Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Is Physical Exercise in Hypoxia an Interesting Strategy to Prevent the Development of Type 2 Diabetes? A Narrative Review
Authors De Groote E, Deldicque L
Received 28 May 2021
Accepted for publication 10 July 2021
Published 11 August 2021 Volume 2021:14 Pages 3603—3616
DOI https://doi.org/10.2147/DMSO.S322249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Estelle De Groote, Louise Deldicque
Institute of Neuroscience, Université catholique de Louvain, Louvain-la-Neuve, Belgium
Correspondence: Louise Deldicque
Institute of Neuroscience, Université catholique de Louvain, Place Pierre de Coubertin, 1 Box L08.10.01, Louvain-la-Neuve, 1348, Belgium
Tel +32 10 47 44 43
Email [email protected]
Abstract:: Impaired metabolism is becoming one of the main causes of mortality and the identification of strategies to cure those diseases is a major public health concern. A number of therapies are being developed to treat type 2 diabetes mellitus (T2DM), but few of them focus on situations prior to diabetes. Obesity, aging and insulin resistance are all risk factors, which fortunately can be reversed to some extent. Non-drug interventions, such as exercise, are interesting strategies to prevent the onset of diabetes, but it remains to determine the optimal dose and conditions. In the search of optimizing the effects of physical exercise to prevent T2DM, hypoxic training has emerged as an interesting and original strategy. Several recent studies have chosen to look at the effects of hypoxic training in people at risk of developing T2DM. Therefore, the purpose of this narrative review is to give an overview of all original articles having tested the effects of a single exercise or exercise training in hypoxia on glucose metabolism and other health-related parameters in people at risk of developing T2DM. Taken together, the data on the effects of hypoxic training on glucose metabolism, insulin sensitivity and the health status of people at risk of T2DM are inconclusive. Some studies show that hypoxic training can improve glucose metabolism and the health status to a greater extent than normoxic training, while others do not corroborate the latter. When an additional benefit of hypoxic vs normoxic training is found, it still remains to determine which signaling pathways and molecular mechanisms are involved.
Keywords: hypoxic training, glucose metabolism, insulin resistance, obesity, prediabetes
Introduction
Type 2 diabetes mellitus (T2DM) has been rising up the past 30 years and is estimated to increase further in the coming years according to the World Health Organization (WHO). This chronic disease together with its complications is a huge public health problem and represents an important cost for the society.1 While finding effective therapeutic strategies in the treatment of diabetes appears to be essential, current worldwide attention is shifting towards the prevention of T2DM. Pre-diabetes is defined as impaired fasting glycaemia or impaired glucose tolerance and is a predictor of future diabetes risk.2 Focusing on pre-diabetic people or even targeting other risk factors prior to the development of pre-diabetes could be an interesting strategy to prevent T2DM. Inactive lifestyle, aging of the population, obesity and insulin resistance are all known risk factors for T2DM,3–5 resulting in skeletal muscle dysfunction, which in turn limits physical activity, alters mobility and decreases locomotion.6 Decreased mobility is associated with a reduction in the energy expenditure and creates a vicious circle of weight gain and reduced quality of life.7 Based on previous evidence, increasing physical activity is a commonly used measure in the prevention of T2DM.8 This approach focuses not only on weight loss but also on other associated complications such as dysregulation of glucose metabolism and cardiovascular disease.8 Different exercise modalities have been evidenced to induce health benefits. The reader is referred to recent reviews on the effects of resistance and endurance exercise, alone or in combination, in T2DM.9–12
While nobody will question the beneficial effects of classical exercise modalities on the prevention of T2DM, it remains to determine the optimal dose and conditions. In the search of optimizing the effects of physical exercise to prevent T2DM, hypoxic training has emerged as an interesting and original strategy.13 Hypoxia refers to a state of low oxygen levels in tissues below physiological levels.14 Systemic hypoxia refers to a decrease in blood oxygen pressure (PO2) generalized to the entire body.15 Hypoxia can vary in duration of exposure, from acute and intermittent to chronic exposure, as well as in intensity, from moderate (fraction of inspired oxygen, FiO2, around 15–16%) to severe (FiO2 11–12%). Whether hypoxic training is really efficient still needs to be better documented. In healthy subjects, hypoxic training may improve glucose metabolism and health status more than normoxic exercise.16–18 Similar data have been found in T2DM individuals.19–21 Hypoxic training would lead to greater weight loss, greater energy expenditure, and improved regulation of lipid metabolism through activation of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC1α).17 Moreover, an increase in the expression of glucose transporter (GLUT) 4 was observed following exposure to hypoxia,22 explaining the improved regulation of glucose metabolism.
As “prevention is better than cure”, several recent studies have chosen to look at the effects of hypoxic training in people at risk of developing T2DM. It is now time to get an overview of all original articles having tested the effects of a single exercise or exercise training in hypoxia on glucose metabolism and other health-related parameters in people at risk of developing T2DM and to possibly conclude on the efficacy of hypoxic training in that specific population.
