Back to Journals » OncoTargets and Therapy » Volume 11
Is long interval from neoadjuvant chemoradiotherapy to surgery optimal for rectal cancer in the era of intensity-modulated radiotherapy?: a prospective observational study
Authors Chang H , Jiang W, Ye WJ, Tao YL, Wang QX, Xiao WW, Gao YH
Received 3 April 2018
Accepted for publication 6 August 2018
Published 21 September 2018 Volume 2018:11 Pages 6129—6138
DOI https://doi.org/10.2147/OTT.S169985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Hui Chang,1,2,* Wu Jiang,1,3,* Wei-Jun Ye,1,2 Ya-Lan Tao,1,2 Qiao-Xuan Wang,1,2 Wei-Wei Xiao,1,2 Yuan-Hong Gao1,2
1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China; 2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 3Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Objectives: To evaluate the impact of interval between neoadjuvant chemoradiotherapy (NACRT) and surgery on therapeutic and adverse effects of surgery, and long-term outcome of patients with locally advanced rectal cancer (RC), in the era of intensity-modulated radiotherapy (IMRT).
Patients and methods: Patients diagnosed with stage II–III RC and treated with IMRT-based NACRT followed by radical surgery were enrolled consecutively from April 2011 to March 2014. The data of all the patients were collected prospectively and grouped according to their NACRT-to-surgery interval. The therapeutic and adverse effects of surgery, and survivals were compared between the patients with interval ≤7 weeks and those with interval ≥8 weeks.
Results: A total of 231 patients were eligible for analysis, including 106 cases with interval ≤7 weeks and 125 cases with interval ≥8 weeks. The therapeutic and adverse effects of surgery were similar between these two groups of patients. However, interval ≥8 weeks appeared to lead to poorer overall, distant-metastasis-free and disease-free survivals, compared with interval ≤7 weeks. The HRs were 1.805, 1.714, and 1.796 (P-values were 0.045, 0.049, and 0.028), respectively.
Conclusion: For patients with locally advanced RC, a long NACRT-to-surgery interval might bring a potential risk of increased distant metastasis rather than a better tumor regression in the era of IMRT.
Keywords: rectal cancer, interval, neoadjuvant chemoradiotherapy, surgery, survival
Introduction
Colorectal cancer, particularly rectal cancer (RC), is the third most common malignancy in People’s Republic of China.1 At initial diagnosis, about 74.0% of RC patients are found to have a locally advanced (stage II–III) disease.2 For these patients, neoadjuvant chemoradiotherapy (NACRT) before surgery is necessary to facilitate R0 resection, sphincter preservation, and improvement of long-term outcome.3 However, there are still problems to be clarified, such as appropriate time interval between NACRT and surgery.
It has been proven that pathologic complete remission (pCR) of RC often takes months after NACRT.4 Hence, a prolonged NACRT-to-surgery interval is supposed to improve the therapeutic effects of surgery. In fact, Garcia-Aguilar et al5 have conducted a Phase II trial to show that an interval of 11 weeks brought a higher pCR rate with comparable postoperative complications, when compared with the classical 6-week interval. A similar correlation between the NACRT-to-surgery interval and the pCR rate was seen in the meta-analyses by Petrelli et al6 and Du et al.7
On the other hand, long NACRT-to-surgery interval has the possibility to increase dissection difficulty and surgery-related complications due to pelvic fibrosis. In the recent Phase III GRECCAR-6 trial, the 11-week interval appeared to cause higher morbidity and more difficult resection, instead of higher pCR rate, than the 7-week interval.8 Moreover, distant metastasis (DM) is now reported to happen in nearly 20.2% of RC patients and to be the major cause of treatment failure.9 There is also a concern that long interval results in delay of postsurgical adjuvant chemotherapy (ACT) and might increase the risk of DM. Therefore, further studies are needed to eliminate the divergence on the length of the NACRT-to-surgery interval.
Additionally, most of the patients in the published studies focusing on the NACRT-to-surgery interval were not irradiated with intensity-modulated radiotherapy (IMRT), which has gradually become the mainstream irradiating technique for RC due to optimized dose delivery and reduced toxicities.10 So, this prospective observational study aimed to explore the impact of NACRT-to-surgery interval on treatment effects of surgery and long-term outcome of RC patients in the era of IMRT.
