Back to Journals » Clinical Ophthalmology » Volume 15
Is Interferon α-2b 1 MillionIU/mL Truly Better Than Tacrolimus 0.03% for Steroid-Resistant VKC ?: Our 2-Year Experience at a Tertiary Health-Care Centre
Authors Gupta S, Singh P, Singh M, Naik M , Srivastava K
Received 28 May 2021
Accepted for publication 5 July 2021
Published 14 July 2021 Volume 2021:15 Pages 2993—2999
DOI https://doi.org/10.2147/OPTH.S322378
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Sukriti Gupta,1 Priyanka Singh,2 Mrityunjay Singh,1 Mayuresh Naik,3 Kartikeya Srivastava1
1Department of Ophthalmology, V.M.M.C & Safdarjung Hospital, New Delhi, 110029, India; 2Department of Ophthalmology, ESI Medical College and Hospital, Faridabad, Haryana, 121012, India; 3Department of Ophthalmology, H.I.M.S.R & H.A.H. Centenary Hospital, New Delhi, 110062, India
Correspondence: Mayuresh Naik
Department of Ophthalmology, H.I.M.S.R & H.A.H. Centenary Hospital, Room no. 3 of Eye OPD, 1st Floor of OPD Building, Near GK-2, Alaknanda, New Delhi, 110062, India
Tel +91-8287344576
Email [email protected]
Purpose: To compare the efficacy of eye-drop interferon (IFN) α-2b 1 millionIU/mL with eye-ointment tacrolimus 0.03% in refractory vernal keratoconjunctivitis (VKC).
Materials and Methods: Fifty patients with VKC refractory to conventional treatment with topical corticosteroids and antihistamines after 4 weeks of regular use were selected retrospectively. Patients were divided into two groups depending on whether they received eye-ointment tacrolimus 0.03% three times a day or eye-drop IFN alpha-2b 1 millionIU/mL three times a day and were followed up for 24 months. The main outcome measures were total subjective symptom score (TSSS) and total objective ocular score (TOSS).
Results: Mean baseline TSSS was 7.24± 1.98 in Group A (tacrolimus group) and 7.84± 1.82 in Group B (IFN group), and it reduced to 1.12± 0.83 in Group A and 0.62± 0.41 in Group B at 6 months, which was statistically significant compared to the baseline score (p< 0.05) as well as between the two groups. Mean baseline TOSS was 6.72± 2.07 in Group A and 6.56± 2.04 in Group B, and it improved to 1 month onwards to 1.52± 0.87 in Group A and 1.0± 0.71 in Group B at 6 months, which was statistically significant compared to the baseline score (p< 0.05) as well as between the two groups. Side effects like stinging and burning sensations were seen in the tacrolimus group only.
Conclusion: Our study suggests that while both eye-drop IFN α-2b 1 millionIU/mL and eye-ointment tacrolimus eye ointment 0.03% are both safe and effective steroid-sparing agents in steroid-resistant VKC. IFN α-2b results in greater improvement in subjective symptoms and objective signs, has fewer side effects in long term and is better tolerated as compared to tacrolimus.
Keywords: refractory vernal keratoconjunctivitis, tacrolimus, interferon α-2b, VKC
Plain Language Summary
What is Already Known About the Subject
The treatment cocktail for vernal keratoconjunctivitis (VKC) usually includes antihistamines, mast cell stabilizers, and non-steroidal anti-inflammatory drugs for mild disease. For moderate to severe sight threatening cases, topical steroids are the treatment of choice but their long-term use may result in side effects like glaucoma, cataract and secondary infections. To prevent such complications, steroid-sparing agents may be a better option.
What are the New Findings and How Would These Results Change Clinical Practice
Interferon (IFN) α-2b leads to greater reduction in both subjective symptoms as well as objective signs than tacrolimus at 6 months which is statistically significant. This improvement, both subjective and objective, persisted even till the end of follow-up period at 24 months.
