Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Islet Transplantation Imaging in vivo
Authors Zheng L, Wang Y, Yang B, Zhang B , Wu Y
Received 17 May 2020
Accepted for publication 29 July 2020
Published 23 September 2020 Volume 2020:13 Pages 3301—3311
DOI https://doi.org/10.2147/DMSO.S263253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Lei Zheng1 ,* Yinghao Wang1 ,* Bin Yang1,2 ,* Bo Zhang,1,2 Yulian Wu1,2
1Department of Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310000, People’s Republic of China; 2Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education, Cancer Institute, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bo Zhang; Yulian Wu
Department of Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310000, People’s Republic of China
Tel/ Fax +86 571 87783563
Email [email protected]; [email protected]
Abstract: Although islet transplantation plays an effective and powerful role in the treatment of diabetes, a large amount of islet grafts are lost at an early stage due to instant blood-mediated inflammatory reactions, immune rejection, and β-cell toxicity resulting from immunosuppressive agents. Timely intervention based on the viability and function of the transplanted islets at an early stage is crucial. Various islet transplantation imaging techniques are available for monitoring the conditions of post-transplanted islets. Due to the development of various imaging modalities and the continuous study of contrast agents, non-invasive islet transplantation imaging in vivo has made great progress. The tracing and functional evaluation of transplanted islets in vivo have thus become possible. However, most studies on contrast agent and imaging modalities are limited to animal experiments, and long-term toxicity and stability need further evaluation. Accordingly, the clinical application of the current achievements still requires a large amount of effort. In this review, we discuss the contrast agents for MRI, SPECT/PET, BLI/FI, US, MPI, PAI, and multimodal imaging. We further summarize the advantages and limitations of various molecular imaging methods.
Keywords: islet transplantation, imaging modality, non-invasive imaging, multimodal imaging, contrast agent
Introduction
Diabetes mellitus is a chronic disorder of blood glucose caused by a deficient insulin secretory response and insulin resistance in peripheral tissues or the progressive destruction of islet β-cells.1,2 β-cell replacement therapy including pancreas and islet transplantation offers the opportunity for ideal glucose control and minimal risks of hypoglycemic episodes. Furthermore, patients who have successfully undergone β-cell replacement therapy can be free from daily insulin injections. Because of less invasiveness and continuous success, islet transplantation is becoming increasingly available in clinical practice and can help achieve insulin independence by restoring normal β-cell function. However, graft rejection and deterioration of functional islet mass make the recipients return to an insulin dependence status, eventually leading to treatment failure. Thus, it is essential to conduct further timely interventions according to the status of the post-transplanted islets. Monitoring the viability and function of transplanted islets is vital for diabetes treatment.
Accurate assessment of β-cell mass (BCM) is considered necessary not only for understanding the pathogenesis and prognosis of diabetes3 but also for monitoring the status of islet grafts during the entire transplantation period. Molecular imaging can be used to evaluate the function of transplanted islets. Effective, non-invasive and vividly visualized molecular imaging methods and novel contrast agent synthesis strategies have been developed for the following reasons: (1) for studying the pathogenesis of diabetes and further optimizing treatment by monitoring the morphology and function of BCM4 and (2) for improving islet transplantation procedures and detecting postoperative complications by monitoring the survival rate of transplanted islet cells. Here we report the recent progresses and challenges in the noninvasive imaging methods in the islet transplantation field.
Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) is an ideal non-invasive imaging method for transplanted islets given its high resolution, deep tissue penetration, tomographic capability, no ionizing radiation, and repeatability.
Superparamagnetic Iron Oxide (SPIO)
To enhance the local contrast, contrast agents are often used in MRI imaging. Superparamagnetic iron oxide (SPIO) has become a widely used MRI contrast agent because of its low toxicity and high sensitivity.5,6 SPIO labeling has been confirmed to have no effect on islet viability and function.7 After labeling and intrahepatic transplantation, the SPIO-labeled pancreatic islets can be easily detected as hypointense regions in the liver on T2-weighted magnetic resonance images.8 Toso et al proved for the first time that it is safe and feasible to use SPIO-labeled islets for monitoring via MRI in clinical practice.9 The SPIO-labeled islets were transplanted into the liver of four patients with type 1 diabetes and these could be detected as hypointensive spots in three patients via MRI. Saudek et al also detected hypointensive spots in eight type 1 diabetes patients by labeling the transplanted islets with Resovist (a carboxydextran-coated SPIO agent) at 24 weeks after transplantation.10 Only a 50% signal was detected at 1 week after transplantation in this trial.