Patients and methods
Patient selection
The patients who were diagnosed as RC pathologically in our hospital from April 1, 2011 to March 31, 2014 were initially considered. A patient would be consecutively enrolled into this study and prospectively observed if he or she met the following enrollment criteria: 1) previously untreated RC; 2) stage cII–III (cT3-4N0M0, cT1-4N1-2M0) disease; 3) age between 18 and 75 years old; 4) Karnofsky performance score >70; 5) complete records of IMRT-based NACRT and surgery; and 6) R0 resection.
The exclusion criteria included: 1) recurrent RC; 2) DMs before or during treatment; 3) prior chemotherapy or radiotherapy; 4) severe heart, lung, liver, or kidney dysfunctions unsuitable for NACRT; 5) prior history of other malignancies; and 6) application of monoclonal antibody.
This study was approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center. Written informed consent was obtained from each patient before treatment.
Diagnosis and staging
The pathologic diagnosis of RC was made through biopsy under rectoscope. Then pretreatment clinical stage was determined through a computed tomography (CT) of chest and abdomen, a magnetic resonance imaging (MRI) of pelvis, an endoscopic ultrasonography, and a whole-body bone scan. Positron emission tomography was performed to confirm suspicious DM lesions. All the patients enrolled were staged according to the TNM staging standard (seventh edition) of the Union for International Cancer Control/American Joint Cancer Committee (UICC/AJCC).11 The baseline carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels were also tested before treatment.
Treatment strategies
The treatment was performed according to the practice guidelines of our hospital and the National Comprehensive Cancer Network (NCCN). The radiotherapy technique for all the patients in this study was IMRT. The patients were first immobilized by an AIO Bellyboard and Pelvic Solution system (AIO solution, Orfit Industries, Wijnegem, Belgium) and simulated with a moderately full bladder. After a CT simulation with 3-mm slice thickness, the target volumes were delineated and the dose prescription was made (more details can be found in our previous work).12 Radiotherapy was done in a conventional fractionation (2 Gy per fraction, 1 fraction per day, 5 days per week), in which the total dose for the planning target volumes of gross tumor volume and clinical target volume were 50 and 46 Gy, respectively. A linear accelerator delivering an 8-MV photon beam was used to perform the IMRT.
The neoadjuvant chemotherapy was performed with a XELOX (capecitabine + oxaliplatin) regimen, 21 days per cycle, for a total of four cycles. Capecitabine was given 1,000 mg/m2 twice daily on the 1st to the 14th day of a chemotherapy cycle. Oxaliplatin was given 130 mg/m2 on the first day (100 mg/m2 concurrently with radiotherapy). The regimen of the ACT after surgery was also XELOX. A total of four cycles were planned to be performed, if there was no contraindication.
The surgery after NACRT was done according to the total mesorectal excision standard. When tumor infiltrated or adhered to the adjacent organs, the surgeons would apply a multivisceral resection, in which partial or total of the attached organs was removed. The postsurgical pathology of each patient was assessed to decide the pathologic stage, also on basis of the seventh edition of the UICC/AJCC TNM staging classification.11
Follow-up
After treatment, the patients were planned to receive follow-up by outpatient interview every 3–6 months in the first 3 years. The main tasks of the outpatient interview included complete physical examination, thoracoabdominal CT, pelvic MRI, serum CEA and CA19-9 assessment, and annual rectoscope and whole-body bone scan (or positron emission tomography). After the third year, the patients were followed up every 6–12 months by outpatient interview or telephone, until death from RC, or December 31, 2017, whichever came first. Causes of deaths were confirmed by death certificates.
Treatment effect evaluation
The treatment effects of surgery were evaluated through the down-T, the pCR, and the sphincter-preserving rates. Of those, pCR was defined as absence of microscopically viable tumor cells both in the primary site and the regional lymph nodes (stage ypT0N0), according to the Dworak standard.13
The long-term outcome of the patients was evaluated through the overall survival (OS), the local recurrence-free survival (RFS), the distant-metastasis-free survival (MFS), and the disease-free survival (DFS). The survivals were defined as the percentage of patients without corresponding events after a certain time period from diagnosis. The events for OS, RFS, and MFS were death, local recurrence (LR), and DM, respectively; the events for DFS included death, LR, and DM. The patients without the corresponding events or those lost to follow-up were regarded censored.
