Back to Journals » Journal of Experimental Pharmacology » Volume 14
Investigation of HPLF-111624 in Modified Experimental Models of Ulcerative Proctitis and Anal Fissure in Rats
Authors Onkaramurthy M, Azeemuddin MM , Baig MR, Kendagann PH, Rafiq M, Babu UV
Received 1 November 2021
Accepted for publication 28 February 2022
Published 27 April 2022 Volume 2022:14 Pages 149—165
DOI https://doi.org/10.2147/JEP.S345599
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Paola Rogliani
Mallappa Onkaramurthy,1 Mohammed Mukhram Azeemuddin,1 Mirza Rizwan Baig,2 Pavan Heggadadevanakote Kendaganna,2 Mohamed Rafiq,1 Uddagiri Venkanna Babu3
1Discovery Sciences Group, Research and Development Center, Himalaya Wellness Company, Bengaluru, Karnataka, India; 2Microbiology and Toxicology Department, Research and Development Center, Himalaya Wellness Company, Bengaluru, Karnataka, India; 3Research and Development Center, Himalaya Wellness Company, Bengaluru, Karnataka, India
Correspondence: Mohamed Rafiq, Email [email protected]
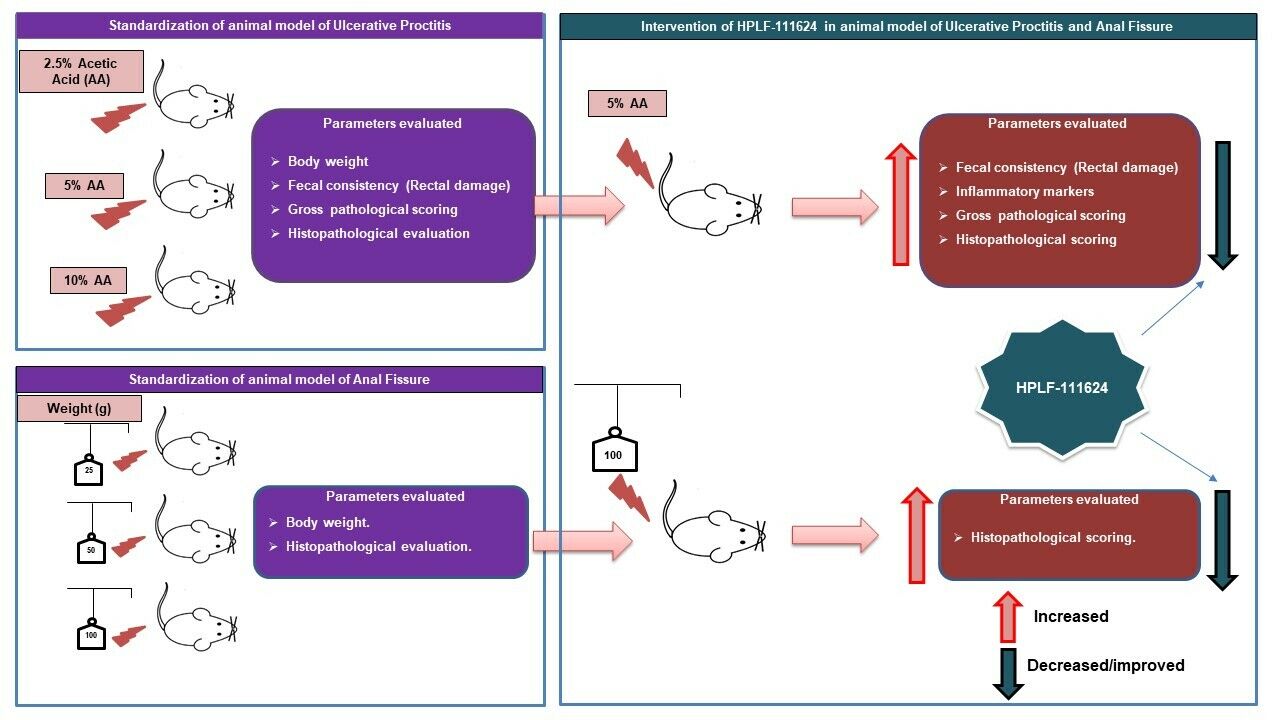
Background: Although ulcerative proctitis (UP) and anal fissure (AF) are common anorectal diseases, there are no appropriate experimental models to screen the drugs intended for these conditions. In this context, existing experimental models mimicking these diseases were modified and the polyherbal formulation, HPLF-111624 was evaluated in these models.
