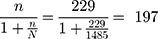Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 12
Isolation and Antibiotic Susceptibility Pattern of Bacterial Uropathogens and Associated Factors Among Adult People Living with HIV/AIDS Attending the HIV Center at Wolaita Sodo University Teaching Referral Hospital, South Ethiopia
Authors Haile Hantalo A , Haile Taassaw K , Solomon Bisetegen F , Woldeamanuel Mulate Y
Received 9 January 2020
Accepted for publication 29 October 2020
Published 27 November 2020 Volume 2020:12 Pages 799—808
DOI https://doi.org/10.2147/HIV.S244619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Admasu Haile Hantalo,1 Kassahun Haile Taassaw,1 Fithamlak Solomon Bisetegen,2 Yimtubezenash Woldeamanuel Mulate3
1Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Wolkite University, Wolkite, Ethiopia; 2Department of Medical Laboratory Sciences, College of Health Sciences, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 3Department of Microbiology, Immunology, Parasitology, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Admasu Haile Hantalo
Department of Medical Laboratory Science, College of Medicine and Health Sciences, Wolkite University, Wolkite, Ethiopia
Tel +251 926 427 907
Email [email protected]
Background: Urinary tract infection remains one of the major public health problems in developing countries, including Ethiopia. Its prevalence is fuelled by human immunodeficiency virus infection which represents a considerable health problem amongst these populations. This study aimed to assess the prevalence, antimicrobial susceptibility pattern and associated factors of bacterial urinary tract infections among adult PLHIV.
Methods: Cross-sectional study was conducted from May to December, 2018 among adult people living with HIV/AIDS in Wolaita Sodo University Teaching and Referral Hospital. The socio-demographic data and clinical data were collected using structured questionnaire. Mid-stream urine sample was collected for bacterial isolation and identification. Antimicrobial sensitivity testing was done by Kirby-Bauer disk diffusion technique. Logistic regression was conducted to check the association between UTI and associated factors.
Results: The overall prevalence of urinary tract infection was 29 (14.1%). The predominant bacteria isolated was E. coli 13 (44.8%) followed by S. aureus 5 (17.2%). Gender, CD4 count, history of catheterization, history of hospitalization, and DM status were independent factors for the occurrence of urinary tract infection. E. coli species were 100% and 84.6% susceptible to ciprofloxacin and norfloxacin, respectively; whereas, there was a complete resistance to amoxicillin-clavulanic acid and ampicillin. K. pneumoniae was pan resistant to gentamicin, amikacin and ampicillin, whereas 100% sensitive to nitrofurantoin. The rate of MDR was 23 (79.3%) with the majority, 16 (69.6%), gram negative and seven (30.4%) gram positive.
Conclusion: The burden of UTI among people living with HIV was considerably high. The findings of this study will help policy makers and other stakeholders as baseline information.
Keywords: urinary tract infection, PLHIV, antibiotic susceptibility pattern, Wolaita, Ethiopia
Introduction
Urinary tract infection (UTI) is the infection of any part of the urinary tract which includes the organs that collect and store urine and release it from the body, the kidneys, ureters and bladder, urethra and accessory structures. It results when microorganisms, usually bacteria from the digestive tract, cling to the opening of the urethra and begin to multiply.1
More than 90% of UTIs are due to enteric gram negative bacteria, mainly Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa and gram-positive bacteria including Staphylococcus aureus.2 Exogenous hospital acquired UTIs can also occur following invasive procedures in the urinary tract, mainly due to urinary catheterization.3
Depending on sex, age, immune status, and the area of the urinary tract that is infected, symptoms and signs of UTI can vary. Pathological conditions that may impede urine flow like enlarged prostate, congenital urinary tract abnormalities, and inflammation have an impact on the occurrence of UTI.3,4 HIV/AIDS results in progressive failure of the immune system leading to life threatening opportunistic infections thriving.5 Globally, an estimated 36.7 million people are living with HIV/AIDS with the highest number (26.7 million) in sub-Saharan Africa from which 1.6 million people are newly infected with HIV.6 On the same report across the globe, from the total number of people infected, 26.5 million were aware of their HIV status and 19.5 million (53%) were on antiretroviral treatment (ART); while 1 million deaths resulted from AIDS were reported. HIV infection is associated with a variety of renal syndromes; patients with low CD4 counts are at risk of the neurological complications of hyperreflexia and hyporeflexia which can lead to urinary stasis and ultimately infection.7 The emergence of antibiotic resistance in the management of UTIs is a serious public health problem globally, particularly in the developing world.8 Since bacterial pathogens of UTIs are variable regionally, infection control and treatment depend on local knowledge of common causative organisms and their antibiotic resistance.9 To the best of our knowledge the prevalence, antimicrobial susceptibility pattern, and associated risk factors of bacterial uropathogens among adult PLHIV in the present study area is unknown. Thus, the current study is planned to determine the prevalence, antimicrobial susceptibility pattern of bacterial uropathogens and associated risk factors among HIV infected patients.
