicenter (eleven US centers) randomized trial, core lab–adjudicated, designed to demonstrate the superiority of JET + PCB versus PTA + PCB in treating FP arterial disease. The study intended to enroll 255 patients, but was stopped early because of poor enrollment due to COVID-19 and concerns about the association of paclitaxel with mortality. The data are thus considered exploratory. A total of 47 patients (48 lesions) with claudication (80.9%) or rest pain/ulcerations (19.2%) were randomly assigned 2:1 to JET + PCB (n=31) or PTA + PCB (n=16). The In.PACT (Medtronic) and Ranger (Boston Scientific) PCBs were used. Freedom from target-lesion revascularization (TLR) was evaluated at 1 year. Analysis was performed on intention to treat.
Results: Mean lesion length was 10.8± 4.3 cm for JET + PCB and 11.2± 7.6 cm for PTA + PCB (P=0.858). There were no other differences in demographic or angiographic variables between the two groups. Procedural success was superior with JET + PCB (87.1%) vs PTA + PCB alone (52.9%; P=0.0147). Overall bailout stenting rate was 17% (0 JET + DCB versus 50% PCB, P< 0.0001). There was no distal embolization requiring treatment. There was no amputation or death in either group. Using KM analysis, the primary end point of freedom from TLR (bailout stent considered a TLR) at 1 year was 100% and 43.8% (P< 0.0001) for JET + PCB versus PTA + PCB, respectively. When bailout stent was not considered a TLR, freedom from TLR was 100% and 93.7%, respectively (P=0.327).
Conclusion: A high rate of freedom from TLR was seen in the JET + PCB arm and the PTA + DCB arm at 1-year follow-up, with a significant reduction in bailout stenting following vessel prepping with the Jetstream.
Keywords: Jetstream, atherectomy, femoropopliteal, vessel prepping, drug-coated balloons, Ranger, In.PACT, randomized trial, dissections, bailout stenting
Introduction
Balloon angioplasty (PTA) of the femoropopliteal (FP) artery carries a high rate of restenosis. Bare-metal self-expanding stents have improved on the patency rate when compared to angioplasty, but the impact on target-lesion revascularization (TLR) remains inconsistent.1 Recently, drug-eluting stents and paclitaxel-coated balloons (PCBs) were shown to improve the long-term patency rate and reduce TLR.2–5 Bailout stenting or primary stenting, however, has its own problems, including stent fractures, restenosis, and thrombosis. Also, stents may adversely affect future endovascular or surgical options, particularly in non-stent zones. Leaving nothing or the least behind is a strategy that has recently gained momentum among endovascular specialists.6 Altering vessel-wall characteristics by either debulking or specialty balloons to gain the maximum luminal area without the need for bailout stenting and to improve on antiproliferative drug uptake is defined as vessel prepping.
Vessel prepping with atherectomy improves technical and procedural outcomes and reduces bailout stenting.7,8 Data also suggest that atherectomy leads to deeper and higher paclitaxel concentrations in the treated vessel,9 which may possibly improve the long-term patency of the FP artery when compared to PCBs only. The impact of atherectomy on the long-term outcome of de novo FP lesions, however, has not been demonstrated when compared to angioplasty alone.10 Small randomized and observational data suggest that the combination of atherectomy and PCB is more effective than PCB alone in treating in-stent restenosis11 and possibly complex de novo arterial disease, including severe calcium and long lesions.12–15
The JET-RANGER study (NCT03206762) was designed to test the hypothesis that Jetstream atherectomy (JET) and PCBs (Ranger balloon or In.PACT) provide superior outcome to PTA and PCBs in de novo FP lesions. The trial was halted early because of poor enrollment, due to the PCB warning on the increase in mortality with drug-coated balloons by the Food and Drug Administration and the COVID-19 pandemic. Therefore, the data in this manuscript are to be considered exploratory. We present the 1-year data on the limited number of patients enrolled in this study.
