Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
Isoniazid Preventive Therapy Uptake and Its Effect on Tuberculosis Incidence Among People Living with HIV in Illubabor and Buno Bedelle Zones, South-West Ethiopia, 2022: A Retrospective Cohort Study
Authors Mulatu G, Gembe M , Chalchisa J , Teklu T , Yismaw W , Dereje D , Wondmagegn H
Received 23 August 2023
Accepted for publication 31 October 2023
Published 6 November 2023 Volume 2023:15 Pages 649—662
DOI https://doi.org/10.2147/HIV.S436787
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Gebremeskel Mulatu,1 Maycas Gembe,1 Jiregna Chalchisa,1 Tigist Teklu,1 Worke Yismaw,1 Debela Dereje,1 Habtamu Wondmagegn2
1Department of Nursing, College of Health Science, Mattu University, Mattu, Ethiopia; 2Department of Anatomy, School of Medicine, Arba Minch University, Arba Minch, Ethiopia
Correspondence: Maycas Gembe, Tel +251 936704900, Email [email protected]
Introduction: Tuberculosis (TB) remains the leading cause of death among human immune deficiency virus (HIV) patients. Based on the 2020 global TB report, Ethiopia was among the 30 high TB and TB/HIV burden countries. This study filled gaps regarding IPT uptake in the study area and representative sample determination for assessing TB incidence and its predictors at public health facilities in Illubabor and Buno Bedelle zones, south-west Ethiopia.
Methods: This retrospective cohort study was conducted among people living with HIV (PLHIV) who were on antiretroviral therapy (ART) at public health facilities in Illubabor and Buno Bedelle zones, south-west Ethiopia. Both isoniazid preventive therapy (IPT) exposed and unexposed PLHIV were followed from the date of ART initiation until the date of TB diagnosis of the most recent visit prior to the end of follow-up. The Cox proportional hazard model was employed to identify variables that predicted the incidence of TB at a P value of < 0.05.
Results: Data were collected on 421 PLHIV, with a response rate of 97.4%. The median (interquartile range (IQR)) age of the study participants was 32 (28– 40) year. The incidence rate of pulmonary TB was 3.1 per 1000 person-months (95% CI: 2.4– 3.9). The incidence rate of TB among IPT-exposed PLHIV was 1.45 per 1000 person-months, but it was 6.2 per 1000 person-months in the unexposed group. Patient’s residence, IPT exposure, baseline ART adherence, baseline hemoglobin level, baseline CD4+ cell, recent hemoglobin level, recent CD4+ cell, recent BMI, and recent WHO HIV clinical stage were independently associated with the incidence of TB.
Conclusion: Healthcare professionals working in ART clinics should routinely assess HIV-positive individuals for changes in clinical indicators and environmental exposures like living conditions, which will help HIV-positive individuals in reducing their risk for TB. Likewise, patients attending ART clinics should receive counseling on a regular basis.
Keywords: tuberculosis, isoniazid preventive therapy, people living with HIV
Introduction
Tuberculosis (TB), an infectious disease caused by the Mycobacterium tuberculosis, has remained a major global public health concern in general and in people living with HIV in particular (PLHIV).1 When an individual is infected with human immunodeficiency virus (HIV), she/he is at increased risk of contracting TB;2 deterioration of immunity in an HIV-positive person is the main determinant factor that leads to TB disease.3 Similarly, TB remains the leading cause of death among acquired immune deficiency syndrome (AIDS) patients.4 The chance of developing active TB among PLHIV is 20 to 37 times higher than those who do not have HIV infection.5 According to the 2022 joint United Nations Program on HIV/AIDS (UNAIDS) report, among all incident episodes of TB, 8% were among people living with HIV, and TB accounted for around one-third of AIDS-related deaths globally.6
After a lot of research and a review of available evidence, the World Health Organization (WHO) recommended the use of isoniazid preventive therapy (IPT) in 2010; it recommended that PLHIV should use IPT for at least 6 months. In this recommendation, WHO specified a simplified algorithm to identify eligible PLHIV for IPT.7 The use of IPT as a TB prevention strategy was first conceptualized by WHO in 1998, particularly targeting PLHIV who live in areas where there is a high prevalence of TB.8 IPT reduces the risk of an early episode of TB or its reoccurrence. IPT should be given to HIV patients irrespective of their immunity status.1,9
In line with the WHO recommendation, the Ethiopian TB, TB/HIV, drug-resistant TB (DR-TB), and leprosy management guideline encourage the use of IPT. According to the guideline, children ≥12 months, adolescents, and adult HIV/AIDS patients who do not report fever, cough, night sweats, and weight loss are unlikely to have active TB and should receive TB preventive therapy (TPT), including IPT. The guideline recommends that HIV-positive children, adolescents, and adults should receive IPT when they are contraindicated to use other TPTs.4,10