Methods
Study Area and Period
The study was conducted among people living with HIV at HIV center of Wolaita Sodo University teaching referral hospital (WSUTRH) which is situated at Wolaita Sodo town, Ethiopia. A total of 205 people living with HIV were included in the study from May to December, 2018.
Study Design and Study Population
Hospital based cross-sectional study was conducted among PLHIV in HIV center of WSUTRH.
Inclusion and Exclusion Criteria
HIV infected adults ≥18 years old were included and patients on antibiotics for more than two weeks prior to the time of enrolment, seriously ill patients, and pregnant mothers were excluded.
Sample Size Determination and Sampling Technique
Single population proportion formula was used to determine sample size by considering the following assumptions: P = 0.107,10 Z score for 95% confidence interval = 1.96, level of precision (d) = 4%. Sample size (no) = 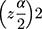 .p(1-p)/d no = ((1.96)2 x 0.107x (1–0.107))/(0.04)2=229, since the total population less than 10,000, correction formula was used.
.p(1-p)/d no = ((1.96)2 x 0.107x (1–0.107))/(0.04)2=229, since the total population less than 10,000, correction formula was used.
By considering 10% non-response rate; the overall sample size is found to be 217.
Study participants were selected using systematic random sampling technique by using Kth value, Kth value = total population/sample size = 1485/217= 7.
After the 1st participant was chosen from 7 early comers to the ART clinic by lottery method, the following were selected every 7 individuals until the required sample size was fulfilled.
Data Collection and Laboratory Processing
Socio-demographic data was collected through interview with a pre-tested structured questionnaire adapted from similar previous National and International studies. The questionnaire contained two parts: socio-demographic characteristics and associated factors. Secondary data on medical and clinical history and current use of anti-tuberculosis drugs were retrieved from patient’s charts. The socio-demographic data, history of exposure for the possible associated factors, and misuse of antibiotic drugs and other relevant information was collected using structured questionnaire. The participants’ current CD4 value and viral load was collected from their medical records.
Sample Collection, Handling, and Transport
A clean catch mid-stream urine (MSU) was collected in sterile, wide mouthed, screw capped containers. They were requested to collect 10mL of urine after instruction on self-collection of midstream urine. The collected urine specimen was divided into two sterile test tubes; one container for microscope investigation and the other for culture inoculation. Female patients were instructed to wash their hands, cleanse the area around the urethral opening with clean water, dry the area with a sterile gauze pad, and collect the middle urine with labia held apart, while male collecting a middle of the urine flow. All urine specimens were placed in a cold box and sent to the Central laboratory within 30 minutes of collection.11 Capillary blood was collected to check blood glucose level of PLHIV.
Sample Processing and Identification
Microscopic Examination of Urine
The urine samples were centrifuged at 2000g for 5 minutes. After centrifugation, the supernatant was discarded and a drop or two of the sediment placed on the grease free slide, cover slip applied and examined under the microscope using the high-power field. Reporting system for microscopic identification is at high magnification for pus cells, red blood cells, epithelial cells, casts, crystals, and yeast cells.