Objective: To establish animal model for UP and AF and to evaluate polyherbal formulation, HPLF-111624 in these disease models.
Methods: An experimental model of UP was selected based on the modification of the ulcerative colitis model using different concentrations of acetic acid. The concentration used for induction were 2.5%, 5% and 10% v/v and different weights used to induce AF were 25 g, 50 g and 100 g, which were selected based on the severity of inflammation, fecal score, gross pathology, and histopathological evaluation. Furthermore, these animal models were used to evaluate the efficacy of HPLF-111624, a polyherbal formulation known to be beneficial in anal diseases.
Results: Acetic acid at 5% produced typical pathological changes that resembled UP, with a significant increase in the fecal score, gross lesion, and histopathological changes. Similarly, among the three weights, physical injury with a 100 g weight produced significant changes in the histopathological score in the model of AF. Intervention with HPLF-111624 at doses of 250 and 500 mg/kg b.wt., showed a reduction in the inflammatory cytokines and a significant improvement in the histopathological findings in both the conditions.
Conclusion: The results showed that the modified experimental models for UP and AF resemble the human pathological conditions and are simple, versatile and may be used for screening drugs intended for these conditions. Intervention with HPLF-111624 was found to be effective in improving the pathological state of UP and AF.
Keywords: anorectal disorders, ulcerative proctitis, anal fissure, inflammation, histopathological evaluation, polyherbal formulation
Graphical Abstract:
Introduction
Anorectal disorders are a group of medical conditions that occur in the rectum and anal canal; they affect around 25% of the United States population. Common anorectal disorders include benign and irritating pruritus ani, with malignancies such as anal fissures (AFs), hemorrhoids, fistula, anal warts, proctitis, and pre-malignancy conditions.1,2
Proctitis is an anorectal disorder that causes inflammation of the rectal mucosa. Based on the etiology of proctitis, it is further classified into ulcerative, radiation, diversion, and infectious proctitis. Ulcerative proctitis (UP) is an idiopathic mucosal inflammation confined to the rectal region; hence, it is a functionally limited form of ulcerative colitis. The incidence of UP has increased up to 55% over the last decade in patients with ulcerative colitis.3,4 Current research evidence suggests that genetic predisposition, environmental factors, intestinal flora, and immune dysregulation are the possible causative factors of UP; however, it is still unclear why the inflammation is limited to the rectum.3 All symptoms of UP are related to the rectum and include bloody stools, diarrhea, and abnormal bowel function. A range of medications are available to help treat the symptoms and reduce remission; however, currently, there is no definitive cure. Medications for the management of UP include mesalamines as suppository, oral formulations, corticosteroids as a foam or enema, oral immunosuppressive drugs, and monoclonal antibodies against tumor necrosis factor-α.4,5 However, these drugs have their own side effects, which probe researchers to find alternative and safe treatment approaches such as herbal treatments.