Methods
Devices
The Jetstream (Boston Scientific) is a rotational and aspiration atherectomy device indicated for use in the peripheral vasculature to treat denovo and non-stent infrainguinal arterial lesions. The Jetstream device uses XC (expandable cutter) and SC (or single cutter) catheters. The XC, as the name implies, can be used blades down and blades up for FP vessels. The SC catheter is typically used for tibial vessels.16
The Ranger PCB catheter (Boston Scientific) is designed for dilating stenotic lesions and applying and delivering paclitaxel to the vessel wall. The balloon uses citrate ester as excipient, with a paclitaxel-coating concentration of 2 μg/mm2. The In.PACT PCB (Medtronic) is also designed to dilate stenotic lesions and deliver paclitaxel to the vessel wall. The balloon uses urea as excipient, with a paclitaxel-coating concentration of 3.5 µg/mm2.
Study Design, Outcomes, and Selection Criteria
JET-RANGER was a prospective, multicenter, randomized study designed to estimate the effect of treating a vessel with plaque excision using JET in combination with a Ranger or In.PACT PCB (JET + PCB) compared to treatment with PCBs alone. The study was approved by the central Advarra Independent Ethics Committee with additional approval by the local institutional review boards at the research sites when required. The study complies with the Declaration of Helsinki. Enrollment started in March 2018 and the last 1-year patient follow-up was in April 2021.
The primary effectiveness outcome was TLR at 1 year, defined as retreatment of the index lesion (extended 1 cm proximal and distal to the lesion). For the primary end point, intraprocedural bailout stenting of the index lesion was considered to meet a TLR end point. The primary safety end point was major adverse events at 30 days, defined as unplanned amputation, total mortality, or TLR.
Secondary outcomes included:
Device outcome — categorized by <50% residual stenosis following the protocol-defined treatment (PTA + PCB or JET + PCB) and prior to bailout stenting at the target lesion, as determined by the Angiographic Core Laboratory.
Procedural outcome — categorized by <30% residual stenosis following the protocol-defined treatment and followed by bailout stenting if needed at the target lesion, as determined by the Angiographic Core Laboratory.
Clinical Patency at 6 months and 1 year — defined as duplex ultrasound patency (PSVR ≤2.4) of target lesion and freedom from clinically driven TLR following index procedure.
TLR with bailout stenting not considered a TLR at 1 year, ie, TLR rate following index procedure.
In-hospital major adverse event rate — defined as all-cause mortality, perforation requiring additional treatment, distal embolization requiring additional mechanical or pharmacological treatment, major bleeding as defined by the Thrombolysis in Myocardial Infarction criteria, vascular access-site complications requiring transfusion and/or surgical repair, acute stent thrombosis, unplanned major or minor amputation.
Major adverse event rate at 30 days — defined as major or minor unplanned amputation of the treated limb, vascular access-site complications requiring transfusion and/or surgical repair, all-cause mortality, acute thrombosis, or clinically driven target-vessel revascularization.
Major adverse events at 1 year — defined as major amputation of the treated limb, all-cause mortality, or TLR.
Change in WIQ Score at 30 days, 6 months, and 1 year, defined as the change in Walking Impairment Questionnaire (WIQ) score compared to pretreatment baseline.
Change in Rutherford clinical category (RCC) at 30 days, 6 months, and 1 year — defined as the change in clinical status indicated by the change in RCC compared to baseline.17
Change in ankle brachial index (ABI) at 6 months and 1 year — defined as the change in the ABI compared to baseline in subjects with compressible arteries. A change of 0.15 or higher was considered significant.
Subject-selection criteria are listed in Table 1.
