Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Is Breast Conserving Surgery Efficacious in Breast Cancer Patients with BRCA1 or BRCA2 Germline Mutation?
Authors Emiroglu S , Özkurt E , Cabıoglu N, Igci A, Saip P, Yazici H, Ozmen T, Ozmen V, Muslumanoglu M, Tukenmez M
Received 18 November 2022
Accepted for publication 8 February 2023
Published 21 February 2023 Volume 2023:15 Pages 163—173
DOI https://doi.org/10.2147/BCTT.S395054
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Selman Emiroglu,1 Enver Özkurt,2 Neslihan Cabıoglu,1 Abdullah Igci,3 Pinar Saip,4 Hulya Yazici,4 Tolga Ozmen,5 Vahit Ozmen,1 Mahmut Muslumanoglu,1 Mustafa Tukenmez1
1Breast Surgery Unit, Department of General Surgery, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey; 2Department of Surgery, Ozel Basari Hospital, Istanbul, Turkey; 3Department of Surgery, American Hospital, Istanbul, Turkey; 4Department of Medical Oncology, Institute of Oncology, Istanbul University, Istanbul, Turkey; 5Division of Gastrointestinal and Oncologic Surgery, Harvard Medical School, Massachusetts General Hospital, Boston, MA, USA
Correspondence: Selman Emiroglu, Breast Surgery Service, Department of General Surgery, Istanbul Faculty of Medicine, Istanbul University, Fatih, Istanbul, 34390, Turkey, Email [email protected]
Background: The optimal surgical therapy for newly diagnosed breast cancer with germline mutations in susceptibility genes is still uncertain for many physicians. In this study, we aimed to determine the efficacy of breast conserving surgery (BCS) in breast cancer patients with BRCA1 or BRCA2 mutation by assessing its outcomes and locoregional recurrence (LR) rates.
Materials and Methods: Seventy-five patients operated with BCS or mastectomy for breast cancer between 2006 and 2017 and had BRCA1 or BRCA2 mutation were included in the study. Effects of the performed breast surgery and clinicopathological characteristics on surgical outcomes, LR rates and survival were analyzed with showing the distribution of BRCA1 and BRCA2 germline mutations.
Results: The median age of the patients was 42 years (20– 77). BRCA1 mutations were found in 46 (61.3%) patients and BRCA2 mutations in 29 (38.7%) patients. Compared to BRCA2 carriers, BRCA1 carriers were more likely to have higher tumor grade (84.8% vs 44.8%; p = 0.001) and non-luminal subtype tumors (67.4% vs 13.8%; p = 0.001). A total of 44 (58.7%) patients underwent unilateral mastectomy and 31 (41.3%) patients underwent BCS. At a median follow-up time of 60 (12– 240) months, LR was observed in 6 patients equally divided in both BCS and mastectomy groups. LR rates were slightly higher after BCS versus mastectomy (9.7% and 6.8%, respectively). Additionally, there were no statistically significant differences in disease-free survival (DFS) and disease-specific survival (DSS) rates after 10 years in the BCS group versus the mastectomy group (p = 0.117 and 0.109, respectively), but in fact, the rates were better in the BCS group.
Conclusion: Our findings indicate that BCS may serve as an efficacious alternative to mastectomy for breast cancer patients with BRCA1 or BRCA2 mutation. Additionally, tumor size, lymph node positivity, and TNM stage should be taken into consideration for a better surgical decision-making.