AF, also known as fissure in ano, is a common anorectal condition with a longitudinal tear in the anal canal, distal to the dentate line. It is further classified into acute fissure and chronic fissure. In acute fissures, with treatment, the symptoms subside within 4 to 6 weeks, while chronic fissures persist for more than 6 to 8 weeks, with signs of edema and fibrosis. People with AF experience sharp pain in the anal region during bowel movements and have bloody stools. The annual incidence of AF is 1.1 per 1000 individuals. A study of 1243 persons concludes that there was no variation between Hispanic and Non-Hispanic individuals in the incidence of AF (1.09 and 1.10 cases per 1000 person-years, respectively). However, Hispanic people of 55–64 age group (1.69 versus 1.08 cases per 1000 person-years; p < 0.01) showed higher incidence of AF, mostly due to a marginally higher occurrence among men (2.00 cases per 1000 person-years among Hispanic men of 55–64 age group).6,7 The main causes of AF are passing of large or hard stool, or prolonged constipation, recurrent diarrhea, and after vaginal delivery . Besides physical injury, other causes of AF include inflammatory bowel disease, granulomatous diseases, HIV, syphilis, and malignancy.1 Treatment approaches for AF include (i) conservative management approaches such as increased fiber intake (eg, psyllium) and warm bathing of the perineum and (ii) medical interventions including application of topical ointments and creams such as glyceryl trinitrate and calcium channel blockers, botulinum toxin injection, and surgical management. However, these medical interventions have their own limitations and side effects.8,9 This has led researchers to embark on finding effective and safe treatment approaches in alternative medicine.
The unavailability of appropriate animal models to screen drugs for both conditions and the lack of adequate literature (noted upon exploring PubMed, Google Scholar, and Scopus databases) related to such animal models pose as barriers to researching new therapies. An experimental model for radiation proctitis is available but there are no animal models available for other forms of proctitis. Radiation therapy is one of the modalities adopted for the treatment of pelvic and abdominal cancer which causes side effects such as proctitis. However, the etiopathology of radiation proctitis is different from that of UP in humans.10–12
In this context, we have modified the experimental model of ulcerative colitis to UP, and oral mucositis model to anal fissure, which closely mimic the human pathological condition. Acetic acid is commonly used phlogistic agent to induce ulcerative colitis in animal research and resembles human ulcerative colitis in its pathophysiological features, histology, and inflammatory mediator profile.13 As UP is a similar disease condition that is limited to the rectal region, we used acetic acid to induce the same. Similarly, the etiology and histopathology of the anal fissure model include simple slit/laceration/abrasion, thrombosis, and inflammation in crypts.14,15 To achieve this, we have used different weights suspended on an electro nickel–plated wire, a physical injury method, to induce a fissure. This technique has been used by researchers to induce the oral mucositis in animals.16 After the experimental models were modified and standardized, “HPLF-111624” was evaluated for UP and AF.
“HPLF-111624” is a well-known standardized herbal formulation of “Himalaya Wellness Company”, Bengaluru, India, recommended for hemorrhoids.17 In this study it was evaluated for its efficacy in experimental models of UP and AF. It is a combination of Commiphora wightii (Arn). Bhandari (Guggulu), Asphaltum (Shilajit) is a complex mixture of organic humic substances, plant and microbial metabolites occurring in the rock rhizospheres of its natural habitat and rich in fulvic acid.18 Azadirachta indica A. Juss. (Nimba), Berberis aristata DC. (Daruharidra), Phyllanthus emblica L. (Amalaki), Terminalia chebula Retz. (Haritaki), Terminalia bellirica (Gaertn.) Roxb. (Vibhitaki), Cassia fistula L. (Aragvadha), Bauhinia variegata L. (Kanchanara), Mesua ferrea L. (Nagkesara) extracts. This combination was selected based on the available evidences in Ayurvedic and modern medicine literature. Each of these herbs is reported to ameliorate various pathological pathways of UP and AF.
Materials and Methods
Chemicals
Acetic acid was procured from S D Fine-Chem Limited from Worli Road, Mumbai, (India) infant feeding tube (catheter) from Ramsons, Gandhinagar, Gujarat, (India), hematology analyzer from Nihon Kohden, model MEK-6550, Gurgaon, Haryana, (India), hematoxylin from Himedia Laboratories (India), eosin from Nice Chemicals Limited (India), Isoflurane from Neon Laboratories (India), and IL-6 and TNF-α ELISA kits from Krishgen Limited (Mumbai, Maharashtra, India).