While both IFN α-2b eye drops 1 millionIU/mL and tacrolimus eye ointment 0.03% are both safe and effective steroid-sparing agents in steroid-resistant VKC, IFN α-2b eye drops result in greater improvement in subjective symptoms and objective signs, have fewer side effects in the long term and are better tolerated as compared to tacrolimus eye ointment.
Introduction
Vernal keratoconjunctivitis (VKC) is a recurrent, bilateral, chronic ocular inflammatory condition that primarily affects boys in their first and second decade, living in warm dry climate countries. It is a severe allergic condition in which both IgE and cell mediated immune mechanisms play a role.1–5 The prevalence of VKC in tropical regions is around 5%.6 VKC persists throughout the year but the symptoms usually get worse during warm seasons.7,8 Most of the patients with VKC have a significant history of atopy and/or a family history of atopy. Although, VKC is a self-limiting disorder which usually resolves after puberty, some patients can have sight threatening complications if it is left untreated.
The treatment cocktail usually includes antihistamines, mast cell stabilizers, and non-steroidal anti-inflammatory drugs for mild disease. For moderate to severe sight threatening cases, topical steroids are the treatment of choice but their long term use may result in side effects like glaucoma, cataract and secondary infections.9 To prevent such complications, steroid-sparing agents may be a better option.
Tacrolimus (FK506) is a non-steroidal macrolide immunosuppressant and, like cyclosporin, it inhibits T-cell activation and IgE dependent histamine release via cyclophilin receptors.10,11 Topical tacrolimus 0.02–0.1% are being used in clinical practice for the treatment of VKC. IFNs are natural proteins that act as immunomodulatory agents. IFN α-2b is a cytokine with endogenous anti-inflammatory and anti-anaphylactic effects. It blocks the IgE-mediated release of histamine, stabilises mast cells and inhibits the arachidonic acid metabolism. IFN is known to exert anti-inflammatory property by inhibiting the release of IL-4 and IL-5 from Th2 cells12 and release of IL-10 from monocytes. Hence, this new drug has proven to be effective in the treatment of VKC.12
Our study was aimed to compare the efficacy of eye drop IFN α-2b 1,000,000 IU/mL to that of tacrolimus eye ointment 0.03% in a group of patients with refractory VKC.
Materials and Methods
Written and informed consent was taken from parents/guardians of all patients. This study was approved by the Institutional Ethics Committee and Institutional Review Board (IEC.IRB/VMMC/SJH/10/2019-17) of V.M.M.C & Safdarjung Hospital, NewDelhi. All procedures performed our study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments.
This retrospective, comparative, observational study was conducted on patients visiting Speciality Cornea Clinic at tertiary health-care hospitals, i.e., V.M.M.C & Safdarjung Hospital, Department of Ophthalmology from 1January 2019 to 31December 2020.
Inclusion Criteria
All patients, 5–15 years of age, having VKC refractory to conventional treatment with topical corticosteroids and topical antihistamines after 4 weeks of regular use were shortlisted. There was a wash-out period of 2 weeks so as to allow the effects of topical corticosteroids to wear off while patients were prescribed topical decongestants and lubricants for temporary symptomatic relief. Of these shortlisted patients, only those who had been prescribed Eye-ointment tacrolimus 0.03% three time a day and eye-drop IFN α-2b 1 millionIU/mL three time a day were selected to be included in final analysis. Patients were matched for age, sex, and any confounding factors and then classified into two groups according to the medication they received.
Exclusion Criteria
Patients with any ocular disease namely uveitis, glaucoma, ocular infection, hypersensitivity to any drug, on any immunosuppressive therapy (topical/ systemic), history of herpetic keratitis, any systemic disease except coexisting asthma/ atopic dermatitis/ allergic rhinitis, and contact lens wearers were excluded from the study.
