Back to Journals » International Medical Case Reports Journal » Volume 14
Iron Deficiency Anemia Refractory to Conventional Therapy but Responsive to Feralgine® in a Young Woman with Celiac Disease
Authors Talarico V , Giancotti L, Miniero R , Bertini M
Received 22 November 2020
Accepted for publication 14 January 2021
Published 16 February 2021 Volume 2021:14 Pages 89—93
DOI https://doi.org/10.2147/IMCRJ.S291599
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Valentina Talarico,1 Laura Giancotti,2 Roberto Miniero,1 Marco Bertini3
1Department of Pediatric, Pugliese-Ciaccio Hospital, Catanzaro, Italy; 2Unit of Pediatrics, University “Magna Graecia”, Catanzaro, Italy; 3R&D Department, Laboratori Baldacci SpA, Pisa, Italy
Correspondence: Valentina Talarico
Department of Pediatrics, Pugliese-Ciaccio Hospital, Viale Pio X, Catanzaro, 88100, Italy
Tel +3402457848
Email [email protected]
Abstract: Iron, which is an important micronutrient in the human body may be deficient in people with celiac disease (CD). Iron deficiency anemia (IDA) may be the presenting feature of celiac disease, also in the absence of diarrhea or weight loss. The treatment of IDA in patient with CD is primarily a gluten-free-diet (GFD), but it is also very important oral iron supplementation until the iron stores have been restored. However, a frequent problem in CD is the poor tolerability and poor efficacy of oral iron preparations. A new product, consisting of the combination of Ferrous Bysglicinate Chelate and Sodium Alginate (Feralgine™), has been demonstrated to be more bioavailable and well tolerated in CD. We present a case report that showed a clear efficacy of this product in a form of IDA refractory to conventional therapy in a woman with CD and we demonstrated a clear increase of serum iron after administration of this new type of ferrous.
Keywords: celiac disease, iron deficiency anemia, ferrous bisglycinate chelate, sodium alginate, OIAT
Introduction
Celiac disease (CD) is an immunologically mediated enteropathy of small bowel triggered in genetically susceptible persons by the ingestion of gluten and related proteins. In Europe and in the United States the prevalence is around 0.3–2.4% in the general population.1 Patients with CD may have various gastrointestinal and/or non-gastrointestinal signs and symptoms, some of which are not specific.1 In CD is usually observed iron malabsorption, because this element is absorbed in the duodenum and in the jejunum. Iron deficiency anemia (IDA) is a frequent finding in patients with overt CD (10–20% of cases). It represents the most common extra-intestinal symptoms of CD while in some patients it may be the only abnormality identified. Conversely, in general population patients presenting with IDA, CD is responsible for the anemia in 5% to 6% of the cases.1–5
Ferrous sulfate (FS) remains the gold standard treatment for oral supplementation in IDA but the treatment is limited by the frequent gastrointestinal side effects, mainly due to the irritation and the chemical reactions with unabsorbed iron compounds.3 FS generally results in poor effectiveness in patients with undiagnosed CD leading to a form of refractory IDA;1–5 furthermore, the effectiveness may be reduced also during the first months of gluten-free diet (GFD) when the mucosa lesions improving is ongoing and this makes CD patients prone to continue to be iron deficient.
The option of a new kind of oral iron replacement treatment well tolerated and well absorbed consists in a particular challenge for patients with bowel diseases giving them the possibility of avoiding parenteral therapy.3,4 Iron amino acid chelates showed a good absorption and tolerability. Many clinical trials demonstrated clinical bioequivalence between FS and Ferrous Bysglicinate Chelate (FBC), although administrated at different iron dosage (ratio 4 to 1respectively).6 Non-heme iron is transported across the apical duodenal membrane by divalent metal transporter 1 (DMT1).3 Iron as FBC has shown to be dissolved more slowly and constantly than iron as FS, justifying a less DMT1 saturation with the consequence of an increase in iron bioavailability.8 Recently, in order to improve the bioavailability a new preparation of FBC has been developed: Ferrous Bysglicinate Chelate Alginate (FBCA). This is a patented co-processed 1 to 1 ratio compound between FBC and Sodium Alginate by applying Spray-Drying technologies.6,7 This compound (named Feralgine®) may represent product with a very interesting and attractive profile by improving metabolism and taste of FBC.7 Furthermore, as FBC, FBCA results are also well tolerated and well absorbed in IDA patients with overt CD.9
Here, we report the case of a young woman with IDA resistant to FS therapy due to underlying CD who responded promptly to FBCA.