Experimental Animals
Inbred male Wistar rats were procured from the central animal house facility (R&D Center, Himalaya Wellness Company, Bengaluru, Karnataka, India). All animals were housed in standard conditions of temperature (22 ± 3°C), relative humidity (55 ± 5%), and light (12 h light/dark cycles) before and throughout the study. They were fed ad libitum with standard rat pellet feed procured from VRK Nutritional Solutions (Pune, Maharashtra, India) and ad libitum water. The investigational protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Himalaya Wellness Company (Protocol no. 197/19) and the animals were treated humanely in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Fisheries, Animal Husbandry and Dairying, Government of India.
Experimental Model of Ulcerative Proctitis
This experiment was carried out in 20 male Wistar rats of body weight 350–400 g and were divided in to 4 groups of 5 each based on the average body weight. Different concentrations of acetic acid (1 mL of 2.5%, 5%, and 10% v/v) in 0.9% saline were used to induce UP in them. Induction was carried out in overnight fasted animals under 2% isoflurane inhalation anesthesia. A 5-mm diameter polyethylene tube was inserted into the rectum (the distance was limited only to the rectum so that induction will limit this portion only) and 1 mL of different concentrations of acetic acid was instilled into the rectum for 30s. Group 1 served as the control (intrarectal administration of saline), group 2 served as the pathological control 1 (1 mL of 2.5% acetic acid), group 3 served as the pathological control 2 (1 mL of 5% acetic acid), and group 4 served as the pathological control 3 (1 mL of 10% acetic acid). The body weight and fecal consistency (rectal damage) of the rats was measured daily. Hematological analysis was carried out on day 7, and finally, all animals were euthanized using 5% of isoflurane inhalation anesthesia, and the rectum was dissected out, weighed, and clinically scored. After scoring, the tissue samples were stored in formalin for further histopathological evaluation.
The fecal examination for diarrhea and rectal bleeding was done daily and scored as follows: 0, normal or negative blood; 1, soft but still formed; 2, soft stool with blood traces; 3, diarrhea and rectal bleeding.11,13
Macroscopic scoring for inflammation in the rectum was done based on the following scores: 0, no ulcer formation; 1, mucosal erythema; 2, mild mucosal edema and bleeding ulcers or bleeding erosion; 3, moderate mucosal, bleeding ulcers, or erosions; 4, severe ulcerations, erosions, and edema with tissue necrosis and perforations.11,13
The rectal sections were analyzed histopathologically for crypt degeneration and necrosis, inflammatory cell infiltration and erosion, and ulcer formation using H&E staining.11,13
Experimental Model of Anal Fissure
AF was modified and standardized using a physical injury method, with the help of an electro nickel–plated wire to induce AF. The electro nickel–plated wires were sterilized with alcohol. Then, different weights were suspended on them and used to form a linear tear in the rectum.
Twenty male Wistar rats of body weight 350–400 g were randomized in to 4 groups of 5 each. AF induction was carried out in overnight fasted rats and isoflurane (2%) was used to anesthetized the rats. Electro nickel–plated wires of 1 mm diameter were suspended with different weights (25 g, 50 g and 100 g) for induction of AF. The pointed end of the wire was placed into the anal region, slightly pressed, and pulled back to form a linear tear or slit or abrasion. The distance was limited only to anal region, so that the AF induction is restricted to the anorectal region only. The induction was confirmed visually by the appearance of a bloodspot in the anal region. This procedure was done in all rats, with uniform weight and pressure to the respective groups. There were 5 rats in each group. Group 1 served as the control (no induction: the electro nickel–plated wire was inserted without weight), group 2 served as the pathological control 1 (a 25 g weight was suspended with the electro nickel–plated wire), group 3 served as the pathological control 2 (a 50 g weight was suspended to the electro nickel–plated wire), and group 4 served as the pathological control 3 (a 100 g weight was suspended to the electro nickel–plated wire) to induce AF. All rats received DM water 10 mL/kg, P.O. daily during the study period.