Procedure and Data Collection
Patients of steroid-resistant VKC who had been put on tacrolimus or IFN α-2b were selected for the study comparison and final analysis. Individuals were then assigned to Group A or Group B as follows:
Group A patients: Eye-ointment tacrolimus 0.03% three times a day.
Group B patients: Eye-drop IFN α-2b 1 millionIU/mL QID.
IFN α-2b eye drop was prepared by diluting 3 millionIU/0.5mL IFN injection (Reliferon © Reliance Formulation Pvt. Ltd) with 2.5 mL artificial tear drops 0.5% Carboxymethylcellulose (RefreshTears © Allergan India Pvt. Ltd.) The IFN α-2b eye drop was stored in the refrigerator (2–8°C) and fresh formulation was prepared after 3 weeks (considering that volume of a drop is 0.05mL so that 1mL has equivalent of 20 drops). After complete general, physical and ocular examination, patients were scored using objective assessment of the signs and symptoms, adapted from similar clinical trials.3,4,13,14 Total subjective symptom score (TSSS) and total objective sign score (TOSS) were recorded according to severity from 0 to 3, with higher score indicating greater severity of the disease (Table 1).13 These scores were used for comparison within each group and between the two groups on each visit. Evaluations done at 1 week, 1 month, 6 months, 12 months, 18 months, 24 months were taken into consideration and any change in TSSS and TOSS and any side, if present were noted.
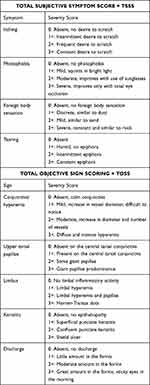 |
Table 1 VKC Scoring System |
Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± SD. Quantitative variables were compared using Independent-t-test for inter-group comparisons and Paired-t-test was used for intra-group comparisons. Qualitative variables were compared using Chi-Square test. A p value of <0.05 was considered statistically significant. The data was entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
Demographic Data
Twenty-five patients included in Group A were treated with tacrolimus 0.03% and 25 patients included in Group B were treated with IFN α-2b eye drops 1 millionIU/mL (Table 2). All were examined at regular visits for 24 months (2 years).
 |
Table 2 Demographic Data of Patients Included in Study |
The mean age of patients was 8.68 ±2.53 years in Group A and 7.92 ± 2.33 years in Group B, the difference between the two groups being not significant statistically (p value = 0.274). Patients had the disease since 3.04 ± 1.21 years, and 2.97 ±2.6 years in Group A and Group B, respectively (p value = 0.810).
Both groups of patients had predominantly tarsal VKC as compared to the limbal or mixed type, but the difference was not statistically significant. Eighteen out of 50 patients enrolled had a history of some associated allergic conditions.
Scoring (TSSS and TOSS)
TSSS was 7.24 ± 1.98 in Group A and 7.84 ± 1.82 in Group B, respectively, at baseline visit (Table 3), the difference being not significant statistically (p value = 0.271). TSSS improved to 1.12 ± 0.83 in Group A and 0.62 ± 0.41 in Group B respectively at 6 months which was statistically significant compared to baseline score (p<0.05) in respective intra-group. Also, the IFN group (Group B) showed more improvement in subjective symptoms as compared to the tacrolimus group (Group A), which was statistically significant at 6 months (p = 0.042). This difference was maintained throughout the period of observation at 24 months.
 |
Table 3 Comparative Data of TSSS and TOSS Between Study Groups |
TOSS was 6.72 ± 2.07 in Group A and 6.56 ± 2.04 in Group B, respectively, at baseline visit, the difference being not significant statistically (p value = 0.785). TOSS improved consistently from 1 month onwards and decreased to 1.52 ± 0.87 in Group A and 1.0 ± 0.71 in Group B, respectively, at 6 months, both of which were statistically significant when compared to baseline (p<0.05) in respective intra-group. Also, the IFN group (Group B) showed more improvement in objective signs as compared to the tacrolimus group (Group A), which was statistically significant at 6 months (p = 0.025). This difference was maintained throughout the period of observation at 24 months.