Case Report
A 22-year-old girl was evaluated at the Regional Center for Diagnosis and Follow-up of the CD of Pediatric Unit of “Magna Graecia” University of Catanzaro because of microcytic anemia unresponsive to oral treatment with FS (60 mg/day). The family medical history was not contributory. The patient’s medical history was notable for non-specific gastrointestinal symptoms such as recurrent abdominal pain and, sometimes, diarrhea, accompanied by mild weakness, that were present at admission to our Unit. The physical examination showed a pale patient with moderate abdominal distension. BMI was normal. The laboratory parameters showed a moderate microcytic hypochromic anemia with a hemoglobin level of 9.1 g/dl, a normal leukocyte count and moderate increase of platelet count (750,000 mmc). Biochemical blood parameters confirmed iron depletion: low levels of iron (35 µg/dL), high Total Iron Binding Capacity (440 μg/dL) and low levels of ferritin (5 ng/mL). As the amount FS administrated the last year considered adequate but ineffective, the patient was considered as a case of IDA resistant to treatment. According to the recent guidelines the possibility of an underlying CD was firstly evaluated.1–3 Positive serology was then detected: IgA endomysial antibody level was 1/320 and the titer of IgA-anti-tissue transglutaminase (IgAtTG) was over 250 U/mL (normal value <15 U/mL). Multiple duodenal biopsies were performed. Histological examination revealed moderate to severe villous atrophy with lamina propria infiltration with chronic mononuclear cells. Intraepithelial cells were increased in number. The final diagnosis was CD (Marsh Oberhuber grade 3c). A strictly GFD was then prescribed. In order to evaluate the absorption of an other than FS iron compound, we performed an oral iron absorption test (OIAT) with FBCA at the dosage of 30 mg of elemental iron. The results confirmed a good iron absorption as the serum iron was 27 µg/dl at baseline (T0) and increased to 93.2 µg/dl after the two hours (T1) (Figure 1). Then, we started treatment with FBCA at the dosage of 30 mg/day. A general status recovery was observed in few days. After 3 months Hb reached 11.5 g/dl; serological IgA endomysial antibody level lowered to 1/160, the titer of IgAtTG lowered to 100 (Table 1). After 6 months iron stores were replaced (ferritin 50 ng/dl). Serology for CD normalized at one year, with complete resolution of gastrointestinal symptoms.
 |
Figure 1 Test OIAT in our patient. |
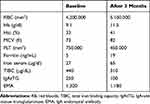 |
Table 1 Laboratory Values at the Beginning and After Three Months of Therapy |
Discussion
In general population, patients with IDA respond promptly to oral FS replacement and when treatment is fully effective hemoglobin reaches normal levels by 2–3 months after therapy has started.3 However, some subjects fail to respond or respond partially to treatment and are considered as cases of refractory IDA. In these patients the possibility of an underlying form of CD must be investigated, as the refractoriness to oral iron supplements is characteristic of IDA in CD.1–5 Our case matched with this scenario having a one-year history of mild IDA treated unsuccessfully with FS while a prompt work-up confirmed the presence of a previously undiagnosed CD.