The study animals were monitored, and their body weights were recorded daily for 7 days. On the last day of the study, all animals were euthanized using isoflurane (5%) inhalation anesthesia, the rectum was collected, grossly observed for visual changes in texture, and stored in formalin for further histopathological evaluation. The anorectal sections were examined histopathologically for any changes in the inflammatory cell infiltration and erosion and ulcer formation, which are the key parameters in evaluating AF in clinical condition.14,15
Evaluation of HPLF-111624 in an Experimental Model of Ulcerative Proctitis
The study was carried out on 32 male Wistar rats weighing 350 to 400 g each. A single dose of 1 mL of acetic acid (concentration 5%, v/v) in 0.9% saline was administered intrarectally to all the animals except control group to induce UP. Control group animals were administered intrarectally with 1 mL of sterilized saline. The procedure for induction of UP was the same as perfromed during model development. After induction, the animals were randomly stratified based on their body weight and divided into 4 groups of 8 each. Group 1 animals served as the control (received DM water 10 mL/kg, P.O.), group 2 animals served as the pathological control (received DM water 10 mL/kg, P.O.), and groups 3 and 4 served as treatment groups (received HPLF-111624 at 250 and 500 mg/kg, P.O., respectively).
The above treatments were carried out for 7 days. The body weight and fecal consistency were measured daily. On the last day (day 7) of the study, all animals received the assigned treatments 1 hour before euthanasia. The blood was collected for hematological analysis and IL-6 and TNF-α estimation. Finally, the animals were euthanized using isoflurane (5%) inhalation anesthesia. Rectum was dissected out, scored, and weighed. Once the scoring was completed, the tissue samples were stored in 10% neutral buffered formalin (NBF) for further histopathological evaluation.
Evaluation of HPLF-111624 in an Experimental Model of Anal Fissure
This experiment was carried out in thirty-two male Wistar rats of body weight 350 to 400 g. The procedure for induction of AF was the same as that conducted during model development. A weight of 100 g tied to the electro nickel–plated wire was used for the induction of AF. After induction, the animals were randomized based on their body weight and grouped into 4 groups, with 8 rats in each group. Group 1 served as the control (DM water 10 mL/kg, P.O.), group 2 served as the pathological control (received DM water at 10 mL/kg P.O.), and groups 3 and 4 served as the treatment groups (received HPLF-111624 at 250 and 500 mg/kg, P.O., respectively). All rats received the assigned treatment once daily for 1 week. All animals were monitored and their body weights were recorded daily for 7 days. On the last day of the study, all animals were euthanized using isoflurane (5%) inhalation anesthesia, anorectal tissue was dissected out and grossly observed, and stored in 10% NBF for further histopathological evaluation. The tissues were evaluated for inflammatory cell infiltration, erosion and ulcer formation.
Statistical Analysis
The values are expressed as mean ± standard error of the mean (SEM). The results were analyzed statistically either using one-way ANOVA followed by post Dunnett’s multiple comparison test and/or one-way ANOVA followed by Kruskal–Wallis test followed by Dunnett’s posttest using GraphPad Prism 6.07 (GraphPad Software Inc, San Diego, CA, USA). A P value < 0.05 was considered as statistically significant.
Results
Experimental Model of Ulcerative Proctitis in Rats
Three different concentrations of acetic acid were used for the induction of UP. The parameters such as body weight,hematology, gross pathology, and histopathological evaluation of the rectal tissue were considered to determine the best dose for induction, because these parameters were relevant to the human pathological condition. Intra rectal instillation of 2.5%, 5%, and 10% acetic acid revealed that there was a dose-dependent increase in the resultant UP.