There were no major side effects noted except a stinging sensation in 3 patients (12%) and a burning sensation in 8 patients (36%) of the tacrolimus group (Group A) which gradually reduced with time. There were no serious adverse effect requiring withdrawal from the study in either group. Intraocular pressures were within normal limits at each visit in both groups. No patient developed exacerbation of symptoms during the study period.
Discussion
VKC is one of the more severe forms of ocular allergy which can cause vision loss, either due to disease itself or due to indiscriminate use of corticosteroids for years. It usually occurs in children, lasts for 2–10 years and is resolved by puberty. Males are affected more than females. It is usually observed in warm climates and hence is common in India. Many patients with VKC have a significant history of atopy, such as atopic dermatitis, asthma or allergic rhinitis.
The pathogenesis of VKC is multifactorial and involves conjunctival infiltration with eosinophils, mast cells, lymphocytes and macrophages. It is Th2 lymphocyte-mediated mechanism and long standing, severe inflammation that can lead to fibrovascular reaction, tissue remodelling, corneal complications and permanent visual impairment.15 This insight has led to use of many drugs like IFN α-2b, tacrolimus, cyclosporine along with conventional therapy like topical steroids, antihistamines, mast cell stabilisers for the treatment of VKC. Topical steroids remain the first line of treatment of VKC, especially in a flare-up, but their use for long term results in serious complications like glaucoma, cataract and ocular infections. Steroid induced glaucoma can potentially lead to blindness and hence, alternative drugs are needed to avoid such complications.
Tacrolimus is an immunosuppressant agent that reduces inflammatory cytokine production and inhibits several immune reactions involved in pathogenesis of VKC. Tacrolimus, in different concentrations and types, has been proven to be safe and effective for the treatment of refractory VKC.16,17 In 2019, Fiorentini et al showed that tacrolimus eye ointment 0.03% can be used as a safe and effective agent for the treatment of refractory VKC in children.18 IFN α-2b is a cytokine with anti-proliferative, anti-inflammatory and immunomodulator effects, which inhibits arachidonic acid metabolism, stabilises mast cells and inhibits IgE-mediated release of histamine. The role of IFN α-2b has been established in allergic asthma, atopic dermatitis and in conjunctival neoplasias. In 2012, Tarun-Vural et al showed that topical IFN α-2b eye drops reduced the signs and symptoms in steroid-resistant-VKC patients and thus, can be used as a promising alternative to steroids for treatment of VKC patients resistant to conventional treatment for short periods. The improvement in signs and symptoms was maintained even 6 months after discontinuation of treatment.19 But the most important drawback of this study was that it had a very small sample size of 12. The second important drawback of this study was that it neither had any comparative nor control group. Similar to our study, in 2017, Zanjani et al conducted a double-masked randomised study in which they compared IFN α-2b and tacrolimus for the treatment of steroid resistant VKC in 40 patients and found that both 0.005% tacrolimus eye ointment and IFN eye drops were equally effective.20 The results of our study are consistent with their results. However, there were certain inherent drawbacks in their study protocol. Firstly, the follow up duration in their study was only 2 months. Secondly, they used tacrolimus eye drops 0.005% formulated from injection tacrolimus, perhaps because such low concentration is not commercially available. Also, they concluded that this ultra-low concentration of tacrolimus was as equally effective as IFN α-2b 1 millionIU/mL, which would logically still leave the comparison of commercially available low-dose tacrolimus eye ointment 0.03% vs IFN α-2b unanswered. Thirdly, as Gupta PC. and Ram J. pointed out, no mention has been made of the number of patients in each group in the entire article. Fourth, there is discrepancy of the demographic data within the article abstract and the main text. Also, the dosage frequency of tacrolimus and IFN in each group has not been elucidated. And finally, contact lens wearers and patients with history of HSV dendritic keratitis were not excluded from the study.21
In our study, each patient was followed up at 1 week to check for compliance and side effects. Since no change in TOSS and TSSS was noted at 1 week, these results were not included in study analysis tables. Both 1 millionIU/mL IFN α-2b eye drops and 0.03% tacrolimus eye ointment showed a significant improvement in TSSS and caused a significant reduction in TOSS from 1 month onwards. Moreover, IFN α-2b caused more reduction in both subjective symptoms TSSS as well as objective signs TOSS than tacrolimus at 6 months which was statistically significant. This improvement, both subjective and objective, persisted even until the end of the follow-up period at 24 months.