Literature Search Strategy
The search terms (Exerc*[Title] OR Train*[Title]) AND (Hypox*[Title] OR Altitud*[Title]) AND (Glucose[Title/Abstract] OR Diab*[Title/Abstract] OR Obes*[Title/Abstract] OR Metab*[Title/Abstract]) were inserted in PubMed and 88 clinical trial articles were returned on the 15 March 2020. A first selection was made based on title relevance and language. Articles not written in English were excluded. Twenty-nine articles remained after this first selection round. After careful reading of the abstracts, only articles dealing with a single session or a whole exercise training period in hypoxia with people at risk of developing T2DM, and reporting data related to glucose metabolism, cardiovascular and/or physical performance were considered, which reduced the number to 12 articles. In line with the narrative style of this review, further literature was searched on the topic and 7 other original articles were added to the selection. The criteria for people at risk of developing T2DM were obese people (body mass index (BMI) > 30 kg⋅m−2), people with the metabolic syndrome according to the NCEP ATP III definition,23 older (>65y) or sedentary (<3h physical activity/week) people with a BMI > 25 kg⋅m−2. Finally, 19 original articles were selected. All studies are summarized in Table 1.
 |  |  |  |
Table 1 Design and Main Results from the Studies Investigating the Effects of a Single Exercise or Exercise Training in Hypoxia vs Normoxia in Subjects at Risk of Developing Diabetes |
Effects of Exercise in Hypoxia on Glucose Metabolism
Single Exercise
While a number of studies have analyzed the effect of exercise in hypoxia in patients with T2DM, fewer studies were performed in populations at risk of developing T2DM. However, this population is of particular interest since T2DM can be reversed up to a certain point.24 It is therefore crucial to develop effective strategies in this population to prevent the development of T2DM. In healthy sedentary subjects, a single exercise performed in hypoxia increased serum insulin levels during and after exercise, which was not the case when exercise was performed in normoxia.25 No exercise-induced c-peptide (a short polypeptide released into the bloodstream as a by-product of insulin formation) modifications compared to the resting measurements were seen on either test day. In overweight men, blood glucose and serum insulin concentrations did not differ between normoxic and hypoxic exercise conditions, consisting in three sessions of 30 min within a 7.5-h period, nor between rest and exercise conditions.26 In pre-diabetic adults, a negative regulation of glucose tolerance was even observed when exercise was performed under hypoxia compared to normoxia.27 In summary, these three studies show a non-positive adaptation of glucose metabolism in response to hypoxic exercise. The release of hyperglycemic hormones such as catecholamines (hormones produced by the adrenal glands in response to various stresses) has been shown to occur after exercise28 and after exposure to hypoxia resulting in a transient decrease in insulin sensitivity29,30 However, an increase in the levels of catecholamines have been shown to be associated with a diminution of the secretion of insulin as well.31 It is therefore quite difficult to attribute the negative effects of hypoxic exercise on glucose tolerance to catecholamines in the three aforementioned studies. Two previous studies in T2DM subjects conversely found that hypoxic cycling for 60 min lead to a reduction in plasma glucose levels directly at the end of exercise and to an increase in insulin sensitivity up to 48 h after exercise in hypoxia but not in normoxia.20,21 The divergent results observed in those studies could be related to the intensity of the exercise. Exercising at the same absolute intensity is perceived to be more difficult in hypoxia than in normoxia.32 Mackenzie et al noted in the limitations of their study that the improvement in insulin sensitivity observed might be due to an increase in the relative intensity of exercise as the same absolute intensity was chosen for both the normoxic and hypoxic groups.20 On the contrary, for both Morishima et al26 and De Groote et al27 equal relative intensities were chosen and no additional positive effects of exercise in hypoxia vs normoxia on glucose metabolism were seen, quite the opposite. Glucose clearance rate was observed to be higher when exercise was performed in normoxia than in hypoxia at the same relative intensity.33 This highlights the importance of the absolute over the relative workload in the regulation of glucose metabolism during exercise. However, more studies in patients suffering from a dysregulation of glucose metabolism are needed to clarify the effects of an acute exercise in hypoxia on glucose homeostasis.
Exercise Training
A larger number of studies looked at the effects of a whole hypoxic training period on glucose metabolism in healthy subjects and patients suffering from glucose metabolism dysregulation. In healthy sedentary subjects, hypoxic exercise training for 4 weeks seems to be more beneficial on glucose metabolism than 2 weeks as the AUC for serum insulin concentrations after glucose ingestion was decreased only in the 4-week, not the 2-week, group compared to before training.34 Moreover, the AUC for blood glucose concentrations after glucose ingestion decreased more after 4 weeks endurance training in hypoxic than in normoxic conditions in healthy sedentary men.35 In obese adolescents, combined endurance and strength training (3x/week during 6 weeks) under hypoxia was efficient at improving glucose tolerance and insulin response to a glucose challenge.36 In obese adults, insulin sensitivity (ISIclamp) improved after a 6-week hypoxic exercise training.37 Those results show that exercising in hypoxia can improve glucose tolerance and insulin sensitivity. While insulin sensitivity improved following hypoxia training, insulin resistance measured by the HOMA IR index decreased after 4 and 6 weeks (3×/week) training in both normoxia and hypoxia in obese adults38 and adolescents,36 respectively. In obese adolescents, exercising in hypoxia once a week during 30 weeks did not further enhance glucose metabolism than exercising in normoxia.39 Two other studies in obese and healthy sedentary people found no additional effect of hypoxia after exercise training for 4 or 8 weeks.38,40 However, training workloads were significantly lower in the hypoxic vs normoxic group, indicating that training in hypoxia led to similar improvements on glucose metabolism as training in normoxia but at a lower workload. Knowing that obese and sedentary people have a higher risk of cardiovascular problems and orthopedic injuries, it would therefore seem advantageous to obtain the same health benefits at a lower intensity. Conversely, other studies found no additional effects of hypoxia to exercise training on glucose metabolism. In obese subjects with sleep apnea, no improvement in glucose metabolism was found after 8 weeks of combined exercise training neither in the normoxic nor in the hypoxic group.41 Similar results were found in obese subjects after 8 weeks of endurance training.42 Finally, in men with the metabolic syndrome, a 6-week endurance training induced a decrease in insulin levels compared to basal levels when the sessions were performed in normoxia but not in hypoxia.43
It would appear, therefore, that the studies diverge as to the additional effect of exercise in hypoxia compared to exercise in normoxia, whether in acute or repeated exercise sessions. In addition, the number and duration of exposures to hypoxia appear to be important parameters to be taken into account. However, the optimal frequency and duration to induce benefits on glucose metabolism is unknown. Another critical parameter is the exercise intensity. The glucose clearance rate is proportional to the absolute intensity of the exercise.33 It is nowadays recommended to exercise at high and even very high intensity in patients. For example, in subjects suffering from the metabolic syndrome, an interval training session at 90% HRmax decreased blood glucose levels further compared to a continuous session at 70% HRmax.44 Up to now, the preponderance of high intensity interval over continuous training to improve glucose metabolism has not been confirmed when training was performed in hypoxia in obese individuals.36,37,45 Further research is warranted to determine the optimal exercise frequency, duration and intensity to be used in hypoxia.