Keywords: BRCA1 or BRCA2 germline mutation, breast conserving surgery, surgical decision, locoregional recurrence
Introduction
Breast cancer (BC) is the most prevalent cancer among women worldwide.1 Approximately, 5–10% of BC cases are hereditary.2 BRCA1 (Breast Cancer 1 gene) and BRCA2 (Breast Cancer 2 gene) are malignancy associated tumor suppressor genes that account for 80% of the highly penetrant inherited BC cases.3 BRCA mutations are associated with hereditary breast and ovarian cancer syndrome. Researchers have reported that BRCA mutation carriers have a life-time risk of BC up to 69–72%, and they have a 10 to 30 times higher risk of developing ovarian cancer compared to the normal population.4
The integration of genetics tests into the care of cancer patients leads the physicians to use the results of germline mutations in breast cancer susceptibility genes for making a better treatment decision.5 Several studies identified that early-stage BC patients who were treated with BCS at a young age had an increased likelihood of ipsilateral breast tumor recurrence after BCS that followed by radiotherapy (BCT).6,7 Family history of BC, genetic predisposition for BC (ie BRCA1 and BRCA2 mutations) or other risk factors are more likely to be in the young BC patients, confounding the role of age and treatment in the clinical outcomes.8 As reported in national comprehensive cancer network clinical practice guidelines for breast cancer, version 3.2022, overall survival (OS) outcomes of BCT or mastectomy for young BC patients are similar and some studies showed improved survival and lesser post-surgical complications with BCS.7
Choosing the appropriate surgical approach for BC patient with BRCA mutation requires consideration of several questions; what is the efficacy of BCS versus mastectomy? Is BCS associated with a higher risk of locoregional recurrence (LR)? What is the risk of contralateral cancer? Is contralateral prophylactic mastectomy beneficial for survival?
This study aimed to determine the efficacy of BCS in BC patients with BRCA1 or BRCA2 mutation by assessing its outcomes and LR rates and to provide answers to the questions asked above. Thereby allowing better surgical decision-making.
Materials and Methods
Patient Selection and Ethical Approval
Between 2006 and December 2017, 5750 patients were diagnosed with BC at Istanbul University’s Istanbul Faculty of Medicine, General Surgery, Division of Breast Surgery, and BRCA1 and BRCA2 genetic tests were performed on 450 patients. In this study, the demographic and clinicopathological data of 75 patients were analyzed. This study has been approved by Istanbul University’s Istanbul Faculty of Medicine (2022/1948). All patients were informed about the study’s purpose, content, and intervention, and their oral and written consent was obtained.
All patients were BRCA1 and/or BRCA2 mutation carriers with small insertion/deletion mutations or rearrangements in BRCA1 and BRCA2. The genetic test results were abstracted from electronic medical records. Genetic tests were performed at the Cancer Genetics Department at Istanbul University’s Oncology Institute. BRCA1 and BRCA2 genes were screened for mutations in fragments between 197 and 823 bp length for Sanger Sequencing and about 450 bp length for NGS using a Multiplicome BRCA MASTR Dx Kit, which has a CE-IVD certificate in the MiSeq Illumina Platform. Rearrangements in BRCA1 and BRCA2 were evaluated by using both the MiSeq NGS platform and multiplex ligation-dependent probe amplification (MLPA) analysis.
Patients Evaluation and Data Collection
Patients were evaluated for demographic characteristics, surgery type, clinicopathological characteristics (surgical margin status, stage, molecular subtype, hormone receptor status, HER2/neu status, etc.), adjuvant and neoadjuvant treatments (chemotherapy, radiotherapy, and hormonal therapy), follow-up time, OS, disease-free survival (DFS) and disease-specific survival (DSS). Patients were followed-up closely, and physical examination findings were recorded at each visit. Dates of death and causes of death were recorded in accordance with the data received from the hospital records and patients’ relatives. LR is defined as a recurrence in the ipsilateral breast/chest wall or regional nodal basin, contrary to the distant site, whereas, local recurrence is a recurrence in the breast.