 |
Table 1 Subject-selection criteria |
Subject Enrollment
Each subject was enrolled in the study after he/she had signed the informed consent form. The point of enrollment was defined as the moment an exchangeable guidewire and treatment catheter crossed the target lesion in the true lumen. Randomization occurred after the subject was enrolled into the study. Randomization was performed in blocks of 6 patients per center at a time. In each block, random numbers were generated to allocate two PTA + PCB for every four JET + PCB (2:1 ratio). Also, within each block PCBs were randomly assigned to either In.PACT balloon or Ranger balloon in a 1:1 ratio. Crossover to the other treatment arm was not allowed. Subjects were informed of the 2:1 randomization between the JET + PCB versus PCB arms but remained blinded as to which treatment they actually received until after their 1-year follow-up visit. It was not possible to blind the investigators due to the substantial treatment differences between the two treatment arms. This shortcoming was partially overcome by quantitative angiographic and duplex ultrasound measurements. Bailout stenting was defined as stenting due to flow limiting dissection D or higher using NHLBI classification of dissections and/or residual narrowing after JET + PCB or PTA + PCB that exceeded 30%. A Clinical Events Committee (CEC) reviewed prespecified adverse events. A Data Safety Monitoring Board (DSMB) was formed and was intended to meet after enrolling the first 60 patients. Given the early termination of the study, the DSMB did not meet.
Procedure
A radiopaque ruler was used to delineate the treated segment for core lab measurements. The subject was enrolled when an exchangeable guidewire crossed the target lesion intraluminally. Upon enrollment, subjects were randomized to PTA + PCB or JET + PCB treatment (1:2 randomization, respectively). Angiographic cine films were recorded, including runoff, at baseline, immediately after treatment with JET or PTA, after PCB, prior to stenting, and after final adjunctive treatment. Bailout stenting criteria were verified by the angiographic core lab (Cardiovascular Imaging Core Laboratory, Beth Israel Deaconess Medical Center, Boston, Massachusetts). Images were captured to minimize vessel overlap and demonstrate the stenosis in its most severe view. Digital subtraction angiography to the tibial vessels was performed to evaluate distal embolization.
Target Lesion
The lesion intended for treatment at the time of the index procedure that met the inclusion criteria and none of the exclusion criteria was considered the target lesion. Only one target lesion was allowed per limb per patient. A patient could not be enrolled twice in the study. If multiple lesions in the target vessel required treatment, pretreatment of the nontarget lesions was performed prior to enrolling the target lesion. Nontarget lesions needed to be proximal to the target lesion. Also, it was considered acceptable to treat nontarget lesion(s) at least 30 days prior to the index procedure. No infrapopliteal lesions were allowed to be treated in the index procedure.
JET + PCB Group
The selection of the JET XC device was left to the discretion of the treating physician. In general; however, the 2.4 XC was recommended for vessel diameter >5 mm and 2.1 XC for 4–5 mm. Wire choice was left to the operator’s discretion, but hydrophilic coronary wires were not allowed. Filters were also encouraged, but the final decision to use them was left to the investigator’s discretion. Following JET atherectomy, dilation of the lesion was done with a 1:1 PTA balloon (semicompliant or noncompliant) inflated only to a pressure that led to full balloon expansion for 90 seconds. This was followed by dilation of the lesion by PCB with a 1:1 balloon size for a total of 3 minutes. PCBs were also chosen by randomization between the Ranger and the In.PACT balloon built into the initial randomization.
The diameter of the study balloon was selected based on the vessel’s normal reference diameter distal to the target lesion. The length of the PCB was selected when feasible to cover approximately 1 cm proximal and 1 cm distal to the treated segment to cover the entire lesion with the PCB. If more than one study balloon was required to cover the entire length of a long lesion, the second study balloon was placed such that there was approximately 1 cm overlap between the balloons placed in a tandem fashion. The inflation pressure of the PCB was at least at nominal inflation pressure or to achieve the full expansion of the balloon above nominal pressure as needed, but not to exceed the rated burst pressure.
PTA + PCB Group
The diameter of the PTA balloon was selected based on the vessel’s normal reference diameter distal to the target lesion. Balloon inflation was performed initially to obtain full balloon expansion for 90 seconds. The length of the PCB was selected when feasible to cover approximately 1 cm proximal and 1 cm distal to the treated segment to cover the entire lesion with PCB. If more than one study balloon was required to cover the entire length of a long lesion, the second study balloon was placed such that there was approximately 1 cm overlap between the balloons placed in a tandem fashion. The inflation pressure of the PCB was at least at nominal inflation pressure or to achieve the full expansion of the balloon above the nominal pressure as needed, but not to exceed the rated burst pressure. The total inflation time was 3 minutes.