Based on the 2020 global TB report,11 Ethiopia was among the 30 high TB and TB/HIV burden countries. Various studies have also shown a decreased incidence of TB among PLHIV who were on IPT compared to those who did not expose to IPT.2,10,12,13 Since PLHIV are at risk for opportunistic TB disease, we hypothesized that all PLHIV should receive IPT regardless of their immunity status. In addition, as far as we are concerned, the level of IPT utilization has not been well studied in the study area. Thus, we uncovered the variation or gap in the uptake of IPT among PLHIV in Illubabor and Buno bedelle zones, south-west Ethiopia. After analyzing the utilization gap in the two zones, we are also planning to conduct qualitative research as to why this utilization gap existed. This study also addressed the effect of IPT in preventing TB among PLHIV in the study area. Furthermore, in this study, we used sample size calculation based on survival analysis, which minimized the methodological gap that occurred among previously conducted studies with regard to TB incidence.2,12,14
Methods and Materials
Study Design
We employed a retrospective cohort study design among PLHIV on antiretroviral therapy (ART) follow-up in Illubabor and Buno Bedelle zones, south-west Ethiopia.
Study Area and Study Period
We conducted this study at public hospital’s ART clinics found in Illubabor and Buno Bedelle zones. Illubabor and Buno Bedelle zones are found in the Oromia Region, south-west Ethiopia. These two zones are known for their forest landscapes. The agricultural lands of the zones are covered by maize cultivation, and the main cash crop in the zones is Coffee. Mettu town and Bedelle town are the administrative towns of the Illubabor and Buno Bedelle zones, respectively. There are five public hospitals in these two zones, namely Mettu Karl Referral Hospital, Bedele General Hospital, Darimu Hospital, Dedesa Hospital, and Chora Hospital. There are also thirty-one health centers in these zones. All public hospitals and nineteen health centers provide ART for PLHIV. Among the nineteen health centers, nine of them started to give ART services in 2020. All public health facilities that provide ART services follow test-and-treat treatment protocol.15,16 TB prevention and treatment service have also been given in these public health facilities. We collected data from the five public hospitals and three health centers found in the two zones. We conducted this study from February 2022 to June 2022.
Study Population
Our study populations were all adult PLHIV attending ART clinics in Illubabor and Buno Bedelle zones. Both IPT-exposed and unexposed PLHIV were followed from the date of ART initiation until the date of TB diagnosis of the most recent visit prior to the end of follow-up. The duration of the follow-up period for a subject was five years and the starting point for follow-up in records was from January 2016 to December 2017. Again, for this study, the study period was from February 15, 2022, to June 20, 2022. We accessed the medical records of PLHIV from February 17, 2022, to March 14, 2022.
Sample Size and Sampling Procedure
The sample size for this study was determined based on the log-rank method for survival analysis. The following assumptions were considered: 5% type one error, power of 80%, hazard rate of 2.16,17 1 to 1 allocation ratio, 0.9 survival probability at the end of follow-up, and 10% contingency for incomplete patient data. Therefore, the final sample size for this study was 428.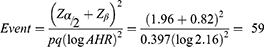
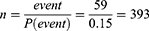
By adding 10% contingency the final sample size will be 432
Where; Zα/2 is reliability coefficient
Zβ is the power of the study
p is probability of survival in the unexposed group
q is probability of event in the unexposed group
To conduct this study, first, we gathered information about the total number of healthcare facilities that have ART clinics in the two zones. Next, we identified health-care facilities that gave ART service for the last five years before data collection, which is our follow-up period for PLHIV. There were 5 public hospitals and 10 health centers that gave ART service for PLHIV; we selected all 5 public hospitals and one-third of the health centers (3 health centers) to collect data necessary for our study. Then, we collected data on the number of PLHIV who attended these health facilities one year before our data collection period, to allocate a proportional number of samples to these health facilities. Finally, necessary data was collected by health professionals working at ART clinics.