Underlying Mechanisms
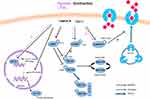
Acute exercise and insulin are known to stimulate glucose uptake via two separate signaling pathways (Figure 1). Glucose uptake in skeletal muscle is mainly possible through facilitative GLUT4, which is the predominant GLUT isoform in skeletal muscle.46 GLUT4, located into intracellular vesicles in the basal state, needs to translocate to the membrane to allow glucose uptake. A single exercise, whether of very high intensity or very long duration, activates AMP-activated protein kinase (AMPK) resulting in the stimulation of glucose transport via the Tre-2/BUB2/cdc 1 domain family 1 (TBC1D1-4)/GLUT4 pathway.47 TBC1D1 and TBC1D4 are two GTPase-activating protein (GAP) of the G protein Rab, located on GLUT4 vesicles and promoting the conversion of Rab-GTP to Rab-GDP are phosphorylated by AMPK.46 This phosphorylation results in an inhibition of their activity by favoring their binding to 14-3-3 protein. This way, TBC1D1-4 are sequestered away from their target removing their inhibition on Rabs and allowing GLUT4 translocation.48
Although additional mechanisms probably need to be elucidated, hypoxia appears to stimulate glucose uptake partially via the same signaling pathways as exercise.49,50 Glucose uptake was shown to be completely blocked in AMPKα2 deficient rodents compared to wild-type under hypoxia and partially blocked under exercise.47 Similar findings were observed in human as a higher phosphorylation of AMPK Thr172 and glucose disappearance rate were observed in a group exercised in hypoxia compared to a group exercised in normoxia.33 In addition to AMPK, exercise and hypoxia were seen to increase intracellular-free calcium,50 which is also involved in the regulation of GLUT4 translocation. Ca2+/calmodulin-dependent protein kinase II (CaMKII), the major multifunctional CaMK in skeletal muscle,51 was suggested to be implicated in contraction and hypoxia-stimulated glucose uptake as the latter was inhibited by the CaMKII blocker KN-93 in rat skeletal muscle.52 KN93 did not prevent the increase in AMPK phosphorylation, which suggested that CaMKII activation by contraction and hypoxia regulated glucose uptake through AMPK-independent mechanisms. A third, although less studied, potential candidate for exercise- and hypoxia-induced glucose uptake is p38 mitogen-activated protein kinase (p38 MAPK),53,54 as the latter has been found to be involved in the regulation of glucose transport as well.55 Although the phosphorylation of p38 MAPK was increased by hypoxia in rat cardiac myocytes,53 hypoxia did not further enhance phospho-p38 MAPK after exercise compared to normoxia in human skeletal muscle.27 Compared to AMPK and CAMKII, the available data do not indicate that p38 MAPK is an important regulator of hypoxia or exercise-induced glucose uptake in skeletal muscle. This does not seem to be the case for the expression of GLUT4 either. Exercise training is known to increase the expression of GLUT4 in human skeletal muscle56 but hypoxia did not appear to induce any further effect in sedentary older men.40
Last but not least, hypoxia induces the stabilization of hypoxia-inducible factor 1 alpha (HIF1α) and the expression of its target genes involved, amongst others, in glycolysis and glucose transport57 such as GLUT1 and phosphofructokinase (PFK). The stimulation of glucose uptake by hypoxia was found to be facilitated by an activation of GLUT1 present at the cell membrane in skeletal muscle myotubes.58 HIF1α is not only stabilized by hypoxia but by exercise as well.59 Logically, it could be assumed that exercise in hypoxia would further stabilize and activate HIF1α compared to exercise in normoxia. However, hypoxia does not necessarily modify exercise-induced stabilization of HIF1α in human skeletal muscle, neither after acute endurance27,60 or resistance61 exercise nor after exercise training.62 Skeletal muscle is a potent tissue to face a lack of oxygen. Probably the inspired fraction of oxygen (FiO2) and/or duration of exposure, i.e the hypoxic dose, used in the different studies was not severe enough to further decrease skeletal muscle oxygenation when combining exercise and hypoxia compared to exercise alone as measured previously.61 Whole-body hypoxia experiment can affect a variety of tissue types, not just skeletal muscle. Therefore, the regulation of glucose homeostasis-related molecular mechanisms in skeletal muscle following exercise in hypoxia is probably independent of the HIF1α pathway.27 Other mechanisms might be responsible for the observed effect of a single exercise or exercise training in hypoxia on glucose tolerance and insulin sensitivity.