Adverse effect evaluation
Indexes of surgery-related adverse effects included the rate of grade 3 postsurgical complications and the intraoperative bleeding volume, the surgery time, and the days of hospitalization during which the surgery was performed. The medical or surgical complications within 90 days after surgery were defined as postsurgical complications and evaluated according to the Clavien–Dindo classification.
Definition and cutoff of variables
During analysis, the patients were grouped according to the NACRT-to-surgery interval, which was defined as the time period from the ending date of NACRT to the date of surgery. The cutoff value of the NACRT-to-surgery interval was 8 (≤7 vs ≥8) weeks, according to the study by Du et al.7
Except the NACRT-to-surgery interval, the candidate prognostic factors in the survival analysis also included age, gender (male vs female), tumor differentiation (poorly differentiated vs moderately-well differentiated), perioperative chemotherapy cycle (≤7 vs 8) and pretreatment anemia (yes vs no), clinical stage (cIII vs cII), CEA, CA19-9, surgery technique (open vs laparoscopic), and pathologic N stage (pN+ vs pN−). The cutoff value of the age was the median age of the whole cohort. Anemia was defined as hemoglobin <130 g/L for males and <120 g/L for females, according to the standard of the WHO.14 The threshold was modified to 110 g/L when a patient was aged ≥65 years old, according to the standard of elderly Chinese described by Peng and Zhang.15 The upper normal limit of CEA and CA19-9 were determined as 5.00 ng/mL and 35.00 U/mL respectively, according to the standard of our hospital.16
Statistical analyses
Continuous data was presented as median with range and compared through a Mann–Whitney U-test. Categorical data were presented as number with proportion (%) and compared through a χ2 test.
The survivals were first calculated by a Kaplan–Meier approach and compared using a log-rank test. The factors exhibiting statistical significance in the univariate analysis were then entered in the multivariate analysis as covariates. The independence of the prognostic factors on predicting survivals was tested through a Cox proportional hazards model. The hazard ratio (HR) and 95% confidence interval (CI) of each variable were calculated. The adjusted survival curves of patients with different NACRT-to-surgery intervals were also depicted.
A two-sided P-value of <0.05 was considered statistically significant. All statistical analyses were done using IBM SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA). The procedure of this study is summarized in Figure 1.
Results
Patient enrollment
Between April 2011 and March 2014, a total of 247 patients diagnosed with untreated stage cII–III RC and treated with IMRT-based NACRT plus surgery were enrolled consecutively. After exclusion of the cases with DM during treatment (N=8) and non-R0 resection (N=8), there were finally 231 patients eligible for analysis. In these patients, 106 cases (45.9%) had an NACRT-to surgery interval ≤7 (range, 4–6) weeks, and 125 cases (54.1%) had an interval ≥8 (range, 8–12) weeks.
Clinicopathological profiles
The median age of the eligible patients was 54 (22–75) years old. So, the cutoff value of the age in survival analysis was also 54 (≤54 vs ≥55) years old. There were 50 (21.6%) and 181 (78.4%) cases with stage cII and cIII disease, respectively. The distribution of the baseline clinicopathological profiles were balanced between the patients with NACRT-to-surgery interval ≤7 weeks and those with interval ≥8 weeks (Table 1).
  | Table 1 Clinicopathological characteristics of the patients |
Treatment effects of surgery
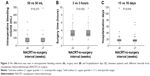
The down-T, the pCR, and the sphincter-preserving rates of the patients with NACRT-to-surgery interval ≤7 weeks were 75.5%, 30.2%, and 67.9%, respectively. The figures of the patients with interval ≥8 weeks were 78.4%, 34.4%, and 76.0%, respectively. There was no statistical difference between the two groups of patients in treatment effects of surgery (Figure 2).
Adverse effects of surgery
The patients with NACRT-to-surgery interval ≤7 weeks had a rate of grade 3 postsurgical complications similar to that of the patients with interval ≥8 weeks (4.7% vs 2.4%, P=0.337). No grade 4 complication was seen. There was no difference in the intraoperative bleeding volume, the surgery time, and the hospitalization days between the two groups of patients (Figure 3).