Each case of BC with BRCA mutation was re-reviewed by a dedicated breast pathologist at our institution to confirm the histologic diagnosis. Estrogen receptor (ER), progesterone receptor (PR), HER2/neu and Ki-67 positivity were assessed using immunohistochemistry (IHC). The histologic classification was based on WHO criteria and histologic grade in the Nottingham system. ER and PR were considered positive if ≥1% cells showed nuclear staining. Cases were considered HER2/neu- positive when they are IHC-3+ or SISH (Silver in situ hybridization)-amplified. The staging was made according to the American Joint Committee on Cancer (7th edition).9
Clear margins were required for BCS; a frozen section diagnosis was performed to judge whether the margins were clear. Patients who underwent BCS and mastectomy and have a large tumor (ie, >5 cm) and/or 4 positive lymph nodes are referred for radiotherapy, whereas patients with 1 to 3 positive lymph nodes may receive radiotherapy if they have other high-risk factors. All patients have been discussed in the multidisciplinary meetings by surgeons, radiologists, pathologists, genetic counselors, radiation and medical oncologists. Based on the decision in the multidisciplinary meetings, patients may receive neoadjuvant or adjuvant chemotherapy or endocrine therapy depending on tumor clinicopathologic characteristics and clinical stage.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) program (version 25.0, IBM Corp., Armonk, NY, USA). Descriptive statistical methods (eg, number, percentage, median) were also used. Kaplan–Meier analysis was used to calculate survival rates. In two-tailed univariate analyses, each parameter was tested using Fisher’s exact test or the chi-square test to assess the associations of documented variables between BRCA1 and BRCA2 carriers depending on the surgical technique. OS was not analyzed because there were no deaths due to non-cancer causes. The effects of various prognostic factors related to tumors and patient characteristics on DFS and DSS were investigated using the Log rank test. In all statistical analyses, a p-value of 0.05 was considered significant.
Results
Patients and Tumors General Characteristics
The median age of the patients was 42 years (20–77 y). There was no statistically significant difference in the age distribution of the patients based on BRCA1 or BRCA2 mutation (p = 0.443). Most patients had invasive ductal carcinoma (73.3%), tumor stage II and III (78.7%) and grade III tumors (69.3%). Moreover, 26.7% of patients had multicentric tumors and 45.3% had LVI. Axillary positivity was present in 49.3% of the patients. BRCA1 patients group had a significantly higher rate of grade III tumors (84.8% vs 44.8%; p=0.001) (Table 1).
 |
Table 1 Demographics and Clinicopathologic Characteristics of BRCA1 and BRCA2 Mutation Carriers |
Thirty-five (46.7%) patients had no family history of BC, 14 (18.7%) patients had a first-degree family history and 26 (34.7%) patients had second- or third-degree family history. BRCA2 carriers were more likely to have a family history of gastrointestinal tract (GIT) cancer (p = 0.041) (Table 1).
Hormone Receptor Status
According to the molecular subtype classification, 41.3% of the tumors were luminal A-B, 12% were luminal-HER2 positive, 22.7% were non-luminal HER2 positive and 24% were triple negative. BRCA2 mutation carriers were more likely than BRCA1 mutation carriers to have hormone receptor positivity and luminal molecular subtypes (86.2% vs 32.6%, respectively; p<0.001) (Table 1).
Surgical Treatment Strategy in Patients
As it shown in Figure 1, 31 patients had BCS (41.3%) and 44 (58.7%) patients underwent unilateral mastectomy. Of the BRCA1 mutation carriers, 43.5% had BCS and 56.5% had unilateral mastectomy, whereas 37.9% of BRCA2 mutation carriers had BCS and 62.1% had unilateral mastectomy (p=0.977). Contralateral prophylactic mastectomy was performed in 27.3% (12/44) of the patients who underwent mastectomy, 6 patients had BRCA1 mutation and 6 patients had BRCA2 mutation (p=0.684). Bilateral salpingo-oophorectomy (BSO) ± total abdominal hysterectomy (TAH) was performed in 18.7% (14/75) of all patients, 9 patients had BRCA1 mutation and 5 patients had BRCA2 mutation (p=0.999) (Table 1).
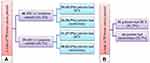 |
Figure 1 (A) All patients’ distribution according to BRCA1 or BRCA2 mutation carrying and surgical treatment strategy. (B) All patients’ distribution according to the surgical treatment strategy. |
Patient’s Follow-Up, OS and Recurrences
At a median follow-up period of 60 (12–240) months. The overall mortality rate was 9.3% (n = 7) and all the seven patients died from cancer causes. LR was observed in 6 patients, 2 had BRCA1 mutation and 4 had BRCA2 mutation. Three LRs were observed in each BCS and mastectomy groups. Five patients were under the age of 50 years, and one was over. In addition, 5 patients had grade III tumors. None of the 6 patients underwent prophylactic TAH-BSO. Four patients received adjuvant chemotherapy, while 2 received neoadjuvant chemotherapy. In both BRCA1 and BRCA2 mutation carriers, there were no statistically significant differences in loco-regional, distant, or contralateral recurrence with respect to several clinicopathological features (p>0.05) (Table 2).