Shift in Energy Sources During Exercise
It is known that endurance training promotes lipid oxidation,63,64 whereas hypoxic conditions tend to promote glucose utilization at the expense of lipids.65 The higher glucose oxidation during exercise in hypoxia may explain, in part, the improvement in glucose tolerance observed in some studies. Respiratory exchange ratio (RER) is often used as a satisfactory non-invasive measurement of substrate utilization.66 In obese adolescents, normoxic, but not hypoxic, training decreased the RER during cycling at 150W36 or 75% of maximal power output,39 indicating a proportionally higher use of glucose during exercise after hypoxic vs normoxic training. Similarly, a higher RER was found during each of the three 30-min trials within a 7.5-h period in hypoxia compared to normoxia.26 Contrary to the previous studies, 4 weeks endurance training (60% VO2max, 3x/week) in overweight to obese adults decreased RER during exercise only in the hypoxic group.38 A longer endurance training period (8 weeks; 3x/week) induced a lower RER during exercise, with no difference between normoxic and hypoxic conditions, in obese men,42 obese men with sleep apnea41 and in older sedentary subjects.40 In the same line, in resting conditions, fat oxidation tended to increase while carbohydrate oxidation tended to decrease after high-intensity interval training in hypoxia in obese women.67 Counterintuitively, after 6 weeks of endurance training in men with the metabolic syndrome, RER values during exercise were higher, indicating a greater fuel provision by carbohydrate oxidation, in the normoxic vs the hypoxic group.43 Altogether, no clear conclusion can be drawn about a possible shift in substrate utilization during exercise after hypoxic vs normoxic training due to the high heterogeneity in the data reported.
Effects of Exercise in Hypoxia on the Health Status
Weight Loss
Weight loss is essential to fight the development of T2DM since obesity is the main factor responsible for this disease.4 Hypoxic training might be more effective at reducing weight than normoxic training in obese individuals due to the potential effect of hypoxic exposure on energy balance.68 Several studies carried out in male and female obese subjects of various ages have shown that exercise training reduced body weight and that the amplitude of this reduction was larger after hypoxic than normoxic training.67,69–71 The benefits of hypoxic training seem to depend on the type of exercise since aerobic interval training led to an increase while sprint interval training led to a decrease in body weight and body mass index (BMI) in obese women.67 However, several studies found no additional effect of hypoxia on exercise training-induced weight loss in obese adolescents,36,39 in obese adults,38,72 in obese men with sleep apnea41 and in men with the metabolic syndrome.43 It should be noted that some studies in individuals at risk of developing T2DM did not observe a decrease in body weight neither after hypoxic nor normoxic training.34,40,42 The lack of weight loss in those studies is probably due to the low exercise intensity used while a higher intensity is known to be more beneficial for weight loss73 and probably as well due to the lack of strict dietary control.
In addition, an increase in energy expenditure, a reduction in appetite with an association of a reduction in food intake as well as an increase in the activity of various endocrine factors relevant to energy balance, namely ghrelin and leptin,69,70 are different factors that may contribute to the possible reduction in body weight observed after hypoxic training. In obese women, an increase in the basal metabolic rate was found after 12 weeks interval training but only in the group trained in hypoxia.74 Similarly, in men with the metabolic syndrome, the energy expenditure after an OGTT increased after 6 weeks endurance training in hypoxia but not in normoxia.43 In addition, while the normoxic group reduced its energy expenditure during a 60-min exercise test after vs before the training period, the hypoxic group kept its energy expenditure during this test constant. Those results indicate that hypoxic training regulates energy expenditure postprandially and during exercise in a favorable way to lose body weight. Based on the fact that hypoxic exposure75 and exercise76 separately control appetite-regulating hormones such as ghrelin and leptin, it has been proposed that hypoxic training could regulate the levels of those hormones to a further extent than normoxic training. However, the number of studies having effectively measured their levels is rather low. In obese men with sleep apnea syndrome, a combined aerobic and strength training in hypoxia, but not in normoxia, induced a reduction in energy intake, independently of any change in leptin levels.41 In sedentary healthy men, energy intake remained unchanged after a 4-week cycling training compared to baseline in both normoxia and hypoxia, despite changes in leptin, ghrelin and glucagon-like peptide-1 levels.35 Fasting serum leptin levels decreased only after normoxic training while a reduction in postprandial leptin levels was measured in both groups. Fasting and postprandial glucagon-like peptide-1 levels increased after training in the normoxic group only. No change in ghrelin levels was measured in any conditions. In summary, contrarily to the aforementioned hypothesis, hypoxic training does not seem to induce any additional effect on hormonal appetite markers compared to normoxic training.35,41 Further studies are necessary to better understand the metabolic changes induced by hypoxic exercise that could influence weight loss, certainly in obese individuals.
Body Composition
Despite no weight loss was observed in some cases, it is still possible that body composition changed in a positive way after hypoxic training. Improving body composition by decreasing body fat and increasing lean body mass is at least as important as losing weight, if not more, and could help to prevent the development of T2DM.77 In obese older men, combined aerobic and strength training in hypoxia decreased whole body fat to a larger extent compared to normoxia.70 Similar observations were also found in obese women after high-intensity interval training67 and obese young adults and adults after endurance training.38,69 Excessive fat mass, especially at the abdominal level, known as visceral fat, is associated with an increased risk of developing T2DM.78,79 In obese women, the decrease in trunk fat mass was larger after aerobic interval training or sprint interval training in hypoxia compared to normoxia.45 This decrease in trunk fat mass was quite persistent as it continued 4 weeks after cessation of training but only in the group trained in hypoxia.74 Of note, other studies found no difference in fat loss between hypoxic and normoxic training conditions either after aerobic training in obese adults72 or after combined aerobic and strength training in obese adolescents36,39 and obese men with sleep apnea syndrome.41 Several studies even did not observe fat loss at all under either condition.35,37,40,42,43 In the latter studies, the lack of effects can be explained by the lack of dietary control and/or by the type of exercise chosen, ie, aerobic training in all studies.