The ophthalmic impact of our study is worth mentioning here. Firstly, the adverse effects noted with tacrolimus were burning and stinging sensation but no such adverse effects were noted with IFN α-2b. There was no increase in intraocular pressure with both tacrolimus and IFN α-2b even after long duration of use. Thus, it is reasonable to believe that both IFN and tacrolimus are equally safe for use in patients with glaucoma or those with steroid-induced raised intraocular pressure. Secondly, IFN α-2b was well tolerated by the patients. As it is more convenient and comfortable to use an eye drop compared to an eye ointment, thus patient compliance and satisfaction was better with IFN α-2b. Both these considerations are significant as the cohort that we are catering to, is the 5- to 15-year-old paediatric group as well as adolescent age group and not only tolerance but also comfort are significant variables in long-term compliance of treatment of steroid-resistant VKC.
Nevertheless, there are a few inherent limitations to the study. Firstly, our study cohort was steroid-resistant and recalcitrant VKC and hence it may be difficult to extrapolate the results of our study to the general population. The second important drawback of our study was that it was a retrospective analysis and the scientific yield of a prospective randomised clinical trial would definitely have been much higher than a retrospective one. Further studies would be warranted to determine if IFN α-2b should be advocated as the main first-line therapy in VKC or whether it should be reserved for severe, recalcitrant and steroid-resistant VKC cases. It is expected that future research would be directed towards newer and better topical steroid-sparing agents that would be safer even in long-term use.
Conclusion
IFN α-2b leads to greater reduction in both subjective symptoms TSSS as well as objective signs TOSS than tacrolimus at 6 months which is statistically significant. This improvement, both subjective and objective, persisted even until the end of follow-up period at 24 months.
Our study suggests that while both IFN α-2b eye drops 1 millionIU/mL and tacrolimus eye ointment 0.03% are both safe and effective steroid-sparing agents in steroid-resistant VKC, IFN α-2b eye drops result in greater improvement in subjective symptoms and objective signs, have fewer side effects in long term and are better tolerated as compared to tacrolimus eye ointment.
Patient and Public Statement
Patients or the public WERE NOT involved in the design, or conduct, or reporting, or dissemination plans of our research.
Ethical Clearance
Obtained from Ethical Clearance Committee, Institution Review Board. IEC.IRB/VMMC/SJH/10/2019-17.
Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from parents/guardians all individual participants included in the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: a severe allergic eye disease with remodelling changes. Pediatr Allergy Immunol. 2014;25:314–322. doi:10.1111/pai.12197
2. Leonardi A, Busca F, Moterle L, et al. Case series of 406 vernal keratoconjunctivitis patients: a demographic and epidemiological study. Acta Ophthalmol Scand. 2006;84:406–410. doi:10.1111/j.1600-0420.2005.00622.x
3. Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited; a case series of 195 patients with long term follow-up. Ophthalmology. 2000;107(6):1157–1163. doi:10.1016/S0161-6420(00)00092-0
4. Pucci N, Novembre E, Lombardi E, et al. Atopy and serum eosinophil cationic protein in 110 white children with vernal keratoconjunctivitis: differences between tarsal and limbal forms. Clin Exp Allergy. 2003;33(3):325–330. doi:10.1046/j.1365-2222.2003.01538.x
5. Lambiase A, Minchiotti S, Leonardi A, et al. Prospective, multicenter demographic and epidemiological study on vernal keratoconjunctivitis: a glimpse of ocular surface in Italian population. Ophthalmic Epidemiol. 2009;16(1):38–41. doi:10.1080/09286580802573177
6. Bremond-Gignac D, Donadieu J, Leonardi A, et al. Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. 2008;92(8):1097–1102. doi:10.1136/bjo.2007.117812
7. Clark AF. Basic sciences in clinical glaucoma: steroids, ocular hypertension, and glaucoma. J Glaucoma. 1995;4(5):354–369. doi:10.1097/00061198-199510000-00010
8. Bielory L. Allergic and immunologic disorders of the eye. Part II: ocular allergy. J Allergy Clin Immunol. 2000;106(6):1019–1032. doi:10.1067/mai.2000.111238
9. Sacchetti M, Baiardini I, Lambiase A, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. Am J Ophthalmol. 2007;144(4):557–563. doi:10.1016/j.ajo.2007.06.028
10. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporine A and FK 506. Immunol Today. 1992;13:136–142. doi:10.1016/0167-5699(92)90111-J
11. Sakuma S, Higashi Y, Sato N, et al. Tacrolimus suppressed the production of cytokines involved in atopic dermatites by direct stimulation of human PBMC system. (Comparison with steroids). Int Immunopharmacol. 2001;1:1219–1226. doi:10.1016/S1567-5769(01)00059-5
12. Mackensen F, Max R, Becker MD. IFN therapy for ocular disease. Curr Opin Ophthalmol. 2006;17:567–573. doi:10.1097/ICU.0b013e328010ab35
13. Bleik JH, Tabbara KF. Topical cyclosporine in vernal keratoconjunctivitis. Ophthalmology. 1991;98(11):1679–1684. doi:10.1016/S0161-6420(91)32069-4
14. Muller GG, Jose NK, de Castro RS. Topical tacrolimus 0.03% as sole therapy in vernal keratoconjunctivitis: a randomized double-masked study. Eye Contact Lens. 2014;40(2):79–83. doi:10.1097/ICL.0000000000000001
15. Bonini S. Atopic keratoconjunctivitis. Allergy. 2004;59(Suppl.78):71–73. doi:10.1111/j.1398-9995.2004.00570.x
16. Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013;13(3):308–314. doi:10.1007/s11882-013-0345-0
17. Pucci N, Caputo R, Di Grande L, et al. Tacrolimus vs. cyclosporine eyedrops in severe cyclosporine-resistant vernal keratoconjunctivitis: a randomized, comparative, double-blind, crossover study. Pediatr Allergy Immunol. 2015;26(3):256–261. doi:10.1111/pai.12360
18. Fiorentini S, Khurram D. Therapeutic effects of topical 0.03% tacrolimus ointment in children with refractory vernal keratoconjunctivitis in Middle East. Saudi J Ophthalmol. 2019;33:117–120. doi:10.1016/j.sjopt.2019.04.001
19. Turan-Vural E, Acar BT, Acar S. The efficacy of topical IFN α 2b treatment in refractory vernal keratoconjunctivitis. Ocul Immunol Inflamm. 2012;20:125–129. doi:10.3109/09273948.2012.656877
20. Zanjani H, Aminifard M, Ghafourian A, et al. Comparative evaluation of tacrolimus versus IFN Α-2b eye drops in the treatment of vernal keratoconjunctivitis. Cornea. 2017;36:675–678. doi:10.1097/ICO.0000000000001200
21. Gupta PC, Ram J. Comment: comparative evaluation of tacrolimus versus interferon alpha-2b eye drops in the treatment of vernal keratoconjunctivitis: a Randomized, Double-Masked Study. Cornea. 2018;37:e1.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