Survival analysis
The median follow-up time of the patients was 47 (range, 10–73) months. A total of 31 cases (13.4%) were lost to follow-up. Until December 2017, there were totally 47 deaths (20.3%), 8 LRs (3.5%), and 63 DMs (27.3%).
In univariate analysis, CEA, CA19-9, NACRT-to-surgery interval, and pathologic N stage presented as possible predictors of the OS, the MFS, and the DFS (Table 2). The patients with NACRT-to-surgery interval ≥8 weeks had a poorer OS (76.0% vs 84.0%, P=0.037), MFS (69.6% vs 80.2%, P=0.036), and DFS (67.2% vs 79.2%, P=0.021) than those with interval ≤7 weeks. Through multivariate analysis, CEA, CA19-9, NACRT-to-surgery, and pathologic N stage were still present to predict the OS, the MFS, and the DFS independently (Table 3). The HRs of NACRT-to-surgery interval on predicting the OS, the MFS, and the DFS were 1.805 (95% CI, 1.016–3.303), 1.714 (95% CI, 1.001–2.934), and 1.796 (95% CI, 1.066–3.025), respectively. The adjusted survival curves are shown in Figure 4.
Discussion
To date, there is no consensus on the best NACRT-to-surgery interval for patients with locally advanced RC. In the latest version of NCCN guidelines, the interval between NACRT and surgery is recommended to be 5–12 weeks,17 which is not an exact value. Many recent studies supported a longer interval. Retrospective studies by Moore et al18 and Tulchinsky et al19 found that the pCR rate increased from 12%–17% to 19%–35% when the NACRT-to-surgery interval was prolonged to 7 weeks. Kalady et al20 reported that an interval ≥8 weeks made the pCR rate increase to 30.8%, and the interval was the sole factor predicting the pCR rate. Sloothaak et al22 and Probst et al21 further made analyses based on large-scale cohorts to confirm a positive correlation between the NACRT-to-surgery interval and the pCR rate. Since good tumor response after NACRT is convinced as a favorable prognosticator of locally advanced RC,23 patients might achieve benefit of long-term outcome through prolonging the interval from NACRT to surgery. Nevertheless, studies by Habr-Gama et al24 and de Campos-Lobato et al25 showed that improved tumor regression from prolonged interval did not necessarily translate into survival benefit.
There were also prospective studies conducted to figure out a proper NACRT-to-surgery interval. But the results were inconsistent. The first prospective study to support the favorable impact of a prolonged interval on the pCR rate was the Lyon R90-01 randomized trial.26 A Phase II trial by Garrer et al27 revealed that patients with NACRT-to-surgery interval of 9–14 weeks had a better 18-month RFS than those with interval of 6–8 weeks (73.8% vs 100.0%, P=0.031). A decreased 3-year LR rate was also seen in patients with interval ≥8 weeks through a study by Zeng et al.28 Conversely, a study by Stein et al29 showed that there was no favorable impact of prolonged interval on the pCR rate. The Istanbul R-01 randomized trial failed to show an improved pCR rate, 5-year LR rate, or OS in patients with 8-week interval, either.30 In the latest Phase III GRECCAR-6 trial, the 11-week interval group exhibited a similar pCR rate (15.0% vs 17.4%, P=0.598) to that of the 7-week interval group. Instead, a higher morbidity was seen in the 11-week interval group (44.5% vs 32.0%, P=0.04) as well as a worse quality of mesorectal resection (78.7% vs 90.0%, P=0.015).8
IMRT has gradually become the primary technique of radiotherapy for locally advanced RC. Therefore, we assessed how NACRT-to-surgery interval affected surgical effects of locally advanced RC, through analyzing a relatively large cohort treated uniformly with IMRT. Our study indicated that short interval (≤7 weeks) was not inferior to long interval (≥8 weeks) in short-term therapeutic effects. The down-T rate (75.5% vs 78.4%, P=0.598), the pCR rate (30.2% vs 34.4%, P=0.496), and the sphincter-preserving rate (67.9% vs 76.0%, P=0.172) were similar between patients with different intervals. The adverse effects were also similar. No difference was seen in the rate of severe postsurgical complications, the intraoperative bleeding volume, the surgery time, or the hospitalization days, between the two groups of patients. Both the intervals were tolerable selections.