In this study, we included all adult PLHIV who were registered for ART from 2016 to 2017. However, we excluded those PLHIV who were transferred into our study healthcare facilities after they had started their ART follow-up in other healthcare facilities outside the study area. We also excluded PLHIV who were already on TB treatment or PLHIV who were on ART less than two years after completion of their TB treatment. We further excluded PLHIV who had incomplete information on their medical record sheet.
Data Collection Procedure and Data Collection Instrument
We developed a data collection tool by revising previously conducted studies on similar study topic.12,17 The data collection tool consisted of socio-demographic variables: patient’s age, sex, residence, marital status, religion, family size, clinical-related variables: WHO clinical staging, cell differentiation 4 (“CD4+”) count, IPT use, etc., and behavioral-related characteristics: substance use, ART adherence.
Patients’ ART registration number was used to easily identify PLHIV on ART and to draw samples at each healthcare facility. We included all PLHIV who had taken ART for the last five years before the data collection period. Data on the main exposure variable (exposure to IPT) along with other predictor variables was looked at from patients’ medical registration folders. Regarding data on time-varying covariates, we considered baseline data as data collected within the first 6 months of initiation of ART, and we considered current data as recent data gathered before the occurrence of TB, loss to follow-up, end of the study period, or death. Diagnosis of TB was made by GeneXpert®, a molecular test for TB diagnosis.
Operational Definitions
Event: time to TB
Censored: PLHIV, who were either lost to follow-up, died, transferred out, or end of the study period.
IPT completed: ART PLHIV who completed the 6-month INH prophylaxis to sterilize latent TB.12
ART adherence:
Good adherence: ≥95% adherence to ART or ≤3 missed doses per month
Fair adherence: 85–94% adherence to ART or 4–8 missed doses per month
Poor adherence: <65% adherence to ART >9 missed doses per month18
Quality Control and Data Analysis
Prior to data collection, a pretest of the data collection tool was done at the data collection site. Since we used secondary data from available records, doing the pretest at these health facilities helped us to know the level of data completeness. Two-day training was given for data collectors; data collectors were BSc nurses working at ART clinics. The training focused on the procedures of data collection, understanding of the study objectives, how to extract data from medical sheets, and how to keep the anonymity of the collected data.
We initially exported the collected data into STATA v.14 software for analysis. We started data analysis by performing summary statistics; descriptive summary statistics was done based on the type and distribution of variables. Then, we checked the assumptions of survival analysis: both the proportional hazard assumption and the assumption of observational independence were checked. To check the proportional hazard assumption, the graph of the -ln(-ln) survival curve was plotted, and the goodness-of-fit test was employed. Parallel curves among categories of predictors and non-significant goodness-of-fit test were used as an indicator of fulfilled proportional hazard assumption. In addition, baseline and recent BMI, baseline and recent “CD4+” count were checked for possible collinearity; there was little collinearity between these variables, variance inflation factor of <5. Model fitness was assessed by the Cox-Snell residual plot. Finally, we employed the Cox proportional hazard model to identify variables that predicted the incidence of TB at a P value of <0.05.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki. We received an ethical clearance letter from Health Science College, Mattu University, with reference no. RPG/139/14. Then, the letter was disseminated to each health facility where the data collection was done. We along with the public health facilities’ administration staff discussed the objectives of this study, and we assured them that the confidentiality of the study participants will be kept. Finally, we received verbal approval to conduct the study. For this study, consent to participate was not applicable.
Result
Study Participants’ Socio-Demographic Characteristics
Among the total respondents (432), data were collected on 421 PLHIV, with a response rate of 97.4%. The data from 11 respondents was not considered because of incompleteness, transferred in cases, or they were already on TB treatment. The median (interquartile range (IQR)) age of the study participants was 32 (28–40) year. The majority of the respondents attended primary education (40.6%), followed by respondents with secondary-level education (31.6%). More than half (58.7%) of the study participants were female. Seventy-one percent of respondents were from urban residents (Table 1).