Most of the studies having looked at body composition only focused on fat mass despite a major role of skeletal muscle mass in glucose homeostasis postprandially, which is particularly important in the prevention of T2DM. Although chronic exposure to hypoxia for several weeks or months generally results in a loss of muscle mass in healthy people,80 anabolic effects have been reported after repeated acute exposures coupled to resistance exercise, which can potentially lead to muscle hypertrophy.81 A greater increase in muscle mass after endurance38 or combined aerobic and strength70 hypoxic than normoxic training has been observed in obese older men and women. Even 4 weeks after the last interval session, the increase in muscle mass was maintained in obese women trained in hypoxia while no change was observed in the normoxic group.67 It should be reported as well that other studies found no difference in muscle mass between hypoxic and normoxic training conditions after aerobic training in obese adults72 or after combined aerobic and strength training in obese adolescents.36,39 Several studies found no gain at all42,43,69 or even a decrease in muscle mass after a whole training period41 under either condition. In summary, to be really conclusive on the effects of hypoxic training on body composition, additional studies with a stricter control of dietary intake are needed.
Physical Performance
Obesity frequently leads to decreased autonomy due to reduced mobility.7 A reduction in maximal exercise capacity is associated with increased mortality.82 It has been pointed out that each increase of one metabolic equivalent of task (MET) in maximal exercise capacity is associated with improved survival.82 Therefore, increasing the maximal exercise capacity of individuals at risk of developing cardiometabolic disease appears to be an attractive strategy for improving their quality of life. In obese individuals, an increase in maximal oxygen uptake (VO2max) and maximal aerobic output was measured after 8 weeks hypoxic but not normoxic training.42 Despite a similar increase in VO2max after 6 weeks hypoxic training in obese adolescents, maximal aerobic output only increased in the hypoxic, not in the normoxic group.36 In the same line, global physical fitness was improved further after 12 weeks hypoxic compared to normoxic training in obese Korean men.70 The benefits of hypoxic training on physical performance were less straightforward or even absent in some other studies in the elderly and patients suffering from chronic diseases.35,38,39,72 The divergent results may be explained by the variations between the hypoxic training programs implemented in the different studies, and more specifically to the number of sessions per week. Exercising once or twice a week might be insufficient for hypoxic training to induce an additional benefit over normoxic training on exercise capacity at moderate intensity (65–70% HRmax).39,41,72 Exercising three times a week, whether for 438 or 12 weeks,70 seems more efficient to detect differences between hypoxic and normoxic training in performance-related parameters.
Lipid Profile
High total cholesterol, triglycerides, LDL and low HDL plasma levels are recognized as important cardiovascular risk factors.83 Diet or exercise training alone are efficient at reducing LDL and cholesterol levels in obese men41 or men with metabolic syndrome.43 In the context of the present review, hypoxic training has been found to decrease triglycerides levels more than normoxic training in obese subjects of all ages,36,71 though this is not a general observation.35,39 The HDL levels do not seem to vary much following exercise training, whether hypoxic or normoxic, in populations at risk of developing T2DM.34,39,43 In summary, in the latter populations, the lipid profile is only barely regulated by exercise training with no distinction between normoxic and hypoxic training. Here as well, no strict dietary control was implemented in the previous studies dealing with exercise training, preventing definitive conclusions to be drawn.
Blood Pressure
Looking at the effect of hypoxic training on blood pressure provides controversial results. Hypoxic, but not normoxic, training reduced systolic and diastolic blood pressure in obese adults after 4 and 8 weeks38,41,69 whereas, conversely, systolic blood pressure was decreased only after normoxic, not hypoxic, training in obese adolescents after 636 and 30 weeks.39 Two other studies have observed a reduction in diastolic blood pressure after exercise training, with no difference between normoxic and hypoxic training.42,43 Although controversial, those results show at least that hypoxia had no negative effect on blood pressure.
Limits and Perspective
Together, the heterogeneity of the subjects and variables investigated makes it difficult to compare the different studies and may help to explain the divergent results reported. Among others, the type, frequency, duration, and intensity of exercise as well as the timing of blood sampling differed between studies. Variables such as inflammation or appetite hormones are often overlooked in the various studies even though they are important markers of obesity and T2DM. In the end, few of the studies listed in Table 1 have examined energy expenditure and energy intake levels. While focusing on a specific diet requires continuous monitoring and is thus rather constraining, it is a crucial parameter to control when dealing with obesity.
Another important parameter to be further analyzed is the impact of keeping the relative vs the absolute exercise intensity constant between hypoxic and normoxic conditions. To date, no study has properly tested this question, certainly not on health-related parameters. This is of the utmost importance when prescribing exercise as a therapeutic strategy. Exercise duration, intensity and frequency need to be accurately defined based on the objective of the training period but how those parameters should be combined remains insufficiently documented up to now. A good periodization and individualization of the training is key not to induce injury and not to give up.