After evaluating the aforementioned parameters at all administered doses, it was observed that 2.5% acetic acid showed mild induction with minor changes (4% decrease on day 7) in the body weight (Table 1), a non significant increase in the fecal consistency score (except day 1 (P < 0.05)) (Figure 1), a non significant difference in hematological test results (Table 1), and a non significant increase in the gross pathology score compared with that in the control animals (Table 2, Figure 2). In addition, the histopathological evaluation of the rectal sections confirmed mild crypt changes, erosion and ulcer formation, and inflammatory cell infiltration compared with that in the control animal (Figure 3).
 |
Table 1 Effect of Different Concentration of Acetic Acid on Body Weight and Hematology Parameters in Ulcerative Proctitis Model |
 |
Table 2 Effect of Different Concentration of Acetic Acid on Rectum Weight, Gross Pathology Scoring and Histology Scoring in Ulcerative Proctitis Model |
 |
Figure 2 Representative gross morphology rectum pictures and the impact of rectal damage induced by different concentrations of acetic acid in the ulcerative proctitis model. |
Animals challenged with 5% acetic acid showed a marked reduction (10% decrease on day 7) in their body weight compared with that in the control animals. Furthermore, there were significant changes in the hematological test results with a significant increase in white blood cell (WBC (P < 0.05)), a significant decrease in red blood cells (RBC (P < 0.01)) and hemoglobin HGB (P < 0.05) (Table 1). There was a significant increase in the fecal score (P < 0.01 and P < 0.05) from day 1 until the completion of the experiment period compared with that in the control animals (Figure 1). Gross pathology showed a visible difference with significant changes in the inflammation score (P < 0.01) and relative rectal weight (P < 0.001) compared with those in the control animals (Table 2, Figure 2). Furthermore, the histopathological analysis revealed significant changes in crypt degeneration and necrosis, inflammatory cell infiltration, and erosion and ulcer formation (P < 0.01) (Table 2, Figure 3), which are the typical pathological markers of UP, compared with that in the control animals.
Animals treated with 10% acetic acid showed similar changes as those observed in animals treated with 5% acetic acid, such as a marked reduction (11% decrease on day 7) in body weight, significant increase in fecal score (P < 0.01 and P < 0.05), and changes in values of hematological parameters (Table 1, Figure 1). Histopathological analysis of the rectal tissues revealed severe pathological changes with severe induction of the disease (Table 2, Figures 2 and 3). Moreover, there was mortality in 10% acetic acid challenged animals (data not shown). Based on these findings, we used 5% acetic acid to induce UP, as 5% acetic acid brought about significant changes in the pathological parameters such as fecal score, hematological values, gross pathology and histopathology without causing mortality.
Experimental Model of Anal Fissure in Rats
The gross pathology (macroscopic observation) and histopathological evaluation were used as parameters to assess the severity of the induction and histopathological changes. The 25 g and 50 g weights suspended on the electro-nickel–plated wires showed mild induction (Figure 4) of the fissure with minimal injury and histopathological evaluation revealed mild to moderate inflammatory cell infiltration ((P< 0.05) at 50-g) and ulcer formation compared with that in the control (Table 3, Figure 5).
 |
Table 3 Histology Scoring Data of Anal Fissure Model |
Gross evaluation showed that the 100 g weight induced a clear linear tear/slit/abrasion in the rectal region compared with that in the control (Figure 4). Further histopathological evaluation showed a significant difference in erosion (P< 0.001) and ulcer formation along with inflammatory cell infiltration (P < 0.01) in the rectal sections compared with that in the control animals (Table 3, Figure 5). These factors helped differentiate the parameters for model standardization. Based on these findings, the 100 g weight was selected for optimal induction of AF and the same load was considered for further study.