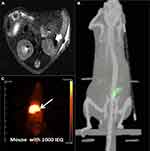 |
Figure 1 Islets transplantation imaging of MRI, SPECT and PET. (A) In vivo MR imaging of recipients having transplanted islets. In vivo spin echo T2-weighted axial MR images of heparin-SPIO-conjugated islets 30 days after xenotransplantation under the renal subcapsular membrane of left kidney in nude mice (300 islet equivalent/mouse). The dark area in the left kidney represents a labeled islet graft. Arrow: transplantation site. Reprinted from Biomaterials. 32(35). Jung MJ, Lee SS, Hwang YH, et al.MRI of transplanted surface-labeled pancreatic islets with heparinized superparamagnetic iron oxide nanoparticles. 9391–9400, Copyright (2011), with permission from Elsevier.21 (B) Transplanted islets under the left kidney capsule of CD1 mice labeled by 111In-tropolone was imaged by three-dimensional reconstructions SPECT/CT. This research was originally published in J Nucl Med. Tai JH, Nguyen B, Wells RG, et al. Imaging of gene expression in live pancreatic islet cell lines using dual-isotope SPECT. J Nucl Med. 2007;49(1):94–102. ©SNMMI. http://jnm.snmjournals.org/content/49/1/94.short.39 (C) PET images were obtained after the [68Ga]DO3A-VS-Cys40-Exendin-4 intravenous injections via the tail in NOD/SCID mice with human-transplanted islets in the liver. The liver with transplanted islets demonstrate prominent tracer uptake (arrow). Reproduced from Junfeng L, Rawson J, et al. Evaluation of [68Ga]DO3A-VSCys40- exendin-4 as a PET probe for imaging human transplanted islets in the liver. Sci Rep. 2019;9:5705. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.56 |
However, the clinical application of SPIO is limited for the following reasons. First, the large amount of iron content in the liver. Second, SPIO is highly sensitive but has low imaging specificity due to hypointense signal contents in the body; thus, it is difficult to quantify.11 Third, SPIO is biodegradable and is easily affected by the surrounding internal environments.12 The Küpffer cells in the liver decompose SPIO quickly, affecting the signal observation of the labeled islets. Fourth, many factors may cause loss of SPIO signal in transplanted islet cells, eg, immune rejection, ischemia and inflammation in the liver after islets transplantation.6,13,14 SPIO-labeled islets are more suitable for real-time imaging of transplanted islets.15
Although Resovist is approved for clinical use as a liver-imaging agent16 and it has been proved that porcine islets can safely and efficiently be labeled with Resovist to monitor kidney capsules of diabetic mice (Figure 1A),17 it has not been applied further because of its instability in clinical trials.12 To solve this problem, SPIO has been further modified, with the conjugation of lipofectamine, poly-L-lysine, polyethyleneimine,18 protamine sulfate,19 and dendron guanidine20 to improve its cell-penetrating ability. Jung et al labeled transplanted islets under the left kidney capsule of nude mice with stable heparin-SPIO to monitor transplanted islet mass by MRI in vivo for 30 days, while treating type 1 diabetes.21 Yang et al reported on a novel β–cell lymphoma (Bcl)-2-functionalized polyethylene glycol (PEG)-ultrasmall superparamagnetic iron oxide (USPIO) as a molecular imaging agent, which is better internalized by islets, for labeling β-cells and visualized the rodent islet cells transplanted under the kidney capsule of mice by MRI for 21 days.22
Fluorine-19 (19F)
Transplanted islets can be quantified using fluorine-19 (19F) under MRI due to lack of endogenous fluoride in the body13 and the high specificity of 19F. It has been reported that 19F has no effect on islet cell viability and function.23 Barnett et al found that transplanted human cadaveric islets labeled with rhodamine-perfluorooctylbromide (PFOB) nanoparticles can be viewed by 19F MRI under the kidney capsules of mice and rabbits.24 Recently, Liang et al reported that 19F MRI is suitable for the high-resolution localization of transplanted cells and pancreatic islets (PIs).25 As a specific contrast agent for glucose transporter-2 (GLUT-2),19F mannoheptulose (MH) contributes to the imaging and tracking of GLUT-2-expressing cells by MRI.26 However, the relatively low clearance of compounds and the relatively low sensitivity of 19F MRI limit its applications.27 Gálisová et al have successfully labeled the PIs with Poly(lactic-co-glycolic acid) nanoparticles (PLGA-NPs) and tracked them by multimodal imaging methods in vivo, addressing the issue of low clearance.11,23
Gadolinium (Gd)
The signal intensity of the MR-positive contrast agent gadolinium (Gd) is stronger than that of SPIO. Biancone et al28 demonstrated that it is possible to image islets under renal capsules after intrahepatic transplantation by labeling with Gd-HP-DO3A in mice. Demine et al have validated peptide P88 targeting β-cells. They conjugated P88 with Gd-DOTA and successfully monitored the transplanted islets in vivo in mice.29 However, because of the side effects of nephrogenic systemic fibrosis, the development of Gd and its derivatives is limited. Reports on the long-term effects of Gd on islet viability and function are not available.
Zn2+ and Mn2+
Some metal ions such as Zn2+ and Mn2+ ions provide an example for the possible evaluation of BCM function because their secretion corresponds to insulin secretion stimulated by glucose.30 Early functional changes of BCM can be identified in diabetic mice by dynamically monitoring manganese ions via MRI;31 further, no long-term effects of Mn2+ on glucose tolerance have been reported. However, further studies regarding this on the appropriate dose of manganese and the toxicity to the body have not been conducted. According to relevant reports, long-term exposure to large doses of manganese can cause extrapyramidal dysfunction and systemic toxicity.32
Theranostic Imaging
Theranostic imaging combines diagnostic imaging with therapy. The term “theranostic” was first coined by Funkhouser et al in 2002.33 Wang et al designed two MR probes by conjugating the therapeutic siRNA (MN-siCaspase-3 and MN-siB2M) with dextran-coated SPIOs. The islets were incubated with the probe before transplantation. The results showed that the transplanted islets under the kidney capsule showed better survival by reducing the expression of caspase-3 in the MN-siCaspase-3 group,34 whereas a significantly delayed onset of hyperglycemia caused by T cell challenge was observed in the MN-siB2M group.35 Barnett et al labeled alginate capsules with perfluorocarbon emulsions for islet imaging and immunoprotection in STZ-induced diabetic mice, and the perfluorocarbons did not affect the permeability or functioning of the islet cells.36 Recently, a nanodrug comprising magnetic nanoparticles (MN, magnetic resonance imaging moiety) conjugated with miR-216a, which targets phosphatase and tensin homolog (PTEN), was synthesized. These nanoparticles could be imagined via MRI and promoted the proliferation of β-cells by downregulating PTEN expression in a type 1 diabetes animal model.37 Although nanoparticles can travel through biological barriers, the tendency of aggregation limits their synthesis.38
Single-Photon Emission Computed Tomography (SPECT)
Single-photon emission computed tomography (SPECT) has developed rapidly in recent years due to its high resolution, depth penetration, and functional evaluation. However, its disadvantages are also obvious: radiation and low spatial and anatomical resolution.