Finally, beyond the health-related effects it might induce, it is of note that hypoxic training does not appear to provoke adverse events. Knowing that a lack of motivation is an important cause of non-adherence to an exercise program in patients, varying the environmental conditions could contribute to increase the adherence. However, hypoxic training implies the use of a quite expensive and technical equipment. If hypoxic exercise is not feasible, exercise in normoxia should be encouraged as it induces an improvement of health parameters as well and is more accessible to the general public.
Conclusion
Taken together, the data on the effects of hypoxic training on glucose metabolism, insulin sensitivity and the health status of people at risk of T2DM are inconclusive. Some studies show that hypoxic training can improve glucose metabolism and the health status to a greater extent than normoxic training while others do not corroborate the latter. When an additional benefit of hypoxic vs normoxic training is found, it still remains to determine which signaling pathways and molecular mechanisms are involved and how it can concretely be implemented in the exercise routine of people at risk of developing T2DM.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Fonds National de la Recherche Scientifique (FNRS, grant F.4504.17) and the Fonds Spécial de Recherche (FSR) from the UCLouvain. The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Glasziou PP, Clarke P, Alexander J, et al. Cost-effectiveness of lowering blood pressure with a fixed combination of perindopril and indapamide in type 2 diabetes mellitus: an ADVANCE trial-based analysis. Med J Aust. 2010;193(6):320–324. doi:10.5694/j.1326-5377.2010.tb03941.x
2. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi:10.1016/S0140-6736(12)60283-9
3. Booth FW, Weeden SH, Tseng BS. Effect of aging on human skeletal muscle and motor function. Med Sci Sports Exerc. 1994;26(5):556–560. doi:10.1249/00005768-199405000-00006
4. Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi:10.1038/35007508
5. Siegel KR, Bullard KM, Imperatore G, et al. Prevalence of major behavioral risk factors for type 2 diabetes. Diabetes Care. 2018;41(5):1032–1039. doi:10.2337/dc17-1775
6. Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. J Exp Biol. 2018;221(13):13. doi:10.1242/jeb.163840
7. Busutil R, Espallardo O, Torres A, Martínez-Galdeano L, Zozaya N, Hidalgo-Vega Á. The impact of obesity on health-related quality of life in Spain. Health Qual Life Outcomes. 2017;15(1):197. doi:10.1186/s12955-017-0773-y
8. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi:10.2337/dc16-1728
9. Codella R, Ialacqua M, Terruzzi I, Luzi L. May the force be with you: why resistance training is essential for subjects with type 2 diabetes mellitus without complications. Endocrine. 2018;62(1):14–25. doi:10.1007/s12020-018-1603-7
10. Qadir R, Sculthorpe NF, Todd T, Brown EC. Effectiveness of resistance training and associated program characteristics in patients at risk for type 2 diabetes: a systematic review and meta-analysis. Sports Med Open. 2021;7(1):38. doi:10.1186/s40798-021-00321-x
11. Verboven M, Van Ryckeghem L, Belkhouribchia J, et al. Effect of exercise intervention on cardiac function in type 2 diabetes mellitus: a systematic review. Sports Med. 2019;49(2):255–268. doi:10.1007/s40279-018-1003-4
12. Miele EM, Headley SAE. The effects of chronic aerobic exercise on cardiovascular risk factors in persons with diabetes mellitus. Curr Diab Rep. 2017;17(10):97. doi:10.1007/s11892-017-0927-7
13. Vogel MBE, Goossens G. Moderate hypoxia exposure: a novel strategy to improve glucose metabolism in humans? EMJ Diabetes. 2015;3(1):73–79.
14. Span PN, Bussink J. Biology of hypoxia. Semin Nucl Med. 2015;45(2):101–109. doi:10.1053/j.semnuclmed.2014.10.002
15. Dinenno FA. Skeletal muscle vasodilation during systemic hypoxia in humans. J Appl Physiol. 2016;120(2):216–225. doi:10.1152/japplphysiol.00256.2015
16. Montero D, Lundby C. Effects of exercise training in hypoxia versus normoxia on vascular health. Sports Med. 2016;46(11):1725–1736. doi:10.1007/s40279-016-0570-5
17. Zoll J, Ponsot E, Dufour S, et al. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J Appl Physiol. 2006;100(4):1258–1266. doi:10.1152/japplphysiol.00359.2005
18. Haufe S, Wiesner S, Engeli S, Luft FC, Jordan J. Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med Sci Sports Exerc. 2008;40(11):1939–1944. doi:10.1249/MSS.0b013e31817f1988
19. Brinkmann C, Bloch W, Brixius K. Exercise during short-term exposure to hypoxia or hyperoxia - novel treatment strategies for type 2 diabetic patients? Scand J Med Sci Sports. 2018;28(2):549–564. doi:10.1111/sms.12937
20. Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2011;27(1):94–101. doi:10.1002/dmrr.1156
21. Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P. Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(4):E546–E555. (). doi:10.1210/jc.2011-2829
22. Dill RP, Chadan SG, Li C, Parkhouse WS. Aging and glucose transporter plasticity in response to hypobaric hypoxia. Mech Ageing Dev. 2001;122(6):533–545. doi:10.1016/S0047-6374(01)00216-0
23. National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. doi:10.1161/circ.106.25.3143
24. Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–2221. doi:10.1016/S0140-6736(09)60619-X
25. Kullmer T, Gabriel H, Jungmann E, et al. Increase of serum insulin and stable c-peptide concentrations with exhaustive incremental graded exercise during acute hypoxia in sedentary subjects. Exp Clin Endocrinol Diabetes. 1995;103(3):156–161. doi:10.1055/s-0029-1211344
26. Morishima T, Mori A, Sasaki H, Goto K. Impact of exercise and moderate hypoxia on glycemic regulation and substrate oxidation pattern. PLoS One. 2014;9(10):e108629. doi:10.1371/journal.pone.0108629
27. De Groote E, Britto FA, Balan E, et al. Effect of hypoxic exercise on glucose tolerance in healthy and pre-diabetic adults. Am J Physiol Endocrinol Metab. 2020;320(1):E43–E54
28. Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38(5):401–423. doi:10.2165/00007256-200838050-00004
29. Peltonen GL, Scalzo RL, Schweder MM, et al. Sympathetic inhibition attenuates hypoxia induced insulin resistance in healthy adult humans. J Physiol. 2012;590(11):2801–2809. doi:10.1113/jphysiol.2011.227090
30. Oltmanns KM, Gehring H, Rudolf S, et al. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med. 2004;169(11):1231–1237. doi:10.1164/rccm.200308-1200OC