 |
Table 1 Socio-Demographic Characteristics of PLHIV Attending at ART Clinics in Illubabor and Buno Bedele Zones, South-West Ethiopia, 2022 |
Clinical and Behavioral Characteristics of the Study Participants
All PLHIV were on ART throughout the follow-up period; test-and-treat HIV treatment protocol, WHO HIV clinical staging and the level of “CD4+” cell count were used as ART initiation criteria. At baseline, the majority (97%) of patients had good adherence to ART, and almost all patients had good adherence to ART at follow-up or recent visits. Opportunistic infection prophylaxis like IPT (or INH), cotrimoxazole, and fluconazole were given for PLHIV. Sixty-two percent of PLHIV were exposed to IPT, and these IPT-exposed PLHIV also took the prophylaxis for 6 months (Figure 1). At baseline, the study participants’ mean (SD) BMI was 20.4 (4), and the mean (SD) of the respondents’ BMI at a recent visit was 21.7 (4.6). The mean (SD) of the respondents’ “CD4+” count at baseline and at recent visits were 458.6 (306.56) and 734.2 (245.4), respectively. The mean (SD) of the study participants’ hemoglobin levels at baseline and recent visits were 13.2 (2.6) and 14.6 (1.2), respectively. At baseline and recent visits, the majority of the patients were at WHO HIV clinical stage I (84%). In regard to respondents’ substance use status, 40.4% of respondents had a history of tobacco use, followed by alcohol and tobacco use (Table 2).
 |
Table 2 Clinical and Behavioral Characteristics of PLHIV Attending at ART Clinics in Illubabor and Buno Bedelle Zones, South-West Ethiopia, 2022 |
 |
Figure 1 IPT exposure status of PLHIV in this study. |
Survival Data Analysis
According to our findings, the majority of PLHIV survived after the median follow-up period. So, the mean survival time for these PLHIV was 58.5 (95% CI: 56.7–60.3). During the follow-up period, 65 patients developed TB, and it took 20,953 person-months to develop the outcome. The incidence rate of pulmonary TB was 3.1 per 1000 person-months (95% CI: 2.4–3.9), or it was 3.7 per 100 person-years (PYs) (95% CI: 2.92–4.74) with a total of 1746.1 person-time. The incidence rate of TB among IPT-exposed PLHIV was 1.45 per 1000 person-months, but it was 6.2 per 1000 person-months in the unexposed group, higher than those exposed to IPT. The following Kaplan–Meier graphs visualize the overall survival probability (Figure 2).
 |
Figure 2 Kaplan–Meier survival probability curve showing time until TB diagnosis among PLHIV in this study. |
The Kaplan–Meier graph was used to visualize differences in survival probability between different categories of variables; Log rank test was also employed to show the statistical significant difference between these variables, a semi-parametric chi-square test used to compare differences in survival probability between different categories of a variable. For instance, according to the Kaplan–Meier survival plot, those individuals belonging to the age group 50 and above and 18–29 had higher survival probability than those individuals belonging to the age groups 30–39 and 40–49, but this difference was not statistically significant, log-rank p-value = 0.54 (Figure 3). However, those PLHIV who were exposed to IPT had a higher survival rate as compared to their counterparts, log-rank p-value = 0.0000 (Figure 4). Furthermore, a life table (Table 3) was used to show the survival probability of PLHIV at an interval of 10 months.
 |
Table 3 Life Table Showing Overall Survival Probability with Ten Years Interval Among PLHIV Attending at ART in Illubabor and Buno Bedele Zones, South-West Ethiopia, 2022 |
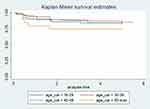 |
Figure 3 Kaplan–Meier curve showing survival probability between different categories of age among PLHIV. |
 |
Figure 4 Kaplan–Meier curve showing survival probability between categories of IPT exposure status. |
Multivariable Data Analysis
We conducted a multivariable data analysis based on the data from bivariate analysis. Those predictor variables that were associated with the dependent variable at a p-value of ≤0.2 were selected for multivariable data analysis. Variables like sex, marital status, residence, family size, substance use, occupation, educational status, IPT exposure, baseline ART adherence, baseline and recent hemoglobin, baseline and recent “CD4+” count, baseline and recent BMI, and baseline WHO HIV clinical staging were included in multivariable Cox regression model.