Herpes simplex virus 1-thymidine kinase (HSV-1tk)-green fluorescent protein (GFP) has successfully been transfected into transplanted β-cells under the kidney capsule and is visualized by SPECT in vivo in animal models (Figure 1B),39 indicating that it is feasible for detecting the gene expression and location of transplanted islets in vivo by SPECT. With regard to other virus vectors, Baculovirus vector was considered to be a powerful vector for studying islet gene delivery in rats.40 Baculovirus vectors can be used to deliver NIS (sodium iodide cotransporter) genes in a non-invasive manner to monitor transplanted islets in vivo by expressing target genes under fluorescent imaging and 125I Nano SPECT/CT imaging. This technology is based on molecular imaging and enables the monitoring of islet survival and distribution after islet transplantation in vivo.
111In-exendin-3 has a higher correlation with β-cell volume than with β-cell area, and it is more reliable for evaluating BCM.41 Eter et al42 showed that islet transplantation has a linear relationship with actual BCM, in terms of 111In-exendin absorption and β-cell volume, in muscle models. However, they did not evaluate the effect of islet cell viability and function with regard to the labeling marker 111In-exendin in their report. Another study43 reported the development of a camelid antibody (nanobody “4hD29”) targeting dipeptidyl peptidase 6 (DPP6) protein of β-cells and having no toxicity toward islets. Further, 4hD29 was labeled with 99mTc to be imaged in mice by SPECT. The abovementioned study also evaluated the correlation between the number of transplanted islets and SPECT signals through 99mTc-labelled 4hD29 markers. This information is useful for the quantitative assessment of transplanted islet function in the future. Radiotracers not only affect the vitality and function of pancreatic islet cells but also cause radiation damage to patients. Thus, non-toxic tracking agents are urgently needed.44
Positron Emission Tomography (PET)
Another nuclear imaging modality, positron emission tomography (PET), has the advantages of high resolution and depth penetration and the disadvantages of radiation and low spatial and anatomical resolution. Compared with SPECT, PET is more sensitive.
A study reported that [18F]-fluoro-2-deoxy-D-glucose (FDG) offers high sensitivity and specificity. The researchers found that the FDG-labeled transplanted islet cells did not accumulate in other organs in pigs,45 suggesting the clinical application of their procedure. FDG-labeled islets were successfully applied to clinical trials without adverse reactions in 2009.46 However, the detection time of [18F]-FDG was limited to 6 h under PET,47 and Islet radioactivity was found to be reduced by nearly 50% by researchers within minutes after transplantation.46–49 Although some reporter genes, such as herpes simplex virus 1 thymidine kinase (HSV-1tk)49 or HSV1-sr39Tk,50 exhibit longer observation times than radiotracers, their further application in humans needs to be explored.
Vesicular monoamine transporter 2 (VMAT2) can be used as targets for transplanted islet identification in PET. [11C] Dihydrotetrabenazine (DTBZ) has been reported to identify the transplanted islets in the muscles of mice by targeting VMAT2.51 [18F] FE-DTBZ-d4 is the primary targeting agent for the imaging of β-cell clusters in clinical studies. Singhal et al52 compared the effects of two PET imaging ligands {(+)-[11C] dihydrotetrabenazine ([11C] DTBZ) and the fluoropropyl analog ([18F] FP-(+)-DTBZ)} on BCM imaging by injecting them into rat models of diabetes and β-cell compensation. [18F] FP-(+)-DTBZ PET imaging evaluated insulin-positive BCM in a non-invasive manner and showed great value in assessing the maintenance or restoration of BCM in mice. In another study,53 18F-FP-(+)-DTBZ was found to significantly improve the dynamic range of pancreatic binding parameters related to β-cell function compared with [11C] DTBZ. Therefore, it can discern the loss of β-cell density in T1DM patients, without depending on difference in pancreatic volume. However, most of these tracers have similar problems such as poor targeting and low expression levels of target molecules.54
The glucagon-like peptide (GLP)-1 receptor agonist exendin-4 has been studied by researchers as an effective probe for PET imaging of islets. To date, several exendin-4 analogs labeled with radioactive metal nuclides, such as 111In, 68Ga, 64Cu, and 99mTc,55 have been evaluated in rodents and non-human primates. Recently, human islets labeled with [68Ga]DO3A-VS-Cys40-Exendin-4 were transplanted into the livers of mice and imaged after 8 weeks via PET. [68Ga]DO3A-VS-Cys40-Exendin-4 was shown to produce significant contrast, which is helpful for further quantitative evaluation of the function of the transplanted islets (Figure 1C).56 Although these tracers have great potential for imaging islets, their applications are limited in clinical practice because islet exposure to high radiation may affect the viability and function of islets. Besides, Abass Alavi reported that PET is not suitable for the quantification of β-cells in native pancreas due to the anatomy of islets, but transplanted islets as a cluster can be imaged.57
Fluorescence Imaging (FI)
Fluorescence imaging (FI) has the advantages of high sensitivity, quantitative evaluation, and no radiation. However, its application is limited by the relatively short period of signal cancellation and poor penetration.