 |
Table 2 Comparison of the Locoregional, Distant and Contralateral Recurrence and Several Clinicopathological Features in BRCA1 and BRCA2 Positive Breast Cancer Patients |
The 6 LR that were observed were as follows: 3 local recurrences (only in the breast), 1 thoracic (pectoral muscle) recurrence and 2 axillary recurrences. In the BCS group, LR was detected in 2 triple negative patients. Systemic metastasis was observed in one patient with a triple negative molecular subtype. Of the 3 LRs seen in the BCS group, 2 were detected within the first 10 years and one was detected after 10 years. Although the surgical margins of 3 patients were negative with local recurrence after BCS, the closest surgical margin in one patient was less than 1 mm and one patient was multicentric (Table 3). Moreover, in the 6 cases with LRs in, there was no significant difference between BCS and mastectomy in terms of recurrence site, LR interval, systemic metastasis rate or systemic metastasis interval.
 |
Table 3 Characteristics of Cases with Loco-Regional Recurrence in BRCA1 and BRCA2 Mutation Carriers |
Patient’s DFS and DSS
However, LR rates were slightly higher in BRCA1 and BRCA2 mutation carriers who underwent BCS than in those who underwent mastectomy (9.7% and 6.8%, respectively). Moreover, 10-year DFS and DSS rates between the BCS and mastectomy groups were closer and the rates were slightly better in the BCS group. In univariate analysis, patients with tumor grade I or II and luminal subtypes had improved DSS rates (p = 0.030 and 0.044, respectively), whereas patients with unicentric tumors had high DFS rates (p = 0.045). Additionally, contralateral prophylactic mastectomy did not improve DFS or DSS compared to BCS (p>0.05) (Table 4).
 |
Table 4 Possible Factors Affecting Disease-Free and Disease-Specific Survival (Univariate Analysis) |
Discussion
In this study, we showed that the prognostic impact of BCS and mastectomy in first primary BC on DFS and DSS was similar within both BRCA1 and BRCA2 mutation carriers. Moreover, there was no difference in terms of recurrence site, LR interval, systemic metastasis rate or systemic metastasis interval. Our study is the first to evaluate the effect of BCS and mastectomy directly both in BRCA1 and BRCA2 mutation carriers in the Turkish population.
Our results agree with American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology guidelines as they recommended that newly diagnosed BC patients with BRCA1 or BRCA2 mutations may be considered for BCT, with local control of the index cancer similar to that of mutation non-carriers.10 Moreover, the surgical approach for high-risk BC patients should consider many factors, including the patient’s age, tumor biology, breast size, genetic risk, oncological history, family history, comorbidities, life expectancy, and the ability to undergo appropriate follow-up.11 In another study done by Huang et al, they showed that BCT may be a safe surgical option for Chinese female BC patients with BRCA1 and/or BRCA2 mutation with taking in consideration tumor size, stage, the number of positive lymph nodes.5 Furthermore, a study by Magnoni et al reported that, during multidisciplinary discussion, in addition to taking recent international guidelines and the patient’s preferences into account, surgical treatment should be personalized based on BC clinicopathological and genetic features.12 Taken together, BCS may serve as an effective rational surgical choice in BRCA1 or BRCA2 mutation carriers as well as tumor size, lymph node positivity, TNM stage, should be taken into consideration during the surgical decision-making.
Numerous retrospective studies have focused on local control after BCS in BRCA1 and/or BRCA2 mutation carriers.13–15 In these studies, with a limited follow-up period, BCS did not increase the risk of local recurrence in mutation carriers compared with non-carriers. Other studies with longer follow-up periods reported that local recurrence was increased in mutation carriers by approximately 10% at 10 years and 15% at 15 years.16,17 Co et al compared the inferior survival outcomes and local recurrence rates of BCS and mastectomy in BRCA mutation carriers across 18 studies. They concluded that BCS should be recommended for patients with breast cancer with BRCA mutations.18 In meta-analysis study, Wang et al19 found that BCS was associated with a significantly higher risk of local recurrence than mastectomy, but no significant effect of BCS on OS, DFS, DSS, or metastasis-free survival was observed. These results agree with ours as BCS may serve as a safe alternative to mastectomy for BRCA mutation carriers BC patients. In addition, mastectomy will be recommended for larger more advanced tumors.