Adjunctive Procedures
Adjunctive procedures were discouraged unless they could cannot be avoided. In the event of a major flow-limiting dissection (type D and higher), perforation, or occlusive complication (eg, recoil), prolonged balloon inflation was recommended initially with a non–drug coated balloon first. All efforts were made to eliminate the need for bailout stent placement. In cases where the results after prolonged balloon inflation were suboptimal (D dissection or higher and >30% residual narrowing), bailout stenting was performed with nitinol self-expanding stents. Adjunctive treatment with cutting balloons or scoring balloons was not allowed. Covered stents were not allowed, except to seal a perforation. The subjects received unfractionated heparin anticoagulation to maintain appropriate intraprocedural clotting time (>250 seconds). If patients had not previously been loaded with an ADP-receptor antagonist, a loading dose was given at the time of the index procedure followed by 3 months’ maintenance dose unless otherwise indicated. The use of cilostazol (Petal) was not allowed during the study. All patients were on aspirin.
Statistical Methods
A total of 255 subjects were planned for enrollment (with a block 2:1 randomization, 167 JET + DCB). However, enrollment dropped severely after the controversy on increased mortality with PCBs and the FDA warning. This halted the study at several sites, as required by local institutional boards. When sites started to resume enrollment, the study was again halted by the COVID-19 pandemic, where elective procedures were placed on hold and patients were medically managed to avoid hospital admissions. Given the limited funds and the extended delays in enrollment, the study had to be halted after 47 patients. Given the overall small number of patients enrolled, the analysis performed is considered exploratory. An intention-to-treat analysis was performed.
Descriptive statistics was performed for all patients, procedures and lesions and by treatment arms. Statistics are presented as counts, percentages, or means ± SD (median) where appropriate. Grubbs’s test was used for outliers. Fisher’s exact, Pearson’s χ2, two-sample t, and Kruskal–Wallis tests were used to test differences for significance (P<0.05). Kaplan–Meier survival analysis for freedom from TLR with and without bailout was performed. Wilcoxon’s test was used for survival comparison between treatment arms to test for significance (P<0.05). Analyses were performed with Minitab 2021 (State College, PA, US) and StatXact 12 (Cytel, Waltham, MA, US).
Results
A total of 47 patients (31 JET + PCB, 16 PTA + PCB) were included. One patient in the JET + PCB arm had two lesions. Figure 1 shows the CONSORT diagram for JET-RANGER. Four patients were lost to follow-up in the JET + PCB arm. There were no patients lost in the PTA + PCB arm. Analysis was conducted on 27 patients in the JET + PCB and 16 patients in the PTA + PCB arm. There was no difference between the two arms in clinical or demographic variables (Table 2). The mean age was 70.5–71.5 years, with 32.3%–43.8% diabetics and 12.9%–31.3% with limb ischemia. Mean lesion lengths were 10.8±4.3 cm and 11.2±7.6 cm (P=0.858), chronic total occlusion 22.6%–43.8% (P=0.182), and calcium PACC classification grades 74.2% and 56.3% (P=0.408) for JET + PCB and PTA + PCB, respectively. There were no differences in the remaining angiographic variables between the two groups (Table 3) either, except for the use of embolic filters, which was significantly higher for JET + PCB vs PTA + PCB (96.8% vs 12.5%, P<0.001).