Fluorescent proteins, such as GFP and cy-5.5, have been used for tracing islets. With the help of the islets of transgenic mice expressing GFP, Hara et al used reflected light confocal imaging to observe the histological and pathological changes of transplanted islets in the portal and surrounding liver tissue after transplantation for 24 h.58 In addition, they found that pancreatic islets were unevenly distributed but arrayed along the large blood vessels by imaging GFP- and red fluorescent protein (RFP)-transgenic mice (Figure 2A).59 Furthermore, Medarova et al found that β-cell apoptosis could be visualized using a near-infrared probe (annexin V Cy5.5) in diabetic mice in vitro and ex vivo.60
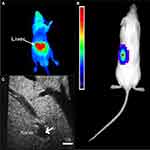 |
Figure 2 Islets transplantation imaging of FI, BLI and US. (A) Transplanted rat islets were detected by PiF fluorescence imaging. Reprinted with permission from Kang NY, Lee JY, Lee SH, et al. Multimodal imaging probe development for pancreatic β cells: from fluorescence to PET. J Am Chem Soc. 2020;142(7):3430–3439. Copyright (2020) American Chemical Society.79 (B) 500 human islets were transduced with Adeno-CMV-Luc and implanted under the left kidney capsule of NOD-SCID mice. A representative CCD image 3 days postimplantation is shown. Reprinted from Mol Ther. 9(3). Lu Y, Dang H, Middleton B, et al. Bioluminescent monitoring of islet graft survival after transplantation. 428–435, Copyright 2004, with permission from Elsevier.65 (C) Intraoperative ultrasound findings of the portal vein. The transplanted islets appeared as hyperechoic clusters in the portal vein (arrows). Reproduced from Sakata N, Goto M, Gumpei Y, et al. Intraoperative ultrasound examination is useful for monitoring transplanted islets: a case report. Islets. 2012;4(5):339–342, reprinted by permission of the publisher (Taylor & Francis Ltd, hhtp://www.tandfonline.com).70 |
Targeted or monoclonal antibodies are used to enhance the islets-to-background ratio in FI. Yohimbine (Yhb)-labeled GLP-1 exhibits advantages such as high specificity for islet cells and rapid blood clearance in vivo, which greatly increase the islets-to-background ratio, making it a suitable Islet targeting agent.61 Recently, Komatsu et al found that surfactants can strengthen Newport Green fluorescence effectively and selectively in live islets without increasing islet toxicity by fluorescence intensity analysis.62
Bioluminescence Imaging (BLI)
Compared with FI, bioluminescence imaging (BLI) allows long-term observation, and also has advantages of quantitative evaluation and high sensitivity, but it is susceptible to the internal environment and has a weak signal.
The BLI technique enables a more sensitive visual monitoring of dynamic changes in islet function for a relatively long period.63,64 Many researchers have verified that the number of transplanted islets is linearly associated with the signal magnitude (Figure 2B).65 Furthermore, Chen et al66 reported on the relationship between islet functional changes and metabolic abnormalities in a mouse transplant model using a BLI system.
A combination of BLI and specific transgenic animal models is highly useful to identify and explore the mechanism of the transplanted islets. Recently, Sekiguchi et al67 showed that BLI of MIP-Luc-VU mice expressing a β-cell-specific reporter allows the detection of changes in BCM. Islet β-cells can be imaged in INS-1-luc BAC transgenic mice under normal and pathological conditions in a noninvasive way by BLI.68
Ultrasonography (US)
Ultrasound imaging has advantages such as ease of procedure, no radiation, and repeatability. However, it also disadvantages including operator dependence, low sensitivity, and low signal. Sakata et al69 used high-frequency ultrasound (HF-US) to visualize transplanted islets and further evaluated ultrasound results and metabolic parameters. They also reported that intraoperative US can be used to image individual islets in the portal vein and can be clinically applied for the functional evaluation of transplanted islets (Figure 2C).70 Imaging of transplanted islets no longer depends on HF-US, and ordinary abdominal ultrasound is also capable for visualization of islets.71 Recently, intraoperative ultrasound has been successfully used for monitoring real-time islet infusion in total pancreatectomy with islet autotransplantation (TPIAT).72 However, HF-US can only visualize aggregated islet clusters and cannot reflect single islets.
Magnetic Particle Imaging (MPI) and Photoacoustic Imaging (PAI)
Magnetic particle imaging (MPI) is a relatively new tomographic imaging technique with no depth attenuation, a high spatiotemporal resolution, and zero background tissue signal that quantitatively images magnetic nanoparticles.73 Wang et al labeled islets with SPIOs and transplanted them in the liver or under the kidney capsule of NOD/SCID mice.74 The signal can be quantitatively detected by MPI two weeks after transplantation. The lack of anatomical background is still the main challenge for MPI. Photoacoustic imaging (PAI) is an emerging biomedical imaging modality with high resolution, diverse endogenous and exogenous contrast, and no ionizing radiation.75 Reproducibility and standardization of photoacoustic images are major obstacles in its clinical implementation. Shi et al reported that PI can be used to track angiogenesis at a subcutaneous islet transplant site in a mouse model.76 PI employed in this study may be used for tracking angiogenesis of transplanted islets.