According to our findings, the following variables predicted the time to occurrence of TB (Table 4): residence, IPT exposure, baseline ART adherence, baseline hemoglobin level, baseline “CD4+” cell, recent hemoglobin level, recent “CD4+” cell, recent BMI, and recent WHO HIV clinical stage. One of the variables that showed association with the dependent variable was residence; those who reside in rural areas were 76% less likely to acquire TB disease than their counterparts at any instantaneous point of time. Study participants who were exposed to IPT were 50% less likely to develop TB at any given time than those individuals who were not exposed to IPT. The risk of opportunistic TB disease at any given time among those PLHIV who had fair or poor adherence to ART was 4.8 times higher than those PLHIV who had good adherence. Respondents who had hemoglobin levels of ≥10 mg/dl at baseline and at recent measurements were 87% and 85% less likely to develop TB than their counterparts. Again, study participants’ “CD4+” count above 350 at baseline and follow-up visits was protective against TB occurrence. Furthermore, those study participants who had a “CD4+” count between 200 and 350 at recent visits were 91% less likely to develop TB at any given time than those who had a “CD4+” count of less than 200. Study participants’ body mass index is the other predictor variable that was associated with the dependent variable; PLHIV who had a BMI of 18.5 and above were 70% less likely to acquire TB at any instantaneous time than those PLHIV who had a BMI of less than 16. In addition, WHO’s HIV clinical stage was the other predictor variable associated with the incidence of TB.
 |
Table 4 Multivariable Cox Regression Analysis of Predictor Variables for Time to TB Among PLHIV Attending at ART Clinics in Illubabor and Buno Bedelle Zones, South-West Ethiopia, 2022 |
Discussion
In this retrospective cohort study, we tried to identify predictors for the incidence of pulmonary TB in two zones found in Oromia Region, south-west Ethiopia. We retrospectively followed 421 PLHIV for 5 years. In this study, the median (IQR) age of study participants was 32 (28–40) year. The overall incidence rate of TB was 3.7 per 100 PY (95% CI: 2.92–4.74) with a total of 1746.1 person time. Sixty-two percent of PLHIV were exposed to IPT; the incidence rate of TB among IPT exposed was lower than those never exposed to IPT (1.7 per 100 PY versus 7.1 per 100 PY). The mean survival time for these PLHIV was 58.5 (95% CI: 56.7–60.3). According to the Log rank test for comparison among categories of predictor variables, those PLHIV who were exposed to IPT had higher survival rates as compared to those PLHIV who were not exposed to IPT. In multivariable Cox regression, variables including patients’ residence, patients’ ART adherence, IPT exposure, baseline hemoglobin level and “CD4+” cell count, follow-up or recent hemoglobin level and “CD4+” cell count, recent BMI, and baseline WHO HIV stage were associated with the incidence of TB.
As compared to previous studies conducted on similar study topic, the incidence rate of TB identified in this study was consistent.2,12,17 Similarities in the incidence rate between the current study and the previous could be due to the fact that some of the exposure characteristics were similar in these studies. In addition, the timing in which these studies were conducted was similar; this also brings about similar outcomes. However, the rate of TB occurrence was lower as compared to a study done by Alemu et al.14 One reason for this variation could be the sample size; there was a large sample size in the former study as compared to this study, which could increase the incidence rate. There was a difference between the previous study12 and the current one regards to the incidence rate of TB among IPT-exposed individuals; the rate of TB incidence among IPT-exposed PLHIV in the former study was lower (0.35 per 100 PY). Since the above study did not provide the uncertainty associated with the incidence rate, we cannot discuss whether or not there was real variation between these two studies.
One of the variables associated with TB is the residence of the study participant; those residing in rural areas were less likely to develop TB as compared to urban residents. Unlike this finding, a study conducted by Pham et al19 showed that being an urban resident was protective against TB. However, our finding was supported by a study conducted by Semu et al.20 In our case, in urban areas, living in a congested and slum urban environment might lead to TB disease, which characterized our study population. IPT exposure was the other predictor variable for TB disease; those PLHIV who took the 6-month IPT were less likely to develop TB than their counterparts. Studies by Kazibwe et al,21 Russom et al,22 Beshaw et al12 and Getu et al17 supported our finding that those PLHIV who did not take or discontinued had higher odds of acquiring TB disease. Therefore, taking IPT, as the main predictor variable of this study, has a significant impact in reducing the burden of TB among PLHIV.