 |
Table 2 Clinical and demographic variables |
 |
Table 3 Angiographic and procedural variables |
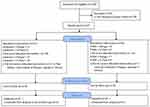 |
Figure 1 CONSORT diagram of JET-RANGER. |
Table 4 displays intraprocedural, 6-month, and 1-year outcomes. Procedural success was superior with JET + PCB (87.1%) vs PTA + PCB alone (52.9%) (P=0.015). There was no distal embolization in either group that required additional therapy besides a vasodilator. Type D dissections and higher were similar between the two groups (6.5% vs 5.9%), but there was more residual narrowing in the PTA + PCB group (50% vs 0). Bailout stenting per patient was 50% in the PTA + PCB group and none in the JET + PCB group (P<0.001), driven exclusively by residual narrowing. There was no in-hospital amputation or death in either group (Table 3). At the 6-month follow-up, proportional TLR was 56.3% versus 0 (with bailout stenting considered a TLR) and 6.3% versus 0 (with bailout stenting not considered a TLR) for PTA + PCB vs JET + PCB, respectively (P<0.001). On KM analysis (Figure 2), the primary end point of freedom from TLR (bailout stent considered a TLR) at 1 year was 100% and 43.8% (P<0.0001) for JET + PCB versus PTA + PCB, respectively. When bailout stent was not considered TLR, freedom from TLR was 100% and 93.7%, respectively (P=0.327). There were no amputations or deaths at 1 year in either arm.
 |
Table 4 Outcomes |
 |
Figure 2 Target-lesion revascularization (TLR) rates without bailout stenting considered a TLR (upper panel) and with bailout stenting considered a TLR (lower panel). |
Discussion
Vessel prepping with atherectomy has been shown to reduce angiographic dissections and bailout stenting in the treatment of FP arterial disease. In addition, atherectomy facilitates drug absorption and increases paclitaxel concentration into the vessel wall. Data from the DEFINITIVE AR study12 suggested that the combination of SilverHawk atherectomy and drug-coated balloons improved patency in longer and calcified disease, although the number of patients studied was too small to show statistical significance. In DEFINITIVE AR, dissections were significantly reduced in the atherectomy + PCB arm when compared to the PTA + PCB arm and technical success was higher with the combination treatment. Similarly, JET-RANGER had higher procedural success in the JET + PCB arm than the PTA + PCB arm. The higher residual narrowing in the PTA + PCB arm was the main predictor of bailout stenting, rather than the presence of flow-limiting dissections. There were two D dissections per core lab that were not stented in the JET + PCB arm, likely not recognized as D dissections by the operators during the procedure. The overall low rate of dissection, however, could be attributed to the relatively prolonged balloon inflation in both arms required by the protocol (average of 250 seconds) and the avoidance of high inflation pressure (average 8 atm). The greater residual narrowing in the PTA + PCB arm cannot be accounted for by undersizing the balloon. The average balloon diameter used was 5.6–5.7 mm and the vessel diameter 5.1–5.3 mm (a ratio of about 1.08:1). Of interest, the procedure time was similar between the two arms of the study. Although operators are concerned about longer procedure time with atherectomy, this was not the case in this study, likely because atherectomy-use time was offset by the longer time needed to perform bailout stenting in the PTA + PCB arm.
In several studies, total occlusions, long lesions, and moderate–severe calcium have been predictors of bailout stenting.18,19 In JET-RANGER, moderate–severe calcified lesions were included (PACCS grades 3/4 in 74%). Despite the early termination of this study, the JET + PCB arm showed a statistically significant higher procedural success and less bailout stenting than the PTA + PCB arm. The primary end point was TLR with bailout stenting as a TLR. The primary end point was based on the hypothesis that the main goal of atherectomy is to reduce stenting and retreatment of the index lesion intraprocedurally, as well as on follow-up. Proportional and KM probability of TLR were 0 and 0 for the JET + PCB arm, respectively. In JET-SCE,20 freedom from TLR was significantly higher with JET + PCB than JET + PTA at 12 months (94.7% vs 68.0%, P=0.002) and 16 months (94.4% vs 54%, P=0.002). The combination of JET with PCB seems to yield excellent freedom from TLR. Interestingly, a high freedom from TLR was also seen in the PTA + DCB arm, and this may have been secondary to the high rate of stenting in this group. Patency data were limited in JET-RANGER because of limited follow-up in the COVID pandemic. Of the eleven patients in the JET + PCB arm that underwent duplex ultrasound, all index lesions per core lab were patent. Of the four patients in the PTA + PCB arm, three were patent.