Davey et al20 compared the safety of BCS and mastectomy in patients with BC with BRCA mutations in 23 studies. DFS and DSS after 5-years, 10-years or 15-years were equivalent in the BCS and mastectomy groups. Bernstein-Molho et al21 studied 255 BC patients with BRCA1 and BRCA2 mutations over a median of 57.7 months. There was no significant difference in the OS. Patients who underwent BCS had higher rates of ipsilateral breast tumor recurrence than those who underwent mastectomy with post-mastectomy radiotherapy. Furthermore, Nilsson et al22 reported that patients who underwent BCS have a higher risk of local recurrence. However, no significant differences in OS, BC death, or distant recurrence were observed between BCS and mastectomy in BRCA mutation carriers. In addition, van den Broek et al23 reported that BRCA mutation carriers who underwent BCS had a similar OS compared to those who underwent mastectomy.
According to our findings, contralateral prophylactic mastectomy did not improve DFS or DSS compared to BCS. In a recent study by Makhnoon et al, they found that no evidence of contralateral prophylactic mastectomy-mediated improvement in OS among women with pathogenic variants in BRCA1 and BRCA2 and the improvement in OS could be explained by the decrease of contralateral breast cancer risk and cancer mortality.24 This result also supported the findings of Fayanju et al.25 Also, Makhnoon et al observed a racial/ethnic difference in the 20-year OS in BC patients who underwent contralateral prophylactic mastectomy.24 In a study by Metcalfe et al, they demonstrated that women with BRCA mutations and treated for stage I or II BC with bilateral mastectomy are less likely to die due to BC than women who are treated with unilateral mastectomy.26
All patients in this study were diagnosed with BC and underwent BCS or mastectomy along with other appropriate treatment protocols. At the same time, BRCA1 and BRCA2 mutation tests were requested. BRCA test results were not available and unnecessary at this stage for the choice of surgical management. This situation is favorable to these patients because patients are more likely to consider mastectomy if they know they have a mutation.
Conclusions
Our findings indicate that BCS may serve as an efficacious rational surgical choice, alternative to mastectomy for BC patients with BRCA1 or BRCA2 mutation. Tumor size, lymph node positivity, and TNM stage should be taken into consideration for a better surgical decision-making.
Data Sharing Statement
The data sets of the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to express our special appreciation and thank to Ph.D. Candidate Asmaa Mahmoud Abuaisha for her help in data analysis and interpretation and writing this manuscript. Also, we would like to thank M.Sc. Atilla Bozdogan for his meticulous statistical analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6(1):R8–R17. doi:10.1186/bcr658
3. Hall JM, Lee MK, Newman B, et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990;250(4988):1684–1689. doi:10.1126/science.2270482
4. Schwartz GF, Veronesi U, Clough KB, et al. Consensus conference on breast conservation. J Am Coll Surg. 2006;203(2):198–207. doi:10.1016/j.jamcollsurg.2006.04.009
5. Huang X, Cai XY, Liu JQ, et al. Breast-conserving therapy is safe both within BRCA1/2 mutation carriers and noncarriers with breast cancer in the Chinese population. Gland Surg. 2020;9(3):775–787. doi:10.21037/gs-20-531
6. Komoike Y, Akiyama F, Iino Y, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006;106(1):35–41. doi:10.1002/cncr.21551
7. Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. doi:10.6004/jnccn.2022.0030
8. Golshan M, Miron A, Nixon AJ, et al. The prevalence of germline BRCA1 and BRCA2 mutations in young women with breast cancer undergoing breast-conservation therapy. Am J Surg. 2006;192(1):58–62. doi:10.1016/j.amjsurg.2005.12.005