In this study, “CD4+” cell count predicted whether or not PLHIV acquired opportunistic TB disease. “CD4+” cell is the main receptor cell for HIV antigen. Consequently, a reduced “CD4+” cell is associated with advanced HIV infection or AIDS. These studies13,14,17,20,23 also tried to uncover the effect of the quantity of “CD4+” cells in determining TB disease. Thus, PLHIV should check their “CD4+” count to prevent TB and other opportunistic diseases. Similarly, in this study, those PLHIV with WHO HIV clinical stage III or IV at baseline were more prone to opportunistic TB disease. This finding is further strengthened by studies conducted in Uganda, East Africa21 and other parts of Ethiopia.12,13,20 Moreover, a study by Getu et al17 particularly stressed that PLHIV with advanced clinical disease had an increased risk for TB. This might again be associated with decreased immunity in HIV-positive individuals, leading to multiple opportunistic comorbidities.
Human immune deficiency virus (HIV) patients’ BMI was also associated with opportunistic TB disease. This study revealed that as the BMI of HIV-positive patients decreases, the likelihood of acquiring TB disease will increase. Body mass index could also be an indirect measure of the nutritional status of these PLHIV. A study entitled “Incidence and determinants of tuberculosis infection among adult patients with HIV attending HIV care in north-east Ethiopia”24 showed similar result about BMI of HIV patients. Furthermore, studies done by Alemu et al14 and Getu et al17 came up with supporting evidence. Based on our findings, baseline adherence to ART was protective against TB; the risk of developing TB was 5 times higher among those who had fair or poor adherence to ART. This finding was in line with the study conducted by Getu et al17 and the study conducted by Mebratu et al.25 Finally, the hemoglobin level of respondents predicted the time to TB disease. This evidence is supported by the study done by Ahmed et al.24 As decreased hemoglobin is an indicator of anemia, anemia itself is a manifestation of immunity deterioration in an individual with HIV. So, in this study, decreased hemoglobin levels might be caused by severe immunity suppression among HIV+ individuals with TB.
As this study is not immune from gaps, there was a limitation in conducting this study. Since this study used information from available sources, some variables were missed. For instance, in this study, the patient’s viral load was not included due to incomplete registration on the individual patient’s medical record sheet. So, to address this gap during our data collection process, we used patients’ “CD4+” count as a proximity measurement of their immunity status.
Conclusion
This retrospective cohort study was conducted to assess the incidence of TB among people living with HIV. Based on our findings, IPT exposure has a significant effect in preventing opportunistic TB disease among PLHIV. Patient’s residence, adherence to ART, the level of hemoglobin and “CD4+” count, patients’ BMI and WHO HIV clinical stage predicted the incidence of TB. Therefore, healthcare professionals working in ART clinics should routinely assess HIV-positive individuals for changes in clinical indicators and environmental exposures like living conditions, which will help HIV-positive individuals in reducing their risk for TB. Likewise, patients attending ART clinics should always seek information regarding their immunity status, and they should receive counseling regarding opportunistic diseases, including TB, on a regular basis.
Acknowledgments
We would like to acknowledge the research and postgraduate office of Mettu University for understanding and allowing us to conduct this study. We would also like to acknowledge the management bodies of public health facilities in Illubabor and Buno Bedelle zones for their cooperation during the conduct of this study.
Funding
We received no funds for this work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. World Health Organization. Global tuberculosis report 2016; 2016
2. Kebede F, Kebede B, Kebede T, Agmasu M, Wang M. Effect of isoniazid preventive therapy on the incidence of tuberculosis among seropositive children attending HIV/AIDS care in two general hospitals, Northwest Ethiopia, 2021. J Trop Med. 2021;2021:9996953. doi:10.1155/2021/9996953
3. Mardani M. TB/HIV co-infection. Iran J Clin Infect Dis. 2007;2(4):167–168.
4. Ethiopian Federal Ministry of Health. Guidelines for Clinical and Programmatic Management of TB, TB / HIV, DR-TB and Leprosy in Ethiopia. TBL Guide; August, 2021:1–249.
5. World Health Organization. G Global Tuberculosis Control. Control; 2010.
6. UNAIDS. Fact Sheet_World Tuberculosis Day; 2022.
7. World Health Organization. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource Constrained Settings; 2011.
8. Godfrey-faussett P. Policy statement on preventive therapy against tuberculosis in people living with HIV; February, 1998.
9. World Health Organization. Implementing the WHO Stop TB Strategy: A Hand Book for National TB Control Programmes; 2008.