Freedom from TLR was comparable between JET + PCB and PTA + PCB when bailout stenting was not included as a TLR. This indicates that the strategy of PTA followed by PCB yields good results at the expense of a high rate of bailout stenting in a set of complex lesions. A high stenting rate post-PTA has been observed in calcified FP arterial disease in multiple studies.21,22 To our surprise, bailout stenting was due to high residual narrowing and not to flow-limiting dissections, possibly due to prolonged balloon inflation in both arms and the cutoff limit of bailout-stenting definition of type D and higher dissections, rather than C and higher. Since the strategy is to leave the least behind with the use of atherectomy, the JET + PCB alternative is an attractive option, particularly in lesions in no-stent zones, such as the distal superficial femoral artery or the popliteal artery.
Mortality with PCB was raised as a concern by Katsanos et al, and led to an FDA warning about the use of these devices. Katsanos et al23 reported all-cause death with PCB/drug-eluting stent at 1 year to be similar between paclitaxel-coated devices and control arms (2.3% versus 2.3%). In our study and with all PCB patients included, there was no death or amputation. In a recent analysis of data from insurance claims in the US and Europe during the 15 months following the publication of the Katsanos et al meta-analysis, Schneider et al noted that there was no significant increase in all-cause mortality with paclitaxel-coated devices.24 Several other studies showed the same findings of no increase in mortality with PCB including data from the In.PACT program, a PCB with higher paclitaxel dose.25–28 Given the small number of patients in our study, no definitive conclusions about mortality increase or decrease can be made with the Ranger or In.PACT balloons. Distal embolization that required any mechanical or lytic treatment was not encountered in either group. Most patients in the JET arm had embolic filters; however, 56.7% of filters had debris in them, consistent with prior studies showing that distal embolization does occur with the JET device and the use of embolic filters is encouraged.29,30
In summary, this study shows that the strategy of Jetstream atherectomy with PCB yields excellent freedom from TLR at 1 year without the need for bailout stenting. Although the results with PTA and PCB were also excellent at 1 year, this came at the cost of a very high rate of bailout stenting in complex lesions with CTO, moderate–severe calcium, and long lesions. The Jeststream device is thus an excellent vessel-prepping device prior to PCB in no-stent zones, such as the distal superficial femoral, common femoral, and popliteal arteries, and in younger patients, where stenting may carry significant complications and reduce surgical options.
Limitations
The study was terminated before enrollment had been completed. Few patients were enrolled, and thus no definitive conclusions could be made as to the superiority of one strategy versus the other. The study, however, demonstrates that atherectomy yields higher procedural success and less bailout stenting, similar to prior published studies. Despite the small number of patients, the data remained highly consistent with other JET + PCB data showing that the overall freedom from TLR is excellent with this combination therapy and with no safety issues of significance at 1-year follow-up. A large randomized trial for atherectomy + PCB versus PTA + PCB is needed to conclusively demonstrate if a long-term outcome benefit is seen with adjunctive vessel prepping with atherectomy prior to PCB.
Data Sharing
The authors do not intend to share individual deidentified data unless requested for a specific pre-specified analysis or for auditing purposes by regulatory bodies. Aggregate data will be released on clinicaltrials.gov and part of an indexed publication and will be accessible to public.
Acknowledgments
The JET-RANGER abstract was previously presented at TCT 2021 and LINC 2022 and published in JACC Interventions supplement. J Am Coll Cardiol. 2021 Nov, 78 (19_Supplement_S) B8. The study was supported by an unrestricted grant from Boston Scientific.
The following JET-RANGER investigators have contributed to the study by recruiting patients into this clinical trial or participated in the CEC committee or core laboratory:
Investigators
Lawrence Garcia, MD. Steward Saint Elizabeth, Boston MA;
Nicholas Petruzzi, MD, Atlantic Medical, NJ;
Mathew Wooster, MD, Medical University South Carolina, SC;
Jack Chamberlin, MD, Alexian Brothers Hospital, IL;
William B. Eaves, MD, Endovascular Technologies, LA;
Richard Kovach, MD, Deborah Heart and Lung, Brown Mills, NJ;
Mohammad Mehdi Ansari, MD, Texas Tech University Health Science, TX;
Esteban Henao, MD, New Mexico Heart Institute, NM;
Faisal Latif, MD, VA Oklahoma, OK;
April Nedeau, MD, Central Maine Medical Center, ME.