Culture and Identification
Well-mixed un-centrifuged urine was cultured on blood agar, Cysteine Lactose Electrolyte Deficient (CLED) medium MacConkey agar (MAC) and Mannitol salt agar. A positive urine culture was defined as colony count ≥105CFU/mL for midstream urine. Pure isolate was sub-cultured onto nutrient broth and incubated aerobically at 37 °C for 12–24 hours for biochemical testing.12 All positive cultures were further identified by their colony characteristics; Gram staining was done to identify Gram positives from Gram negatives. A single isolated bacterium was also inoculated onto nutrient agar slant and stored in a refrigerator after 24 hours of incubation for the maintenance of the isolated bacteria. Gram negative bacterial identification was done following standard procedures, by using a series of biochemical tests which include Kligler iron agar, Simmons citrate agar, lysine iron agar, urea, glucose and lactose fermentation, lysine decarboxylation, gas and H2S production, motility tests, and indole.12 Cultures from MacConkey and blood agar were sub-cultured onto nutrient agar for carrying out the appropriate biochemical tests. Gram staining, catalase, coagulase, and novobiocin were used to identify gram positive bacteria (Oxoid Ltd. Company, UK).
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was done using the Kirby-Bauer disk diffusion method on Mueller Hinton agar (Oxoid Ltd.) prepared with 4mm thickness.13
Panels of eleven antimicrobial disks including ampicillin (10µg), amoxicillin-Clavulanic acid (10µg), cefoxitin (30µg), ciprofloxacin (5µg), gentamicin (10µg), norfloxacin (10µg), cefepime (30µg), ceftriaxone (30µg), nitrofurantoin (F) 300µg, amikacin (10µg), and azithromycin (30µg) (Oxoid Ltd.) were used for susceptibility tests. Then, the bacterial isolates were classified as sensitive (S), intermediate (I), or resistance (R) by comparing against the inhibition zone diameter of interpretative standards as indicated in the CLSI guideline.13
Results
Socio-Demographic Factors
From 217 study participants who were approached, 205 consented to be involved in the study, with a response rate of 94.5%. Out of 205 PLHIV included in study, 124 (60.5%) were females. The majority of study participants, 94 (45.6%), were in age range of 28–37, while 11 (5.4%) were older than 48 years old. Most of the HIV infected patients, 159 (77.6%), were urban dwellers (Table 1).
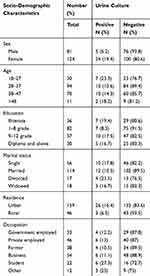 |
Table 1 UTI and socio-demographic characteristics among PLHIV (N=205) attending ART Clinic, WSUTRH, Ethiopia, 2018 |
Culture Results
Urinary tract infections were detected in 29 (14.1%). From total positive culture results, 24 (19.4%) were found in females and 5 (6.2%) in males. Age group 18–27 years had the highest number of bacterial isolates, 7 (23.3%). There was high occurrence of bacterial isolate among people with no formal education, 7 (19.4%), followed by grade 9–12, 10 (17.5%), and the least was identified among 1–8 grade level, 7 (8.5%). Prevalence of bacterial isolate was more common among students, 6 (27.3%) and least among farmers, 4 (10.5%) and regarding income and UTI, individuals with low-income level (19.7%) showed high prevalence of bacterial isolates as shown in Table 1.
As shown in Table 2, out of 205 study participants, 169 (82.4%) were currently using ART, while the remaining 36 (17.6%) have not started treatment. From a total of 205 study participants, 34 (16.6%) were symptomatic while 171 (83.4%) were asymptomatic. Eighteen people with diabetes were identified from the total of 205 participants and among those, UTI was found in 7 (38.9%). Thirty-two adults had a history of hospitalization of all the PLHIV, among which 9 (28.1%) had UTI. Five individuals who had history of catheterization were identified from the total study participants and among those UTI was discovered in 3 (60%) and the remaining 2 did not have UTI. Concerning CD4, 55 (26.8%) had CD4+ cell count < 200cell/mm3 and among those 19 (34.5%) had significant bacterial growth while the remaining 36 (65.5%) did not have significant bacteriuria.