10. Zeleke ED, Ejigu DA, Assefa DG, et al. Isoniazid Preventive Therapy for Prevention of Tuberculosis Among People Living with HIV in Ethiopia: a Systematic Review of Implementation and Impacts. Int J Environ Res Public Health. 2022;20(1):621.
11. WHO. Tuberculosis Report. Vol. XLIX. Baltimore Health News; 2020:8.
12. Beshaw MA, Balcha SA, Lakew AM. Effect of Isoniazid Prophylaxis Therapy on the Prevention of Tuberculosis Incidence and Associated Factors Among HIV Infected Individuals in Northwest Ethiopia: retrospective Cohort Study. HIV AIDS. 2021;13:617–629.
13. Geremew D, Endalamaw A, Negash M, Eshetie S, Tessema B. The protective effect of isoniazid preventive therapy on tuberculosis incidence among HIV positive patients receiving ART in Ethiopian settings: a meta-analysis. BMC Infect Dis. 2019;19(1):405. doi:10.1186/s12879-019-4031-2
14. Alemu A, Yesuf A, Zerihun B, Getu M, Worku T, Bitew ZW. Incidence and determinants of tuberculosis among HIV-positive individuals in Addis Ababa, Ethiopia: a retrospective cohort study. Int J Infect Dis. 2020;95:59–66.
15. Havlir D, Lockman S, Ayles H, Larmarange J, Chamie G. What do the Universal Test and Treat trials tell us about the path to HIV epidemic control ?. J Int AIDS Soc. 2020;23(2):e25455.
16. Support WHO, Country FOR. PROGRESS REPORT 2016; 2016.
17. Getu A, Wolde HF, Animut Y, Kibret AA, Quinn F. Incidence and predictors of Tuberculosis among patients enrolled in Anti-Retroviral Therapy after universal test and treat program, Addis Ababa, Ethiopia. A retrospective follow -up study. PLoS One. 2022;17(8):1–13. doi:10.1371/journal.pone.0272358
18. FMOH Ethiopia. National Consolidated Guidelines for Comprehensive HIV Prevention, Care and; 2018;1–238.
19. Pham BN, Abori N, Silas VD, et al. Tuberculosis and HIV/AIDS-attributed mortalities and associated sociodemographic factors in Papua New Guinea: evidence from the comprehensive health and epidemiological surveillance system. BMJ Open. 2022;12(6):e058962. doi:10.1136/bmjopen-2021-058962
20. Semu M, Fenta TG, Medhin G, Assefa D. Effectiveness of isoniazid preventative therapy in reducing incidence of active tuberculosis among people living with HIV/AIDS in public health facilities of Addis Ababa, Ethiopia: a historical cohort study. BMC Infect Dis. 2017;17(1):1–8. doi:10.1186/s12879-016-2109-7
21. Kazibwe A, Oryokot B, Mugenyi L, et al. Incidence of tuberculosis among PLHIV on antiretroviral therapy who initiated isoniazid preventive therapy: a multi-center retrospective cohort study. PLoS One. 2022;17(5):1–13. doi:10.1371/journal.pone.0266285
22. Russom M, Woldu HG, Berhane A, Jeannetot DYB, Stricker BH, Verhamme K. Effectiveness of a 6-month isoniazid on prevention of incident tuberculosis among people living with HIV in Eritrea: a retrospective cohort study. Infect Dis Ther. 2022;11(1):559–579. doi:10.1007/s40121-022-00589-w
23. Darraj MA, Abdulhaq AA, Yassin A, et al. Tuberculosis among people living with HIV/AIDS in Jazan Region, Southwestern Saudi Arabia. J Infect Public Health. 2021;14(11):1571–1577. doi:10.1016/j.jiph.2021.09.009
24. Ahmed A, Mekonnen D, Shiferaw AM, Belayneh F, Yenit MK. Incidence and determinants of tuberculosis infection among adult patients with HIV attending HIV care in north-east Ethiopia: a retrospective cohort study. BMJ Open. 2018;8(2):e016961. doi:10.1136/bmjopen-2017-016961
25. Mebratu W, Wedajo S, Mohammed S, Endawkie A, Damtew Y. Prevalence and associated factors of tuberculosis among isoniazid users and non-users of HIV patients in Dessie, Ethiopia. Sci Rep. 2022;12(1):1–10. doi:10.1038/s41598-022-16437-3.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