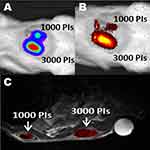 |
Figure 3 Trimodal imaging of transplanted pancreatic islets in scaffolds. Representative (A) bioluminescence, (B) fluorescence, and (C) axial F-19/H-1 MR images of 3000 and 1000 pancreatic islets transplanted into scaffolds on days 4. Reproduced from Gálisová A, Herynek V, Swider E, et al. A trimodal imaging platform for tracking viable transplanted pancreatic islets in vivo: F-19 MR, fluorescence, and bioluminescence imaging. Mol Imaging Biol. 2019;21(3):454Y464. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.11 |
Multimodality Imaging
A single imaging modality cannot provide all the required data.77 MRI is limited by low sensitivity, and SPECT/PET techniques have a poor resolution, and BLI and FI have weak tissue penetration.78 Multimodal imaging can combine the advantages of separate imaging modalities to overcome the limitations of single imaging methods. In recent years, multimodal imaging has shown rapid development. Barnett et al labeled human cadaveric islets with rhodamine-perfluorooctylbromide (PFOB) and rhodamine-perfluoropolyether (PFPE) nanoparticles to visualize islets under kidney capsules of mice and rabbits via MRI, US, or CT imaging.24 PFOB and PFPE did not affect the viability and glucose responsiveness of human islets. Furthermore, this study contributes to real-time image-guided cell infusion. Gálisová et al developed a PLGA-NP poly(lactic-co-glycolic acid) platform, wherein PLGA-NP poly(lactic-co-glycolic acid) encapsulated with perfluoro-15-crown-5-ether and the near-infrared fluorescent dye indocyanine green and obtained detailed information about localization, size, and viability of transplanted islets by multimodal imaging (Figure 3).11 Recently, a new pancreatic β-cell probe, PiF (pancreatic islet fluorinated probe), was developed. This probe can not only image the islets in the pancreas but also monitor the intraportal transplanted islets by simple tail vein injection without the prelabeling of islets.79 At the same time, the probe PiF reduced the preparation time for tissue staining protocols from one day to 2 h.79 Although multimodal imaging can obtain more comprehensive data from transplanted islets, multimodal imaging nanoparticles are more complex and expensive to be synthesized and have a larger molecular weight. The effect of these nanoparticles on the transplanted islet cells and the human body and their stability still need further study.
Conclusion
As the islet transplantation technology continues to show great value in type I diabetes, imaging and functional evaluation of transplanted islets are constantly improving and developing. There are many imaging modalities for tracing and quantitatively evaluating transplanted islets, but many shortcomings persist at present (Table 1).
 |
Table 1 Advantages and Disadvantages of Imaging Modalities of Islets |
In my opinion, MRI is currently an ideal non-invasive imaging modality for the visualization of transplanted islets for its high resolution, deep tissue penetration, low toxicity, and repeatability. MRI has higher sensitivity for islet detection and can image single islets with SPIO labeling. Further, compared to islet transplantation in the liver, more subtle changes of islets can be detected under the renal capsule and grafts do not migrate here.17 Microencapsulated islets have solved the problems of a shortage of donor organs and xenograft immune rejection.80 Moreover, theranostic imaging of MRI provides better islet protection via monitoring. Research on various nanoparticles has promoted the development of multimodal imaging of transplanted islets. As an essential component of multimodal imaging, MRI obtains more detailed quantitative evaluation data of transplanted islets. Thus, MRI has great potential for islet transplantation imaging in the future.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no. 81570698) and the Natural Science Foundation of Zhejiang Province of China (no. Q16H180002).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Robertson RP, Harmon J, Tran POT, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl Supplement 1):S119–S124. doi:10.2337/diabetes.53.2007.S119
2. Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in β-cell function. Nature. 2001;414(6865):788–791. doi:10.1038/414788a