31. Galbo H. Hormonal and Metabolic Adaptation to Exercise. Georg Thieme Verlag; 1983.
32. Álvarez-herms J, Julià-Sánchez S, Gatterer H, et al. Anaerobic training in hypoxia: a new approach to stimulate the rating of effort perception. Physiol Behav. 2016;163:37–42. doi:10.1016/j.physbeh.2016.04.035
33. Wadley GD, Lee-Young RS, Canny BJ, et al. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab. 2006;290(4):E694–E702. doi:10.1152/ajpendo.00464.2005
34. Morishima T, Hasegawa Y, Sasaki H, Kurihara T, Hamaoka T, Goto K. Effects of different periods of hypoxic training on glucose metabolism and insulin sensitivity. Clin Physiol Funct Imaging. 2015;35(2):104–109. doi:10.1111/cpf.12133
35. Morishima T, Kurihara T, Hamaoka T, Goto K. Whole body, regional fat accumulation, and appetite-related hormonal response after hypoxic training. Clin Physiol Funct Imaging. 2014;34(2):90–97. doi:10.1111/cpf.12069
36. De Groote E, Britto FA, Bullock L, et al. Hypoxic training improves normoxic glucose tolerance in adolescents with obesity. Med Sci Sports Exerc. 2018;50(11):2200–2208. doi:10.1249/MSS.0000000000001694
37. Mai K, Klug L, Rakova N, et al. Hypoxia and exercise interactions on skeletal muscle insulin sensitivity in obese subjects with metabolic syndrome: results of a randomized controlled trial. Int J Obes. 2019;44(5):1119–1128
38. Wiesner S, Haufe S, Engeli S, et al. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity. 2010;18(1):116–120. doi:10.1038/oby.2009.193
39. Britto FA, De Groote E, Aranda J, Bullock L, Nielens H, Deldicque L. Effects of a 30-week combined training program in normoxia and in hypoxia on exercise performance and health-related parameters in obese adolescents: a pilot study. J Sports Med Phys Fitness. 2020;60(4):601–609. doi:10.23736/S0022-4707.20.10190-7
40. Chobanyan-Jurgens K, Scheibe RJ, Potthast AB, et al. Influences of hypoxia exercise on whole-body insulin sensitivity and oxidative metabolism in older individuals. J Clin Endocrinol Metab. 2019;104(11):5238–5248. doi:10.1210/jc.2019-00411
41. Gonzalez-Muniesa P, Lopez-Pascual A, de Andres J, et al. Impact of intermittent hypoxia and exercise on blood pressure and metabolic features from obese subjects suffering sleep apnea-hypopnea syndrome. J Physiol Biochem. 2015;71(3):589–599. doi:10.1007/s13105-015-0410-3
42. Chacaroun S, Borowik A, Ygi V-E, et al. Hypoxic exercise training to improve exercise capacity in obese individuals. Med Sci Sports Exerc. 2020;52(8):1641–1649. doi:10.1249/MSS.0000000000002322
43. Klug L, Mahler A, Rakova N, et al. Normobaric hypoxic conditioning in men with metabolic syndrome. Physiol Rep. 2018;6(24):e13949. doi:10.14814/phy2.13949
44. Tjonna AE, Lee SJ, Rognmo O, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi:10.1161/CIRCULATIONAHA.108.772822
45. Camacho-Cardenosa A, Camacho-Cardenosa M, Brazo-Sayavera J, Burtscher M, Timon R, Olcina G. Effects of high-intensity interval training under normobaric hypoxia on cardiometabolic risk markers in overweight/obese women. High Alt Med Biol. 2018;19(4):356–366. doi:10.1089/ham.2018.0059
46. Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93(3):993–1017. doi:10.1152/physrev.00038.2012
47. Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7(5):1085–1094. doi:10.1016/S1097-2765(01)00251-9
48. Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295(1):E29–E37. doi:10.1152/ajpendo.90331.2008
49. Azevedo JL, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44(6):695–698. doi:10.2337/diab.44.6.695
50. Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol. 1991;70(4):1593–1600. doi:10.1152/jappl.1991.70.4.1593
51. Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574(Pt 3):889–903. doi:10.1113/jphysiol.2006.111757
52. Wright DC, Geiger PC, Holloszy JO, Han DH. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am J Physiol Endocrinol Metab. 2005;288(6):E1062–E1066. doi:10.1152/ajpendo.00561.2004
53. Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate p65PAK, p38Mitogen-Activated Protein Kinase (MAPK), and Stress-Activated Protein Kinase (SAPK) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1997;239(3):840–844. doi:10.1006/bbrc.1997.7570
54. Parker L, Trewin A, Levinger I, Shaw CS, Stepto NK. The effect of exercise-intensity on skeletal muscle stress kinase and insulin protein signaling. PLoS One. 2017;12(2):e0171613. doi:10.1371/journal.pone.0171613
55. Somwar R, Perreault M, Kapur S, et al. Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: potential role in the stimulation of glucose transport. Diabetes. 2000;49(11):1794. doi:10.2337/diabetes.49.11.1794
56. Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes. 2000;49(7):1092–1095. doi:10.2337/diabetes.49.7.1092
57. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15(1):551–578. doi:10.1146/annurev.cellbio.15.1.551
58. Behrooz A, Ismail-Beigi F. Stimulation of glucose transport by hypoxia: signals and mechanisms. Physiology. 1999;14(3):105–110.