Treatment of IDA in CD consists of two principal activities. The first one is a GFD program, but it is well known that it takes place 6–12 months to normalize duodenal surface and let full iron absorption. The second one is to supply oral iron. Depending iron absorption on months necessary for the correction of intestinal mucosa by a good adherence to GFD, correction of IDA necessary result a long time consuming process.1,2,4,5
According to the common good clinical practice as soon as CD diagnosis was obtained, our patients started a GFD.1 Considering our previous results with FCBA in CD patients we programmed to prescribe this preparation.9
The response to oral iron therapy may be evaluated by reticulocyte count that is increased within 5 to 10 days. However, in order to avoid repeated blood sampling, currently the efficacy of the treatment is evaluated at least one month after iron therapy by measuring hemoglobin levels. The possibility to evaluate iron absorption after administration of a single dose might be helpful in programming iron treatment, especially in patients with CD. Interesting, measurement of iron absorption has been rekindled by reports that absorption of a single dose of nonradioactive iron may be evaluated by essay of the plasma iron concentration in the next 2–4 hours after the administration (OIAT). However, despite this procedure is easy to perform and provides clear information on the benefit of oral iron replacement, it is not routinely performed in the work-up of IDA.10–12
Using OIAT we have previously first evidenced that FBC is well absorbed and tolerated in children with overt CD as well as in those in GFD.12 Furthermore, our study showed that most celiac children who received FBC supplementation had full recovery of their iron stores, in association with a strict adherence to a GFD.13
Vernero et al evidenced the FBCA (30 mg/day of elemental iron) effectiveness in IDA patients with inflammatory bowel disease.14 Similar results have been obtained by Ame et al studying 12 patients with IDA.15 A good absorption of FBCA has been reported by Rondinelli et al performing OIAT in 14 adults with IDA (increase of mean serum iron from 11.21±10.66 to 111.00±51.56 µg/dl).15 In a previous study we investigated by OIAT the absorption of iron present in FBCA in 14 adults CD patients with IDA compared to 12 non-CD patients with IDA. In CD patients mean serum iron increased from 28.21 g/dL to 94.14 g/dL while in non-CD patients with IDA mean serum iron increased from 34.91 g/dL to 118.83 g/dL.The results confirmed as FBCA was well absorbed in patients with overt CD before starting GFD as well as in non-CD patients.9 The rate of iron absorption was similar to the ones observed by others studying OIAT with FS in IDA patients.9–11
In our patient FBCA resulted well absorbed in overt CD before GFD as we have previously observed in the other CD patients.9 Subsequently, the anemia was almost entirely corrected after 3 months of treatment with GFD and FBCA, while the iron stores were replaced after other 3 months. This incremental rate is similar to that one expected for non-CD IDA patients with adequate FS replacement but shorter than that expected in most of CD patients in GFD treated with FS.1–5 In our case we confirm that FBCA is well absorbed in patients with overt CD yet during the first months of GFD when intestinal mucosa is not complete repaired.
The mechanism that could explain the good absorption of FBCA in CD remains unclear. Many studies have shown that aminoacid-chelated iron is better absorbed in intestine, compared to inorganic iron. In particular, it has been demonstrated that iron chelated with glycine is better and more quickly absorbed than FS. The intestinal absorption of non-heme iron is mediated by DMT1 that is less expressed in CD as a consequence of the mucosa’s lesions. This event may explain why iron is less absorbed in this disease. Recent studies in pigs showed that FBC increases transcript expression of DMT1 and the PepT1, a heme-iron transporter.16 The author suggested that FBC might be absorbed as heme iron, via the PepT1. Other studies have also shown that FBC is absorbed intact by intestinal cells; this observation underlines the possibility that absorption process of the iron as FBC may occur also independently of the presence of DMT1.17–22 Moreover, latest findings of a constant and slow release of iron from FBCA might explain the high bioavailability of iron, also in CD patients.8
In conclusion, this case report confirms the efficacy of FBCA in patients with overt CD, being well absorbed yet during the first months of GFD. This evidence supports the possibility that this preparation might be considered as a treatment of choice in celiac patients with IDA. Furthermore, our case confirms as the OIAT may be helpful in programming iron treatment in patients with IDA in order to choose the best compound for the patient. Finally, the present case report confirms as screening of CD should be done as a routine investigation in any patient with IDA.
We received written informed consent by the patient for the publication of this manuscript; no institutional consent was required for publication of the case.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest and any financial support.
References
1. Lebwoh B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391(10115):70–81. doi:10.1016/S0140-6736(17)31796-8
2. Mahadev S, Laszkowska M, Sundström J, et al. Prevalence of celiac disease in patients with iron deficiency anemia-a systematic review with meta-analysis. Gastroenterology. 2018;155(2):374–382. doi:10.1053/j.gastro.2018.04.016
3. Camaschella C. Iron deficiency. Blood. 2019;133(1):30–39. doi:10.1182/blood-2018-05-815944
4. Stefanelli G, Viscido A, Longo S, Magistroni Latella G. Persistent iron deficiency anemia in patients with celiac disease despite a gluten-free diet. Nutrients. 2020;12(8):2176–2195. doi:10.3390/nu12082176
5. Freeman HJ. Iron deficiency anemia in celiac disease. World J Gastroenterol. 2015;21(31):9233–9238. doi:10.3748/wjg.v21.i31.9233
6. Gervasi GB, Baldacci M, Bertini M. Feralgine® a new co-processed substance to improve oral iron bioavailability, taste and tolerability in iron deficiency patients. Arch Med. 2016;8(4):13–16.