We also assessed the influence of NACRT-to-surgery interval on long-term outcome, and attained results which were a bit different from those of many previous studies. After a follow-up of nearly 4 years, we showed in this study that the patients with long interval appeared to have a worse OS (76.0% vs 84.0%, P=0.037), MFS (69.6% vs 80.2%, P=0.036), and DFS (67.2% vs 79.2%, P=0.021) than those with short interval. And through multivariate analysis, long interval maintained its independence on predicting a poorer OS (HR =1.805, P=0.045), MFS (HR =1.714, P=0.049), and DFS (HR =1.796, P=0.028). In other words, long NACRT-to-surgery interval resulted in an increased risk of DM and cancer death. The explanation might be that prolongation of NACRT-to-surgery interval inevitably prolonged the interval from NACRT to ACT as well. As we know, DM is now the leading cause of RC-related death.9 Although necessity of ACT remains controversial, it is believed that systemic chemotherapy of sufficient intensity is needed to eradicate DM effectively. A total of 6-month (8-cycle) XELOX chemotherapy is proposed by the NCCN guidelines to be performed periopratively.17 Therefore, ACT of 4–6 cycles is often needed after surgery, especially in patients with a residual tumor after NACRT.31 Delay of the ACT may provide sufficient time for subclinical DM lesions to grow to a size which can no longer be eradicated by the currently available cytotoxic agents. There have been studies revealing that a 2- or 3-month delay of ACT from surgery could negatively influence both the cancer-specific and the all-cause mortality of colorectal cancer,32–34 though no study directly reported the impact of a delayed ACT from NACRT. Hence, a long NACRT-to-surgery interval could probably impair elimination of DM and survival of RC patients. Additionally, in the recent Stockholm III trial, 4–8 weeks emerged as a reasonable NACRT-to-surgery interval which brought an acceptable DM rate and OS.35 It was in accordance with our results. However, a longer interval (8–12 weeks) was not assessed in that study.
Actually, it might be more appropriate to apply an individualized interval, which could be decided mainly by the possibility of pCR and the risk of DM. And since pathologic stage is the most important predictor of DM and can only be known after surgery,36 it may be more practical to determine NACRT-to-surgery interval through prediction of pCR. There have been studies focusing on predicting pCR at initial diagnosis. The reported predictors included tumor size, clinical N stage, and some well-known biomarkers such as p53, p21, Ki67, and VEGF.37,38 Further study is needed to build an accurate, practical predicting system to determine individualized interval for each locally advanced RC patient.
Indeed, there are still three main limitations in this study. First, it was not a controlled study with random allocation of the patients. Second, it was a single-institutional experience. Third, circumferential resection margin was not included in survival analysis of the study cohort because it was not routinely assessed in our hospital before 2017. Thus, a multicenter randomized controlled trial is needed to further validate the results of this study, before popularization to clinical use.
Conclusion
For patients with locally advanced RC, a long NACRT-to-surgery interval might bring a potential risk of increased DM instead of a better tumor regression, in the era of IMRT. Prolonged interval should be allowed in patients with caution before more evidence can be attained.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1–12. | ||
Yu HC, Luo YX, Peng H, et al. Association of perioperative blood pressure with long-term survival in rectal cancer patients. Chin J Cancer. 2016;35:38. | ||
Sineshaw HM, Jemal A, Thomas CR, Mitin T. Changes in treatment patterns for patients with locally advanced rectal cancer in the United States over the past decade: An analysis from the National Cancer Data Base. Cancer. 2016;122(13):1996–2003. | ||
Wang Y, Cummings B, Catton P, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: long term outcome of 271 patients. Radiother Oncol. 2005;77(2):126–132. | ||
Garcia-Aguilar J, Smith DD, Avila K, et al. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254(1):97–102. | ||
Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263(3):458–464. | ||
du D, Su Z, Wang D, Liu W, Wei Z. Optimal Interval to Surgery After Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Systematic Review and Meta-analysis. Clin Colorectal Cancer. 2018;17(1):13–24. | ||
Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol. 2016;34(31):3773–3780. | ||
Guren MG, Kørner H, Pfeffer F, et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993–2010. Acta Oncol. 2015;54(10):1714–1722. | ||
Samuelian JM, Callister MD, Ashman JB, Young-Fadok TM, Borad MJ, Gunderson LL. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82(5):1981–1987. | ||
Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (UICC International Union Against Cancer). 7th ed. New York: Wiley-Blackwell; 2009. | ||
Gao YH, Lin JZ, An X, et al. Neoadjuvant sandwich treatment with oxaliplatin and capecitabine administered prior to, concurrently with, and following radiation therapy in locally advanced rectal cancer: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2014;90(5):1153–1160. | ||
Kaytan-Saglam E, Balik E, Saglam S, et al. Delayed versus immediate surgery following short-course neoadjuvant radiotherapy in resectable (T3N0/N+) rectal cancer. J Cancer Res Clin Oncol. 2017;143(8):1597–1603. | ||
World Health Organization. The Global Prevalence of Anaemia in 2011. Geneva, Switzerland: World Health Organization; 2015. | ||
Peng XL, Zhang C. The exploration of diagnostic standard of anemia in aged people. Journal of Clinical Hematology. 2000;5:217–218. [Article in Chinese]. | ||
Lu Z, Peng J, Wang Z, et al. High preoperative serum CA19-9 level is predictive of poor prognosis for patients with colorectal liver oligometastases undergoing hepatic resection. Med Oncol. 2016;33(11):121. | ||
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Rectal Cancer (version 4. 2017). NCCN.org. Fort Washington, PA: National Comprehensive Cancer Network. Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed March 19, 2018. | ||
Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47(3):279–286. | ||
Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15(10):2661–2667. | ||
Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):213–220. | ||
Probst CP, Becerra AZ, Aquina CT, et al. Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg. 2015;221(2):430–440. | ||
Sloothaak DA, Geijsen DE, van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100(7):933–939. | ||
Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32(15):1554–1562. | ||
Habr-Gama A, Perez RO, Proscurshim I, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(4):1181–1188. | ||
de Campos-Lobato LF, Geisler DP, da Luz Moreira A, Stocchi L, Dietz D, Kalady MF. Neoadjuvant therapy for rectal cancer: the impact of longer interval between chemoradiation and surgery. J Gastrointest Surg. 2011;15(3):444–450. | ||
Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17(8):2396. | ||
Garrer WY, El Hossieny HA, Gad ZS, Namour AE, Abo Amer SM. Appropriate Timing of Surgery after Neoadjuvant ChemoRadiation Therapy for Locally Advanced Rectal Cancer. Asian Pac J Cancer Prev. 2016;17(9):4381–4389. | ||
Zeng WG, Zhou ZX, Liang JW, et al. Impact of interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer on surgical and oncologic outcome. J Surg Oncol. 2014;110(4):463–467. | ||
Stein DE, Mahmoud NN, Anné PR, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum. 2003;46(4):448–453. | ||
Saglam S, Bugra D, Saglam EK, et al. Fourth versus eighth week surgery after neoadjuvant radiochemotherapy in T3-4/N0+ rectal cancer: Istanbul R-01 study. J Gastrointest Oncol. 2014;5(1):9–17. | ||
Maas M, Nelemans PJ, Valentini V, et al. Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer. 2015;137(1):212–220. | ||
Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107(11):2581–2588. | ||
Cheung WY, Neville BA, Earle CC. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis Colon Rectum. 2009;52(6):1054–1064. | ||
des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46(6):1049–1055. | ||
Glimelius B, Martling A. What conclusions can be drawn from the Stockholm III rectal cancer trial in the era of watch and wait? Acta Oncol. 2017;56(9):1139–1142. | ||
van Gijn W, van Stiphout RG, van de Velde CJ, et al. Nomograms to predict survival and the risk for developing local or distant recurrence in patients with rectal cancer treated with optional short-term radiotherapy. Ann Oncol. 2015;26(5):928–935. | ||
Garland ML, Vather R, Bunkley N, Pearse M, Bissett IP. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal Dis. 2014;29(3):301–307. | ||
Hur H, Kim NK, Min BS, et al. Can a biomarker-based scoring system predict pathologic complete response after preoperative chemoradiotherapy for rectal cancer? Dis Colon Rectum. 2014;57(5):592–601. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.