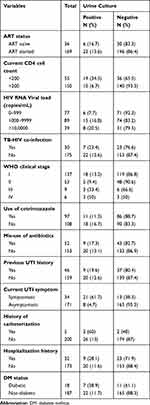 |
Table 2 UTI and clinical characteristics among PLHIV (N=205) attending ART Clinic, WSUTRH, Ethiopia, 2018 |
Most of the participants, 89 (43.4%), had viral load range of 1000–9999. The occurrence of UTI was high (20.5%) among individuals with viral load of >10, 0000. Forty-six PLHIV had a previous history of UTI from which UTI was found in 9 (19.6%) participants. Thirty participants had current TB/HIV coinfection and from those UTI was found in 7 (23.4%) participants. Regarding WHO clinical stage of HIV, the majority, 137 (66.8%), of participants were in clinical stage I and the least, 6 (2.9%), were clinical stage IV (Table 2).
Microscopic Findings
The majority of microscopic examination showed epithelial cells, 90 (43.9%), followed by pus cells, 50 (24.4%).
Etiologic Agents
A total of 29 uropathogenic bacterial species in five genera including Escherichia, Staphylococcus, Proteus, Klebsiella, and Pseudomonas were identified, of which 21 (72.4%) were Gram negative and 8 (27.6%) were gram positive. E. coli, 13 (44.8%), were the most common, followed by S. aureus, 5 (17.3%) (Figure 1).
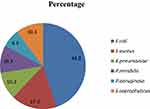 |
Figure 1 Distribution of bacterial isolates among PLHIV (n=205) attending ART clinic, WSUTRH, Ethiopia, 2018. |
Risk Factors Associated with Urinary Tract Infections
After excluding the confounding factors, sex [AOR=8.53:95% CI, 1.970–36.929] and CD4 count <200/mm3 with [AOR=0.59, 95% CI, 0.009, 0.368] revealed significant association with UTI. Current symptom of UTI and history of catheterization with AOR (95% CI) 0.35[0.010, 0116] p-value = 0.000 and AOR (95% CI) 0.048[0.003, 0.828], P-value = 0.014 respectively became the independent factor with UTI. The likelihood of developing UTI among those who had no history of hospitalization and being non-diabetic was 0.33 and 0.21 times lower respectively than those who had history of hospitalization and being diabetic (Table 3).
 |
Table 3 Factors associated with UTI among PLHIV (N=205) attending ART Clinic, WSUTRH, Ethiopia, 2018 |
Antimicrobial Susceptibility Pattern of Bacterial Uropathogens
The antimicrobial susceptibility pattern of E. coli species were 100%, 84.6%, 76.9%, 69.2% and 62.2% susceptible to ciprofloxacin, norfloxacin, amikacin, nitrofurantoin, and cefepime respectively. There was higher rate resistance to amoxicillin-clavulanic acid (100%) and ampicillin (100%) K. pneumoniae was 100% sensitive to nitrofurantoin but completely resistant to gentamicin, amoxicillin-clavulanic acid, and ampicillin. All P. mirabilis isolates susceptible to norfloxacin, gentamicin, and ciprofloxacin and totally resistant to amoxicillin-clavulanic acid and ampicillin (Table 4). S. aureus showed 100%, 80%, 60%, and 60% sensitivity to nitrofurantoin, ciprofloxacin, gentamicin, and azithromycin respectively, but it showed pan resistance for ampicillin and cefoxitin. S. saprophyticus depicted total resistance for ampicillin, amoxicillin-clavulanic acid, and cefoxitin, but it became 100% sensitive for gentamicin (Table 5)
 |
Table 4 Antimicrobial susceptibility patterns of gram-negative bacterial isolates (N= 21) among PLHIV attending ART Clinic, WSUTRH, Ethiopia, 2018 |
 |
Table 5 Antimicrobial susceptibility patterns of gram-positive bacteria isolates (N=8) among PLHIV Attending ART Clinic, WSUTRH, Ethiopia, 2018 |
Multiple Drug Resistance Patterns of Uropathogenic Bacterial Isolates
As revealed in the Figure 2, out of 29 uropathogenic isolates, 23 (79.3%) isolates were MDR of which gram negative accounts for 16 (69.6%) and gram positive 7 (30.4%). Among gram negatives all K. pneumoniae isolates and two P. mirabilis isolates were resistant to six classes of antibiotics. Regarding gram positives, single isolates of S. saprophyticus and S. aureus were resistant to six and five class of antibiotics respectively.