CEC Committee
Jon Robken, MD, Interventional Cardiology;
Param Singh, MD, Interventional Cardiology;
Vijay Ranjendran, MD, Interventional Cardiology.
Core Laboratories
Angiographic core lab: Cardiovascular Imaging Core Laboratory, Beth Israel Deaconess Medical Center, Boston, MA;
Duplex ultrasound core lab: VasCore Vascular Ultrasound Core Laboratory, Massachusetts General Hospital, Boston, MA.
Disclosure
Dr Nicolas Shammas receives educational and research grants from Boston Scientific, Angiodynamics, VentureMed, Phillips, and Bard/BD and is on the speakers’ bureaux of Janssen, Lilly, Esperion, Kiniksa, Bayer, and Boehringer Ingelheim. The authors report no other conflicts of interest in this work.
References
1. Shammas NW, Banerjee S. Should we routinely stent the femoropopliteal artery? An interventionalist’s perspective. J Invasive Cardiol. 2015;27(11):E258–61.
2. Albrecht T, Speck U, Baier C, Wolf KJ, Bohm M, Scheller B. Reduction of stenosis due to intimal hyperplasia after stent supported angioplasty of peripheral arteries by local administration of paclitaxel in swine. Invest Radiol. 2007;42(8):579–585. doi:10.1097/RLI.0b013e31804f5a60
3. Scheller B, Speck U, Abramjuk C, Bernhardt U, Bohm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circ Aug. 2004;110(7):810–814. doi:10.1161/01.CIR.0000138929.71660.E0
4. Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7(1):10–19. doi:10.1016/j.jcin.2013.05.022
5. Liistro F, Grotti S, Porto I, et al. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery). JACC Cardiovasc Interv. 2013;6(12):1295–1302. doi:10.1016/j.jcin.2013.07.010
6. Banerjee S, Jeon-Slaughter H, Armstrong EJ, et al. Clinical outcomes and cost comparisons of stent and non-stent interventions in infrainguinal peripheral artery disease: insights from the excellence in peripheral artery disease (XLPAD) registry. J Invasive Cardiol. 2019;31(1):1–9.
7. Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360° Trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26(8):355–360.
8. Shammas NW, Coiner D, Shammas GA, et al. Percutaneous lower-extremity arterial interventions with primary balloon angioplasty versus Silverhawk atherectomy and adjunctive balloon angioplasty: randomized trial. J Vasc Interv Radiol. 2011;22(9):1223–1228. doi:10.1016/j.jvir.2011.05.013
9. Tzafriri AR, Garcia-Polite F, Zani B, et al. Calcified plaque modification alters local drug delivery in the treatment of peripheral atherosclerosis. J Control Release. 2017;264:203–210. doi:10.1016/j.jconrel.2017.08.037
10. Wu Z, Huang Q, Pu H, et al. Atherectomy combined with balloon angioplasty versus balloon angioplasty alone for de novo femoropopliteal arterial diseases: a systematic review and meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. 2021;62(1):65–73. doi:10.1016/j.ejvs.2021.02.012
11. Gandini R, Del Giudice C, Merolla S, et al. Treatment of chronic SFA in-stent occlusion with combined laser atherectomy and drug-eluting balloon angioplasty in patients with critical limb ischemia: a single-center, prospective, randomized study. J Endovasc Ther. 2013;20(6):805–814. doi:10.1583/13-4308MR.1
12. Zeller T, Langhoff R, Rocha-Singh KJ, et al. DEFINITIVE AR investigators. directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: twelve-month results of the DEFINITIVE AR study. Circ Cardiovasc Interv. 2017;10(9):e004848. doi:10.1161/CIRCINTERVENTIONS.116.004848
13. Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc Intervent Radiol. 2014;37(4):898–907. doi:10.1007/s00270-014-0904-3