3. Reiner T, Thurber G, Gaglia J, et al. Accurate measurement of pancreatic islet -cell mass using a second-generation fluorescent exendin-4 analog. Proc Natl Acad Sci U S A. 2011;108(31):12815–12820. doi:10.1073/pnas.1109859108
4. Yang L, Ji W, Xue Y, et al. Imaging beta-cell mass and function in situ and in vivo. J Mol Med (Berl). 2013;91(8):929–938. doi:10.1007/s00109-013-1056-7
5. Wang Y-XJ. Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg. 2011;1(1):35–40. doi:10.3978/j.issn.2223-4292.2011.08.03
6. Medarova Z, Vallabhajosyula P, Tena A, et al. In vivo imaging of autologous islet grafts in the liver and under the kidney capsule in non-human primates. Transplantation. 2009;87(11):1659–1666. doi:10.1097/TP.0b013e3181a5cbc0
7. Koblas T, Girman P, Berkova Z, et al. Magnetic resonance imaging of intrahepatically transplanted islets using paramagnetic beads. Transplant Proc. 2005;37(8):3493–3495. doi:10.1016/j.transproceed.2005.09.142
8. Jirak D, Kríz J, Herynek V, et al. MRI of transplanted pancreatic islets. Magn Reson Med. 2004;52(6):1228–1233. doi:10.1002/mrm.20282
9. Toso C, Vallee J-P, Morel P, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8(3):701. doi:10.1111/j.1600-6143.2007.02120.x
10. Saudek F, Jirák D, Girman P, et al. Magnetic resonance imaging of pancreatic islets transplanted into the liver in humans. Transplantation. 2010;90(12):1602–1606. doi:10.1097/TP.0b013e3181ffba5e
11. Gálisová A, Herynek V, Swider E, et al. A trimodal imaging platform for tracking viable transplanted pancreatic islets in vivo: F-19 MR, fluorescence, and bioluminescence imaging. Mol Imaging Biol. 2019;21(3):454Y464. doi:10.1007/s11307-018-1270-3
12. Wei W, Ehlerding EB, Lan X, et al. Molecular imaging of β-cells: diabetes and beyond. Adv Drug Deliv Rev. 2019;139(139):16–31. doi:10.1016/j.addr.2018.06.022
13. Liu Y, Song B, Ran X, et al. Molecular imaging of pancreatic islet transplantation. Exp Clin Endocrinol Diabetes. 2014;122(2):79–86. doi:10.1055/s-0033-1363232
14. Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4(4):259–268. doi:10.1038/nri1332
15. Ahrens ET, Bulte JWM. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol. 2013;13(10):755–763. doi:10.1038/nri3531
16. Kim HS, Choi Y, Song IC, Moon WK. Magnetic resonance imaging and biological properties of pancreatic islets labeled with iron oxide nanoparticles. NMR Biomed. 2009;22(8):852–856. doi:10.1002/nbm.1398
17. Kim HS, Kim H, Park KS, et al. Evaluation of porcine pancreatic islets transplanted in the kidney capsules of diabetic mice using a clinically approved Superparamagnetic Iron Oxide (SPIO) and a 1.5T MR scanner. Korean J Radiol. 2010;11(6):673–682. doi:10.3348/kjr.2010.11.6.673
18. Toso C, Vallee JP, Morel P, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant. 2008;8:701e6.
19. Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med. 2006;12(1):144e8. doi:10.1038/nm1316
20. Evgenov NV, Medarova Z, Pratt J, et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes. 2006;55(9):2419e28. doi:10.2337/db06-0484
21. Jung MJ, Lee SS, Hwang YH, et al. MRI of transplanted surface-labeled pancreatic islets with heparinized superparamagnetic iron oxide nanoparticles. Biomaterials. 2011;32(35):9391e9400. doi:10.1016/j.biomaterials.2011.08.070
22. Yang B, Cai H, Qin W, et al. Bcl-2-functionalized ultrasmall superparamagnetic iron oxide nanoparticles coated with amphiphilic polymer enhance the labeling efficiency of islets for detection by magnetic resonance imaging. Int J Nanomedicine. 2013;8:3977–3990. doi:10.2147/IJN.S52058
23. Herynek V, Gálisová A, Srinivas M, et al. Pre-microporation improves outcome of pancreatic islet labelling for optical and 19F MR imaging. Biol Proced Online. 2017;19(1):6. doi:10.1186/s12575-017-0055-4
24. Barnett BP, Ruiz-Cabello J, Hota P, et al. Use of perfluorocarbon nanoparticles for non-invasive multimodal cell tracking of human pancreatic islets. Contrast Media Mol Imaging. 2011;6(4):251–259. doi:10.1002/cmmi.424
25. Liang S, Louchami K, Holvoet B, et al. Tri-modal in vivo imaging of pancreatic islets transplanted subcutaneously in mice. Mol Imaging Biol. 2018;20(6):940–951.
26. Liang S, Louchami K, Kolster H, et al. In vivo and ex vivo 19-fluorine magnetic resonance imaging and spectroscopy of beta-cells and pancreatic islets using GLUT-2 specific contrast agents. Contrast Media Mol Imaging. 2016;11(6):506–513. doi:10.1002/cmmi.1712