 |
Figure 2 Multidrug resistance patterns of uropathogenic isolates among PLHIV attending ART clinic, WSUTRH, Ethiopia, 2018. |
Discussion
The overall prevalence of UTI in the current study was 14.1%. This finding is in agreement with previous studies elsewhere.10,14–17 On the contrary, findings disparately higher than this study were also noted including 93.8%18 in Nigeria, and 77.5%19 and 41.7%20 in India. On the other hand, the prevalence of UTI in the current study was higher than previous studies conducted in Iran 8.06%,21 London 5.7%,22 and Bangalore 7.1%7 among HIV seropositive participants. Variation in prevalence of UTI across studies could be multifactorial in which differences in study set up, social habits of the community, socioeconomic status, standard of personal hygiene, sexual activity, history of UTI, diabetes mellitus, anatomic or functional genitourinary tract abnormalities and health education practices play a pivotal role.3,23,24
Our finding shows that E. coli 13 (44.8%) was the predominant isolate. This finding corroborated with previous findings in Addis Ababa 49%,14 Gondar 56.1%,10 Jimma 54.3%,15 India 52.04%,9 and Nigeria 47.3%.25 But this finding was higher than other studies conducted in Saudi Arabia E. coli 19.5%26 and South Africa E. coli (17.9%).27 On the other hand, there are previous findings where Klebsiella were the most prevalent isolate according to studies in the country 28%.15,28 The preponderance of E. coli could be due to having of unique structure that helps these species for attachment to uroepithelial cells allowing multiplication and tissue invasion. The overall variation of bacterial species may indicate a changing pattern in local prevalence of uropathogen depending upon age, sex, catheterization, study participants, and hospitalization.29,30
The second most common uropathogen in this study was S. aureus (17.3%). This finding is in line with commonly isolated bacteria in other studies of the general population elsewhere in the world and in Ethiopia.25,31,32
The prevalence of UTI among participants with CD4+ count <200/mm3 (34.5%) was higher than participants whose CD4+ count >200/mm3 (6.7%). This finding is in line with other studies which showed a high prevalence of UTI 26.3% in Nigeria,23 50.4% in Nigeria,24 24.6% in Gondar10 and, 36% in Addis Ababa.14 The results may imply that the more immune compromised the patient, the higher the risk of UTI and possibly more vulnerable to other opportunistic infections.33 Out of 18 confirmed diabetic mellitus patients, UTI was identified among seven (38.9%) participants. This finding was higher than a study from Gondar and Addis Ababa which reported 17.8%34 and 10.9% respectively.15
All gram negative bacteria (E. coli, K. pneumoniae, P. mirabilis, and P. aeruginosa) showed total resistance for ampicillin and amoxicillin-clavulanic acid in the present study. Similar findings to this study were also reported in Addis Ababa where both antibiotics showed complete resistance.14 Likewise, comparative findings were also documented in Nigeria36 where 88% of the E. coli isolates showed resistance for ampicillin, and 77% for P. aeruginosa. There was also a higher rate of resistance to ampicillin (90%) and amoxicillin-clavulanic acid (100%) in Nigeria.37
The high resistance to ampicillin and amoxicillin-clavulanic acid may be due to easy access and availability as well as uncontrolled and indiscriminate use of these antibiotics.
Norfloxacin and ciprofloxacin were effective antibiotics for E. coli with >80% sensitivity in this study. Likewise, similar findings were also noted in Gondar (100% and 92.3%),10 Jimma (100%, 84%),16 and Addis Ababa (100% and 90%), Ethiopia.15 This is comparable with the study done in Ghana which showed 83.3% sensitivity to the E. coli.38 In contrast to our study, the study done in Addis Ababa, E. coli showed high sensitivity (100%) to ceftriaxone, nitrofurantoin, and amikacin.14 P. mirablis depicted total sensitivity to norfloxacin, ciprofloxacin, and gentamicin in the current study, which was also reported in Addis Ababa where they became 100% sensitive to ciprofloxacin and gentamicin.39 This finding is similar with a study done in Gondar which showed 100% sensitivity to ceftriaxone, ciprofloxacin, and gentamicin.34 Regarding P. aeruginosa, all isolates showed 100% susceptibility to ceftriaxone, cefotaxime, gentamicin, nitrofurantoin, and ciprofloxacin. Ceftriaxone resistance level of 77% and 100% were also reported in Nigeria36 and south west Ethiopia.