7. Baldacci M, Gervasi GB, Bertini M. Iron deficiency anemia and iron deficiency: are alginates a good choice to improve oral iron bioavailability and safety? Transl Sci. 2018;4:1–3.
8. Chetoni P et al. Evaluation of the dissolution process from TecnoFER Plus – July 2019 – Data on file Laboratori Baldacci SpA.
9. Giancotti L, Talarico V, Mazza GA, et al. Feralgine™ a new approach for iron deficiency anemia in celiac patients. Nutrients. 2019;11(4):887–893. doi:10.3390/nu11040887
10. Hacibekiroglu T, Akinci S, Basturk AR, et al. A forgotten screening test for iron deficiency anemia: oral iron absorption test. Clin Ter. 2013;164(6):495–497. doi:10.7417/CT.2013.1627
11. Santarpia L, Pagano MC, Cuomo R, Alfonso L, Contaldo F, Pasanisi F. Iron absorption following a single oral dose of ferrous sulfate or ferric gluconate in patients with gastrectomy. Ann Nutr Metab. 2013;63(1–2):55–59. doi:10.1159/000351447
12. Kobune M, Miyanishi K, Takada K, et al. Establishment of a simple test for iron absorption from the gastrointestinal tract. Int J Hematol. 2011;93(6):715–719. doi:10.1007/s12185-011-0878-8
13. Mazza GA, Pedrelli L, Battaglia E, Giancotti L, Miniero R. Oral iron absorption test with ferrous bisglycinate chelate in children with celiac disease. Minerva Pediatr. 2019;71(2):139–143. doi:10.23736/S0026-4946.16.04718-6
14. Vernero M, Boano V, Ribaldone DG, Pellicano R, Astegiano M. Oral iron supplementation with Feralgine® in inflammatory bowel disease: a retrospective observational study. Minerva Gastroenterol Dietol. 2019;65(3):200–203. doi:10.23736/S1121-421X.19.02572-8
15. Ame AC, Campa E. FERALGINE® a new oral iron therapy for iron deficiency anemia: preliminary clinical results on a case series of 12 anemic patients. Res Rev. 2016;3:29–35.
16. Rondinelli MB, Di Bartolomei A, De Rosa A, Pirelli L. Oral Iron Absorption Test (OIAT): a forgotten screening test for iron absorption from the gastrointestinal tract. A casa series of Iron Deficiency Anemia (IDA) patients treated with FERALGINE®. J Blood Disord Med. 2017;2(1).
17. Ferrari P, Nicolini A, Manca ML, et al. Treatment of mild non-chemotherapy-induced iron deficiency anemia in cancer patients: comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomed Pharmacother. 2012;66(6):414–418. doi:10.1016/j.biopha.2012.06.003
18. Barisani D, Parafioriti A, Bardella MT, et al. Adaptive changes of duodenal iron transport proteins in celiac disease. Physiol Genom. 2004;17(3):316–325.
19. Yanatori I, Kishi F. DMT1 and iron transport. Free Radic Biol Med. 2019;133:55–63. doi:10.1016/j.freeradbiomed.2018.07.020
20. Zhuo Z, Yu X, Li S, Fang S, Feng J. Heme and non-heme iron on growth performances, blood parameters, tissue mineral concentration, and intestinal morphology of weanling pigs. Biol Trace Elem Res. 2018;187(2):411–417. doi:10.1007/s12011-018-1385-z
21. Liao ZC, Guan WT, Chen F, et al. Ferrous bisglycinate increased iron transportation through DMT1 and PepT1 in pig intestinal epithelial cells compared with ferrous sulphate. J Anim Feed Sci. 2014;23(2):153–159. doi:10.22358/jafs/65704/2014
22. Yu X, Chen L, Ding H, Zhao Y, Feng J. Iron transport from ferrous bisglycinate and ferrous sulfate in DMT1-knockout human intestinal caco-2 cells. Nutrients. 2019;11(3):485. doi:10.3390/nu11030485
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