27. Srinivas M, Böhm-Sturm P, Aswendt M, et al. In vivo 19F MRI for cell tracking. J Vis Exp. 2013;25:e50802.
28. Biancone L, Crich SG, Cantaluppi V, et al. Magnetic resonance imaging of gadolinium-labeled pancreatic islets for experimental transplantation. NMR Biomed. 2007;20(1):40–48. doi:10.1002/nbm.1088
29. Demine S, Balhuizen A, Debaille V, et al. Imaging of human insulin secreting cells with Gd-DOTA-P88, a paramagnetic contrast agent targeting the beta cell biomarker FXYD2γα. Molecules. 2018;23,:2100. doi:10.3390
30. Laurent D, Vinet L, Lamprianou S, et al. Pancreatic β-cell imaging in humans: fiction or option? Diabetes Obes Metab. 2016;18(1):6–15. doi:10.1111/dom.12544
31. Meyer A, Stolz K, Dreher W, et al. Manganese-mediated MRI signals correlate with functional β-cell mass during diabetes progression. Diabetes. 2015;64(6):2138–2147. doi:10.2337/db14-0864
32. Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose-dependent and temporal evolution of T1 contrast. Magn Reson Med. 2005;53:640–648. doi:10.1002/mrm.20368
33. Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug Chem. 2011;22:1879–1903. doi:10.1021/bc200151q
34. Wang P, Yigit MV, Medarova Z, et al. Combined small interfering RNA therapy and in vivo magnetic resonance imaging in islet transplantation. Diabetes. 2011;60:565–571. doi:10.2337/db10-1400
35. Wang P, Yigit MV, Ran C, et al. A theranostic small interfering RNA nanoprobe protects pancreatic islet grafts from adoptively transferred immune rejection. Diabetes. 2012;61(12):3247–3254. doi:10.2337/db12-0441
36. Barnett BP, Ruiz-Cabello J, Hota P, et al. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology. 2011;258(1):182–191. doi:10.1148/radiol.10092339
37. Wang P, Liu Q, Zhao H, et al. miR-216a-targeting theranostic nanoparticles promote proliferation of insulin-secreting cells in type 1 diabetes animal model. Sci Rep. 2020;10(1):5302. doi:10.1038/s41598-020-62269-4
38. Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2016;24(3):179–191. doi:10.3109/1061186X.2015.1051049
39. Tai JH, Nguyen B, Wells RG, et al. Imaging of gene expression in live pancreatic islet cell lines using dual-isotope SPECT. J Nucl Med. 2007;49(1):94–102. doi:10.2967/jnumed.107.043430
40. Liu S, Pan Y, Lv J, et al. Feasibility of baculovirus-mediated reporter gene delivery for efficient monitoring of islet transplantation in vivo. Nucl Med Biol. 2014;41(2):171–178. doi:10.1016/j.nucmedbio.2013.10.009
41. Eter WA, Parween S, Joosten L, et al. SPECT-OPT multimodal imaging enables accurate evaluation of radiotracers for β-cell mass assessments. Sci Rep. 2016;6(1):24576. doi:10.1038/srep24576
42. Eter WA, Van der Kroon I, Andralojc K, et al. Non-invasive in vivo determination of viable islet graft volume by 111in-exendin-3. Sci Rep. 2017;7(1):7232. doi:10.1038/s41598-017-07815-3
43. Demine S, Ribeiro RG, Thevenet J, et al. A nanobody-based nuclear imaging tracer targeting dipeptidyl peptidase 6 to determine the mass of human beta cell grafts in mice. Diabetologia. 2020;63(4):825–836. doi:10.1007/s00125-019-05068-5
44. Jodal A, Schibli R, Béhé M. Targets and probes for non-invasive imaging of β-cells. Eur J Nucl Med Mol Imaging. 2017;44(4):712–727. doi:10.1007/s00259-016-3592-1
45. Eich T, Eriksson O, Lundgren T. Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med. 2007;356(26):2754–2755. doi:10.1056/NEJMc070201
46. Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9(12):2816–2824. doi:10.1111/j.1600-6143.2009.02844.x
47. Toso C, Zaidi H, Morel P, et al. Positron-emission tomography imaging of early events after transplantation of islets of langerhans. Transplantation. 2005;79(3):353–355. doi:10.1097/01.TP.0000149501.50870.9D
48. Eich T, Eriksson O, Sundin A, et al. Positron emission tomography: a real-time tool to quantify early islet engraftment in a preclinical large animal model. Transplantation. 2007;84(7):893–898. doi:10.1097/01.tp.0000284730.86567.9f
49. Arifin DR, Bulte JWM. Imaging of pancreatic islet cells. Diabetes Metab Res Rev. 2011;27(8):761–766. doi:10.1002/dmrr.1248
50. Lu Y, Dang H, Middleton B, et al. Long-term monitoring of transplanted islets using positron emission tomography. Mol Ther. 2006;14(6):851–856. doi:10.1016/j.ymthe.2006.08.007
51. Simpson NR, Souza F, Witkowski P, et al. Visualizing pancreatic β-cell mass with [11C]DTBZ. Nucl Med Biol. 2006;33(7):855–864. doi:10.1016/j.nucmedbio.2006.07.002
52. Singhal T, Ding Y-S, Weinzimmer D, et al. Pancreatic beta cell mass PET imaging and quantification with [11C]DTBZ and [18F]FP-(+)-DTBZ in rodent models of diabetes. Mol Imaging Biol. 2011;13(5):973–984. doi:10.1007/s11307-010-0406-x
53. Normandin MD, Petersen KF, Ding Y-S, et al. In vivo imaging of endogenous pancreatic -cell mass in healthy and type 1 diabetic subjects using 18F-fluoropropyl-dihydrotetrabenazine and PET. J Nucl Med. 2012;53(6):908–916. doi:10.2967/jnumed.111.100545
54. Eriksson O, Alavi A. Imaging the islet graft by positron emission tomography. Eur J Nucl Med Mol Imaging. 2012;39(3):533–542. doi:10.1007/s00259-011-1928-4