S. aureus showed 80% and 100% sensitivity for ciprofloxacin and nitrofurantoin whereas all of the isolates were resistant to ampicillin and 80% are cefoxitin resistant. This finding was comparable to other studies conducted in Ethiopia.10,32,39 S. saprophyticus became 100% sensitive for gentamicin and completely resistant for ampicillin, amoxicillin-clavulanic acid, and cefoxitin in the current study. In agreement with our study, S. saprophyticus showed 100% sensitivity to gentamicin in Addis Ababa14 with complete resistance to ampicillin and cefoxitin. It was also in agreement with the study conducted in Jimma16 and Iran21 where all isolates were fully resistant to ampicillin. The similarity and differences between reports on resistance patterns may be due to the distribution of resistant strains across the country.
In the current study, twenty-three (79.3%) uropathogenic bacterial isolates showed multidrug resistance (MDR). This finding was in line other studies done in Addis Ababa 78.4%,14 74%.40 MDR pattern in this study was lower than previous studies conducted in Gondar, Ethiopia 91.7%34 and 95%.32 In contrast, our finding of MDR pattern was more common than the study conducted in India 70.0%. This changing MDR pattern in the country and across the country may be due to difference in antibiotic use practices, including self-medication, high frequency of antibiotic use, and indiscriminate use. MDR pattern was more common in gram negative 69.6% than gram positive bacteria 30.4%.
The strength of our study was that it evaluated urine samples for uropathogenic bacteria and highlighted the pattern of antimicrobial resistance that provides precise scientific data for appropriate treatment, prevention, and control of UTI. However, the study was done at one hospital; it may not represent UTI in all HIV infected patients. Other causative agents of UTI (anaerobic bacteria, viruses, and fungus) in HIV-positive patients were not done due to a lack of testing facilities.
Conclusion
The prevalence of UTI in this study was considerably high. Sex, CD4 count, history of catheterization, and hospitalization were risk factors significantly associated with UTI. E. coli and S. aureus were the major causative agents of UTI. Isolation of MDR bacteria shows the emerging challenge to treat UTI. The health professionals should be aware of local resistance patterns to consider updating empirical treatment for UTI. Measures including health education, continuous monitoring of bacteria, and antimicrobial surveillance are crucial among this group of individuals to mitigate infection and antimicrobial resistance. Further study is needed on large number of PLHIV from several health facilities to infer prevalence of UTI for all HIV patients.
Abbreviations
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CFU, colony forming unit; CLED, cystine lactose electrolyte deficient; CLSI, Clinical and Laboratory Standards Institute; HIV, human immunodeficiency virus; MDR, multidrug resistance; MSU, midstream urine; UTI, urinary tract infection; WHO, World Health Organization; WSUTRH, Wolaita Sodo University Teaching and Referral Hospital.
Data Sharing Statement
The data presented in this study contain confidential and private information about the patients and their locations. To share this information is ethically not allowed. However, the analyzed data without patient’s personal information and hospital and private labs can be requested from the corresponding author if required. All data generated and analyzed during this study period were included in the manuscript.
Ethical Approval and Consent to Participate
Ethical clearance was obtained from the ethics committee of the Department of Microbiology, Immunology and Parasitology, College of Health Sciences, Addis Ababa University. Official permission was obtained from study site. Written informed consent was obtained from each study participant. Urine culture and antimicrobial sensitivity test results were reported to the attending physician for subsequent treatment and follow up. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank Addis Ababa University, Department of Microbiology, Immunology and Parasitology Faculty of Medicine for sponsoring the research and Wolaita Sodo University Teaching Referral Hospital Central Laboratory for unreserved material and reagent supply that made the study possible. We extend our gratitude to physicians and laboratory staff, for their support in screening of symptomatic study participants and organizing the preconditions of sample collection and data collection. We also extend profound gratitude to the study participants without whom this research work would not have been possible.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Alka N, Priti S, Shanta SN. Bacterial pathogens in urinary tract infection and antibiotic susceptibility pattern. J Phar Bioth Sci. 2012;21:1–3.