9. Krammer J, Pinker-Domenig K, Robson ME, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2017;163(3):565–571. doi:10.1007/s10549-017-4198-4
10. Tung NM, Boughey JC, Pierce LJ, et al. Management of hereditary breast cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol. 2020;38(18):2080–2106. doi:10.1200/JCO.20.00299
11. Corso G, Magnoni F. Hereditary breast cancer: translation into clinical practice of recent American Society of Clinical Oncology, American Society of Radiation Oncology, and Society of Surgical Oncology recommendations. Eur J Cancer Prev. 2021;30(4):311–314. doi:10.1097/CEJ.0000000000000624
12. Magnoni F, Sacchini V, Veronesi P, et al. Surgical management of inherited breast cancer: role of breast-conserving surgery. Cancers. 2022;14(13):3245. doi:10.3390/cancers14133245
13. Pierce LJ, Strawderman M, Narod SA, et al. Effect of radiotherapy after breast-conserving treatment in women with breast cancer and germline BRCA1/2 mutations. J Clin Oncol. 2000;18(19):3360–3369. doi:10.1200/JCO.2000.18.19.3360
14. Seynaeve C, Verhoog LC, van de Bosch LM, et al. Ipsilateral breast tumour recurrence in hereditary breast cancer following breast-conserving therapy. Eur J Cancer. 2004;40(8):1150–1158. doi:10.1016/j.ejca.2004.01.017
15. Verhoog LC, Brekelmans CT, Seynaeve C, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998;351(9099):316–321. doi:10.1016/s0140-6736(97)07065-7
16. Fowble B. Ipsilateral breast tumor recurrence following breast-conserving surgery for early-stage invasive cancer. Acta Oncol. 1999;38(Suppl 13):9–17. doi:10.1080/028418699432716
17. Kreike B, Hart AA, van de Velde T, et al. Continuing risk of ipsilateral breast relapse after breast-conserving therapy at long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;71(4):1014–1021. doi:10.1016/j.ijrobp.2007.11.029
18. Co M, Liu T, Leung J, et al. Breast conserving surgery for BRCA mutation carriers-a systematic review. Clin Breast Cancer. 2020;20(3):e244–e250. doi:10.1016/j.clbc.2019.07.014
19. Wang C, Lin Y, Zhu H, et al. Breast-conserving therapy for breast cancer with BRCA mutations: a meta-analysis. Breast Cancer. 2022;29(2):314–323. doi:10.1007/s12282-021-01312-2
20. Davey MG, Davey CM, Ryan ÉJ, Lowery AJ, Kerin MJ. Combined breast conservation therapy versus mastectomy for BRCA mutation carriers - A systematic review and meta-analysis. Breast. 2021;56:26–34. doi:10.1016/j.breast.2021.02.001
21. Bernstein-Molho R, Laitman Y, Galper S, et al. Locoregional treatments and ipsilateral breast cancer recurrence rates in BRCA1/2 mutation carriers. Int J Radiat Oncol Biol Phys. 2021;109(5):1332–1340. doi:10.1016/j.ijrobp.2020.11.058
22. Nilsson MP, Hartman L, Kristoffersson U, et al. High risk of in-breast tumor recurrence after BRCA1/2-associated breast cancer. Breast Cancer Res Treat. 2014;147(3):571–578. doi:10.1007/s10549-014-3115-3
23. van den Broek AJ, Schmidt MK, van ‘t Veer LJ, et al. Prognostic impact of breast-conserving therapy versus mastectomy of BRCA1/2 mutation carriers compared with noncarriers in a consecutive series of young breast cancer patients. Ann Surg. 2019;270(2):364–372. doi:10.1097/SLA.0000000000002804
24. Makhnoon S, Gutierrez Barrera AM, Bassett R, Afrough A, Bedrosian I, Arun BK. Contralateral prophylactic mastectomy among women with pathogenic variants in BRCA1/2: overall survival, racial, and ethnic differences. Breast J. 2022;2022:1447545. doi:10.1155/2022/1447545
25. Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg. 2014;260(6):1000–1010. doi:10.1097/SLA.0000000000000769
26. Metcalfe K, Gershman S, Ghadirian P, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. 2014;348:g226. doi:10.1136/bmj.g226
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