55. Selvaraju RK, Velikyan I, Johansson L, et al. In vivo imaging of the glucagonlike peptide 1 receptor in the pancreas with 68Ga-labeled DO3A-exendin-4. J Nucl Med. 2013;54(8):1458–1463. doi:10.2967/jnumed.112.114066
56. Junfeng L, Rawson J, Chea J, et al. Evaluation of [68Ga]DO3A-VSCys40- exendin-4 as a PET probe for imaging human transplanted islets in the liver. Sci Rep. 2019;9:5705. doi:10.1038/s41598-019-42172-3
57. Alavi A, Werner TJ. Futility of attempts to detect and quantify beta cells by PET imaging in the pancreas: why it is time to abandon the approach. Diabetologia. 2018;61(12):2512–2515. doi:10.1007/s00125-018-4676-1
58. Hara M, Yin D, Dizon RF, et al. A mouse model for studying intrahepatic islet transplantation. Transplantation. 2004;78(4):615–618. doi:10.1097/01.TP.0000128838.54074.74
59. Hara M, Dizon RF, Glick BS, et al. Imaging pancreatic β-cells in the intact pancreas. Am J Physiol Endocrinol Metab. 2006;290(5):E1041–E1047. doi:10.1152/ajpendo.00365.2005
60. Medarova Z, Bonner-Weir S, Lipes M, et al. Imaging -cell death with a near-infrared probe. Diabetes. 2005;54(6):1780–1788. doi:10.2337/diabetes.54.6.1780
61. Steyn LV, Ananthakrishnan K, Anderson MJ, et al. A synthetic heterobivalent ligand composed of glucagon-like peptide 1 and yohimbine specifically targets β cells within the pancreas. Mol Imaging Biol. 2015;17(4):461–470. doi:10.1007/s11307-014-0817-1
62. Komatsu H, Omori K, Kandeel F, Mullen Y. Surfactants improve live cell imaging of human pancreatic islets. Pancreas. 2018;47(9):1093–1100. doi:10.1097/MPA.0000000000001139
63. Patel M, Gleason A, O’Malley S, et al. Non-invasive bioluminescence imaging of β-cell function in obese-hyperglycemic [ob/ob] mice. PLoS One. 2014;9(9):e106693. doi:10.1371/journal.pone.0106693
64. Virostko J, Radhika A, Poffenberger G, Dula AN, Moore DJ, Powers AC. Bioluminescence imaging reveals dynamics of beta cell loss in the non-obese diabetic (NOD) mouse model. PLoS One. 2013;8(3):e57784. doi:10.1371/journal.pone.0057784
65. Lu Y, Dang H, Middleton B, et al. Bioluminescent monitoring of islet graft survival after transplantation. Mol Ther. 2004;9(3):428–435. doi:10.1016/j.ymthe.2004.01.008
66. Chen X, Zhang X, Larson CS, et al. In vivo bioluminescence imaging of transplanted islets and early detection of graft rejection. Transplantation. 2006;81(10):1421–1427. doi:10.1097/01.tp.0000206109.71181.bf
67. Sekiguchi Y, Owada J, Oishi H, et al. Noninvasive monitoring of ^|^beta;-cell mass and fetal ^|^beta;-cell genesis in mice using bioluminescence imaging. Exp Anim. 2012;61(4):445–451. doi:10.1538/expanim.61.445
68. Katsumata T, Oishi H, Sekiguchi Y, et al. Bioluminescence imaging of β cells and intrahepatic insulin gene activity under normal and pathological conditions. PLoS One. 2013;8(4):e60411. doi:10.1371/journal.pone.0060411
69. Sakata N, Kodama T, Chen R, et al. Monitoring transplanted islets by high-frequency ultrasound. Islets. 2011;3(5):259–266. doi:10.4161/isl.3.5.17058
70. Sakata N, Goto M, Gumpei Y, et al. Intraoperative ultrasound examination is useful for monitoring transplanted islets: a case report. Islets. 2012;4(5):339–342. doi:10.4161/isl.22384
71. Sakata N, Yoshimatsu G, Tsuchiya H, et al. Imaging of transplanted islets by positron emission tomography, magnetic resonance imaging, and ultrasonography. Islets. 2013;5(5):179–187. doi:10.4161/isl.26980
72. Noory M, Renz JF, Rosen PL, Patel H, Schwartzman A, Gruessner RWG. Real-time, intraoperative doppler/ultrasound monitoring of islet infusion during total pancreatectomy with islet autotransplant: a first report. Transplant Proc. 2019;51(10):3428–3430. doi:10.1016/j.transproceed.2019.08.041
73. Knopp T, Gdaniec N, Möddel M. Magnetic particle imaging: from proof of principle to preclinical applications. Phys Med Biol. 2017;62(14):R124–R178. doi:10.1088/1361-6560/aa6c99
74. Wang P, Goodwill PW, Pandit P, et al. Magnetic particle imaging of islet transplantation in the liver and under the kidney capsule in mouse models. Quant Imaging Med Surg. 2018;8(2):114–122. doi:10.21037/qims.2018.02.06
75. Steinberg I, Huland DM, Vermesh O, Frostig HE, Tummers WS, Gambhir SS. Photoacoustic clinical imaging. Photoacoustics. 2019;14:77–98. doi:10.1016/j.pacs.2019.05.001
76. Shi W, Pawlick R, Bruni A, et al. Photoacoustic imaging of angiogenesis in a subcutaneous islet transplant site in a murine model. J Biomed Opt. 2016;21(6):66003. doi:10.1117/1.JBO.21.6.066003
77. Kim J, Piao Y, Hyeon T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem Soc Rev. 2009;38(2):372–390. doi:10.1039/B709883A
78. Burke BP, Cawthorne C, Archibald SJ. Multimodal nanoparticle imaging agents: design and applications. Phil Trans R Soc A. 2017;375(2107):20170261. doi:10.1098/rsta.2017.0261
79. Kang N-Y, Lee JY, Lee SH, et al. Multimodal imaging probe development for pancreatic β cells: from fluorescence to PET. J Am Chem Soc. 2020;142(7):3430–3439. doi:10.1021/jacs.9b11173
80. Mettler E, Trenkler A, Feilen PJ, et al. Magnetic separation of encapsulated islet cells labeled with superparamagnetic iron oxide nano particles. Xenotransplantation. 2013;20(4):219–226. doi:10.1111/xen.12042
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