2. Heyns CF, Smit SG, Merwe AD, Zarrabi AD. Urologic aspects of HIV & AIDS. Nat Rev Urol. 2013;10:713–722. doi:10.1038/nrurol.2013.230
3. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults. 2009 international clinical practices guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663.
4. World Health Organization. Antimicrobial Resistance. Global Report on Surveillance. Geneva; 2014.
5. Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–484. doi:10.1146/annurev.med.60.041807.123549
6. United Nations program on AIDS/World Health Organization. Global AIDS Update; 2016
7. Rashmi KS, RaviKumar KL, Bhagyashree HN. asymptomatic bacteriuria in HIV/AIDS Patients: occurrence and risk associated with low CD4 counts. J Evolution Med Dent Sci. 2013;2:3358–3366. doi:10.14260/jemds/705
8. Jordi V, Tibor P. Update on Antibacterial Resistance in Low Income Countries: factors Favoring the Emergence of Resistance. Open Inf Dis J. 2010;4:38–54.
9. Biradar SK, Doddamani PK. Prevalence and antibiogram of uropathogens in a tertiary care hospital. World J Pharmaceut Res. 2013;2:1534–1543.
10. Alemu A, Dagnew M, Alem M, Gizachew M. Uropathogenic bacterial isolates and their antimicrobial susceptibility patterns among HIV/AIDS patients attending Gondar University Specialized Hospital Gondar, Northwest Ethiopia. J Microbiol Res Rev. 2013;1(4):42–51.
11. Cheesbrough M. District Laboratory Practice in Tropical Countries. Part 2. New York: Cambridge University Press; 2006.
12. Cheesbrough M. District Laboratory Practice in Tropical Countries. Part 1. New York: Cambridge University Press; 2009.
13. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
14. Fenta GM, Legesse MH, Weldearegay GM. Bacteriuria and their antibiotic susceptibility patterns among people living with HIV attending Tikur Anbessa Specialized and Zewditu Memorial Hospital ART Clinics, Addis Ababa, Ethiopia. J Bacteriol Parasitol. 2016;7:292. doi:10.4172/2155-9597.1000292
15. Yeshitela B, Gebre-Selassie S, Feleke Y. Asymptomatic bacteriuria and symptomatic urinary tract infections (UTI) in patients with diabetes mellitus in Tikur Anbessa Specialized University Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50(3):239–249.
16. Debalke S, Cheneke W, Tassew H, Awol M. Urinary tract infection among Antiretroviral Therapy Users and Nonusers in Jimma University Specialized Hospital, Jimma, Ethiopia. Int J Microbiol. 2014;2014:1–6. doi:10.1155/2014/968716
17. Olowe OA, Ojo-Johnson BB, Makanjuola OB, Olowe RA, Mabayoje VO. Detection of bacteriuria among human immunodeficiency virus seropositive individuals in Osogbo, South-west Nigeria. Eur J Microbiol Immunol (Bp). 2015;5(1):126–130. doi:10.1556/EuJMI-D-14-00036
18. Iroha I, Nwakeze E, Ejikeugwu C, et al. Frequency and antibiogram of uropathogens isolated from urine samples of HIV infected patients on antiretroviral therapy. Am J Biosci. 2013;1(3):50–53. doi:10.11648/j.ajbio.20130103.11
19. Xavier TF, Auxilia A, Kannan M. Isolation and characterization of UTI pathogens from HIV positive patients of Karur District, Tamil Nadu, India. Int J Curr Microbiol Appl Sci. 2015;4(1):558–563.
20. Banu A, Jyothi R. asymptomatic bacteriuria in HIV positive individuals in a tertiary care hospital. J HIV Hum Reprod. 2013;1(2):54–57.
21. Rahem K, Ayub S, Hesamaddin SA, Hale K. Antibiotic susceptibility of bacterial strains isolated from urinary tract infections in Karaj Iran. Jundishapur J Microbiol. 2013;6(1):86–90.
22. Evans JK, McOwan A, Hillman RJ, Forster GE. Incidence of symptomatic urinary tract infections in HIV seropositive patients and the use of co-trimoxazole as prophylaxis against Pneumocystis carznn pneumonia. Genitourin Med. 1995;71:120–122.