59. Gorgens SW, Benninghoff T, Eckardt K, et al. Hypoxia in combination with muscle contraction improves insulin action and glucose metabolism in human skeletal muscle via the hif-1alpha pathway. Diabetes. 2017;66(11):2800–2807. doi:10.2337/db16-1488
60. Slivka DR, Heesch MW, Dumke CL, Cuddy JS, Hailes WS, Ruby BC. Human skeletal muscle mRNAResponse to a single hypoxic exercise bout. Wilderness Environ Med. 2014;25(4):462–465. doi:10.1016/j.wem.2014.06.011
61. Gnimassou O, Fernandez-Verdejo R, Brook M, et al. Environmental hypoxia favors myoblast differentiation and fast phenotype but blunts activation of protein synthesis after resistance exercise in human skeletal muscle. FASEB J. 2018;32(10):5272–5284. doi:10.1096/fj.201800049RR
62. De Smet S, D’Hulst G, Poffe C, Van Thienen R, Berardi E, Hespel P. High-intensity interval training in hypoxia does not affect muscle HIF responses to acute hypoxia in humans. Eur J Appl Physiol. 2018;118(4):847–862. doi:10.1007/s00421-018-3820-4
63. Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000;72(2 Suppl):558s–563s. doi:10.1093/ajcn/72.2.558S
64. Dumortier M, Brandou F, Perez-Martin A, Fedou C, Mercier J, Brun JF. Low intensity endurance exercise targeted for lipid oxidation improves body composition and insulin sensitivity in patients with the metabolic syndrome. Diabetes Metab. 2003;29(5):509–518. doi:10.1016/S1262-3636(07)70065-4
65. Favier FB, Britto FA, Freyssenet DG, Bigard XA, Benoit H. HIF-1-driven skeletal muscle adaptations to chronic hypoxia: molecular insights into muscle physiology. Cell Mol Life Sci. 2015;72(24):4681–4696. doi:10.1007/s00018-015-2025-9
66. Farinatti P, Castinheiras Neto AG, Amorim PR. Oxygen consumption and substrate utilization during and after resistance exercises performed with different muscle mass. Int J Exerc Sci. 2016;9(1):77–88.
67. Camacho-Cardenosa A, Camacho-Cardenosa M, Burtscher M, et al. High-intensity interval training in normobaric hypoxia leads to greater body fat loss in overweight/obese women than high-intensity interval training in normoxia. Front Physiol. 2018;9:60. doi:10.3389/fphys.2018.00060
68. Kayser B, Verges S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obesity Rev. 2013;14(7):579–592.
69. Kong Z, Zang Y, Hu Y. Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. 2014;18(3):591–597. doi:10.1007/s11325-013-0922-4
70. Park H-Y, Jung W-S, Kim J, Lim K. Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: a randomized controlled trial. Geriatr Gerontol Int. 2019;19(4):311–316.
71. Netzer NC, Chytra R, Kupper T. Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 2008;12(2):129–134. doi:10.1007/s11325-007-0149-3
72. Gatterer H, Haacke S, Burtscher M, et al. Normobaric intermittent hypoxia over 8 months does not reduce body weight and metabolic risk factors-a randomized, single blind, placebo-controlled study in normobaric hypoxia and normobaric sham hypoxia. Obes Facts. 2015;8(3):200–209. doi:10.1159/000431157
73. Boutcher SH. High-intensity intermittent exercise and fat loss. J Obes. 2011;2011:868305. doi:10.1155/2011/868305
74. Camacho-Cardenosa A, Camacho-Cardenosa M, Olcina G, Timon R, Brazo-Sayavera J. Detraining effect on overweight/obese women after high-intensity interval training in hypoxia. Scand J Med Sci Sports. 2019;29(4):535–543. doi:10.1111/sms.13380
75. Lippl FJ, Neubauer S, Schipfer S, et al. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity. 2010;18(4):675–681.
76. Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab. 2010;95(4):1609–1616. doi:10.1210/jc.2009-2082
77. Yki-Järvinen H, Koivisto VA. Effects of body composition on insulin sensitivity. Diabetes. 1983;32(10):965–969. doi:10.2337/diab.32.10.965
78. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738.
79. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–1667. doi:10.1161/CIRCULATIONAHA.107.739714
80. Hoppeler H, Kleinert E, Schlegel C, et al. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med. 1990;11(Suppl 1):S3–S9. doi:10.1055/s-2007-1024846
81. Feriche B, García-Ramos A, Morales-Artacho AJ, Padial P. Resistance training using different hypoxic training strategies: a basis for hypertrophy and muscle power development. Sports Med Open. 2017;3(1):12. doi:10.1186/s40798-017-0078-z
82. Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi:10.1161/CIR.0000000000000461
83. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215–225. doi:10.1177/1753944717711379
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.