14. Cioppa A, Stabile E, Popusoi G, et al. Combined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: one-year single centre clinical results. Cardiovasc Revasc Med. 2012;13(4):219–223. doi:10.1016/j.carrev.2012.04.007
15. Scheer F, Lüdtke CW, Kamusella P, et al. Combination of rotational atherothrombectomy and Paclitaxel-coated angioplasty for femoropopliteal occlusion. Clin Med Insights Cardiol. 2015;8(Suppl 2):43–48. doi:10.4137/CMC.S15231
16. Shammas NW, Shammas NW. JETSTREAM atherectomy: a review of technique, tips, and tricks in treating the femoropopliteal lesions. Int J Angiol. 2014;25(4):266–270. doi:10.1055/s-0034-1390083
17. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–538. doi:10.1016/S0741-5214(97)70045-4
18. Shammas NW, Coiner D, Shammas G, Jerin M. Predictors of provisional stenting in patients undergoing lower extremity arterial interventions. Int J Angiol. 2011;20(2):95–100. doi:10.1055/s-0031-1279683
19. Tepe G, Brodmann B, Werner M, et al. Disrupt PAD III investigators. intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized disrupt PAD III trial. JACC Cardiovasc Interv. 2021;14(12):1352–1361. doi:10.1016/j.jcin.2021.04.010
20. Shammas NW, Shammas GA, Jones-Miller S, et al. Long-term outcomes with Jetstream atherectomy with or without drug coated balloons in treating femoropopliteal arteries: a single center experience (JET-SCE). Cardiovasc Revasc Med. 2018;19(7Pt A):771–777. doi:10.1016/j.carrev.2018.02.003
21. Tepe G, Laird J, Schneider P, et al. IN.PACT SFA trial investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131(5):495–502. doi:10.1161/CIRCULATIONAHA.114.011004
22. Kokkinidis DG, Jeon-Slaughter H, Khalili H, et al. Adjunctive stent use during endovascular intervention to the femoropopliteal artery with drug coated balloons: insights from the XLPAD registry. Vasc Med. 2018;23(4):358–364. doi:10.1177/1358863X18775593
23. Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7(24):e011245. doi:10.1161/JAHA.118.011245
24. Schneider PA, Varcoe RL, Secemsky E, et al. Update on paclitaxel for femoral-popliteal occlusive disease in the 15 months following a summary level meta-analysis demonstrated increased risk of late mortality and dose response to paclitaxel. J Vasc Surg. 2020;73(1):311–322. doi:10.1016/j.jvs.2020.07.093
25. Schneider PA, Laird JR, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol. 2019;73(20):2550–2563. doi:10.1016/j.jacc.2019.01.013
26. Schneider PA, Brodmann M, Mauri L, et al. Paclitaxel exposure: long-term safety and effectiveness of a drug-coated balloon for claudication in pooled randomized trials. Catheter Cardiovasc Interv. 2020;96(5):1087–1099. doi:10.1002/ccd.29152
27. Steiner S, Schmidt A, Zeller T, et al. COMPARE: prospective, randomized, non-inferiority trial of high- vs. low-dose paclitaxel drug-coated balloons for femoropopliteal interventions. Eur Heart J. 2020;41(27):2541–2552. doi:10.1093/eurheartj/ehaa049
28. Schroeder H, Meyer D-R, Lux B, et al. Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: outcomes from the ILLUMENATE first-in-human study. Catheter Cardiovasc Interv. 2015;86(2):278–286. doi:10.1002/ccd.25900
29. Sharis EM, Shammas NW, Shammas GA, Jones-Miller S. Wirion EPS filter with jetstream atherectomy of femoropopliteal arterial disease: a single center experience. Cardiovasc Revasc Med. 2020;21(1):96–99. doi:10.1016/j.carrev.2018.11.014
30. Shammas NW, Pucillo A, Jenkins JET, et al. WIRION embolic protection system in lower extremity arterial interventions: results of the pivotal WISE LE trial. JACC Cardiovasc Interv. 2018;11(19):1995–2003. doi:10.1016/j.jcin.2018.05.025
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