23. Essien UC, Iheukwumere CC, Davou GI, et al. Prevalence and predictors of asymptomatic urinary tract infection among HIV positive patients in Jos, North Central Nigeria. Int J Curr Microbiol Appl Sci. 2015;4(9):454–462.
24. Skrzat-Klapaczyńska A, Matøosz B, Bednarska A, Paciorek M, Firląg-Burkacka E, Horban A. Factors associated with urinary tract infections among HIV-1 infected patients. PLoS One. 2018;13(1):e0190564. doi:10.1371/journal.pone.0190564
25. Akinbami A, Shojobi IB, Ajibola S, et al. Prevalence of asymptomatic bacteriuria in HIV infected patients in a Tertiary Hospital in Lagos, Nigeria. World J AIDS. 2013;3:105–110. doi:10.4236/wja.2013.32014
26. Mona AA, Hanem AM, Dai Abdullah AH, Ashwaq MA. Prevalence and predisposing factors of urinary tract infections among pregnant women in Abha General Hospital. IJSBAR. 2013;11(1):18–29.
27. Iweriebor BC, Obi CL, Akinyemi O, Ramalivhana NJ, Hattori T, Okoh AI. Uropathogens isolated from HIV-infected patients from Limpopo province, South Africa. Afr J Biotechnol. 2012;11:10598–10604. doi:10.5897/AJB10.2413
28. Fatuma A, Aruna R. Prevalence of asymptomatic bacteriuria and associated risk factors among antenatal women attending a tertiary care hospital. J Med Allied Sci. 2011;1(2):74–78.
29. Sefton AM. The impact of resistance on the management of urinary tract infections. Int J Antimicrob Agents. 2000;16:489–491. doi:10.1016/S0924-8579(00)00282-X
30. Omoregie R, Eghafona NO. Urinary tract infection among asymptomatic HIV patients in Benin City, Nigeria. Br J Biomed Sci. 2009;66(4):190–193. doi:10.1080/09674845.2009.11730272
31. Frank PN, Chukwugozim UR, Okerentugba P, Okonko O. HIV-1 & −2 Co-infections with multi-drug resistant (MDR) uropathogensise Port Harcourt, Nigeria. Nat Sci. 2013;11(11).
32. Agersew A, Moges F, Shiferaw Y, et al. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. 2012;5(197).
33. Nilanjan C, Anirban M, Santanu S, et al. Current trends of opportunistic infections among HIV seropositive patients from Eastern India. Jpn J Infect Dis. 2008;61:49–53.
34. Yismaw G, Asrat D, Woldeamanuel Y, Unakal C. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia. Eur J Exp Biol. 2012;2(4):889–898.
35. Kemajou TS, Ajugwo AO, Oshoma CE, Enabulele OI. Antibiotic resistance of bacterial isolates from HIV positive patients with urinary tract Infection (UTI) in Portharcourt, Nigeria. J AIDS Clin Res. 2016;7:594. doi:10.4172/2155-6113.1000594
36. Ibadian O, Michael F, Peada OA, Facpb UG. Urinary tract infection in Adolescent/Young Adult Nigerians with acquired human immuno deficiency disease in Benin City. A peer review. J Biomed Sci. 2006;5(2):55–60.
37. Boaitey YA, Nkrumah B, Idriss A, Kofi Tay SC. Gastrointestinal and urinary tract pathogenic infections among HIV seropositive patients at the Komfo Anokye Teaching Hospital in Ghana. BMC Res Notes. 2012;5:454. doi:10.1186/1756-0500-5-454
38. Getu Y, Ali I, Lema T, Belay H, Yeshetela B. Bacteriuria and antimicrobial susceptibility pattern among HIV patients attending ALERT Center, Addis Ababa, Ethiopia. Am J Health Res. 2017;5(3):76–82. doi:10.11648/j.ajhr.20170503.14
39. Addisu A, Daniel A, Yimtubezinash W, Yirgu G, Ahmed A, Tadele M. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2008;46(3):227–235.
40. Murugesh K, Deepa S, Ravindranath C, Venkatesha D. Multi drug resistant uropathogens in HIV: are they a threat to community? Int J Sci Stud. 2014;2(3):38–42.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.