During the entire period of model development and standardization, we did not observe marked changes in the body weight of the pathological group animals compared with that in the control group animals (data not shown).
Evaluation of HPLF-111624 in an Experimental Model of Ulcerative Proctitis
All animals were weighed daily from day 1 to day 7. There was a marked decrease (12%) in body weight of the pathological control animals compared with that in the control animals at the end of the study period. The body weight in HPLF-111624-treated (250 and 500 mg/kg) animals decreased by 2% and 3% respectively, compared with that of the control animals (Table 4).
 |
Table 4 Effect of HPLF-111624 on Body Weight Change and Hematology Parameters in Acetic Acid Induced Ulcerative Proctitis Model |
The fecal consistency was scored daily in the experimental period. The stools were collected on a thick paper, observed for changes such as bleeding and diarrhea and scored. The fecal consistency score was maximum in the pathological control group on day 1 with rectal bleeding and diarrhea (P< 0.001) and was significant compared with that of the control group. Although the fecal consistency score reduced in the pathological control animals during the overall experimental period from day 1 to 7, it was statistically significant (P < 0.001) compared with that in the control animals. The animals treated with HPLF-111624 at 250 mg/kg exhibited a decrease in the fecal consistency score from day 1 to 7, and this trend was significant on day 3 (P < 0.01); 2, 4, 5 6 and 7 (P< 0.05). The animals treated with HPLF-111624 (500 mg/kg) showed a significant decrease in the fecal consistence score (P < 0.01) on day 3, 4 and 5 (P < 0.05), compared with the pathological control group (Figure 6).
Intra rectal acetic acid instillation produced marked changes in the hematological parameters of rats. Induction at 5% acetic acid concentration significantly (P < 0.001) increased the WBC count. However, intervention with HPLF-111624 at 250 mg/kg and 500 mg/kg doses significantly lowered the WBC count (P < 0.05) compared with that in the pathological control group. Furthermore, 5% acetic acid administration significantly lowered the RBC count (P < 0.05) and hemoglobin concentration (P < 0.05) compared with that in the control animals. The HPLF-111624-treated (250 and 500 mg/kg) animals showed improvement in RBC count and HGB concentration compared with that in the pathological control animals (Table 4).
Acetic acid caused severe gross lesions such as ulcers and edematous inflammation in the rectum. It showed a significant increase in the rectal score (P < 0.001) in the pathological control group compared with control. Intervention with HPLF-111624 at 250 and 500 mg/kg doses for 7 days brought about a significant (P < 0.01 and P < 0.05, respectively) decrease in the gross scoring compared with that in the pathological control group (Table 5, Figure 7).
 |
Table 5 Effect of HPLF-111624 on Rectum Weight, Gross Pathology Scoring, Histology Scoring and Serum Inflammatory Marker Level in Ulcerative Proctitis Model |
Acetic acid at 5% concentration caused severe rectal inflammation with a significant increase in the relative rectal weight (P < 0.001) in the pathological control group. Treatment with HPLF-111624 at doses of 250 and 500 mg/kg for 7 days significantly decreased the rectal weight (P < 0.01 and P < 0.05, respectively) compared with that in the pathological control group (Table 5).
The histological examination of the rectal sections of the control group animals showed normal morphology with clear epithelial histoarchitecture, whereas the rectal sections of the pathological group animals treated with 5% acetic acid showed severe damage with significant changes in the crypt degeneration (P < 0.001) accompanied by marked inflammatory cell infiltration with severe epithelial erosion and ulcer formation. Further HPLF-111624-treated animals exhibited a marked amelioration in the tissue histology with minimal damage to the mucosa. It also brought about mild-to-moderate protection by countering the acetic acid-induced UP. Furthermore, histopathological evaluation of the rectal sections revealed that HPLF-111624 brought about a significant decrease in crypt degeneration (P < 0.05) accompanied by a decrease in the inflammatory cell infiltration (P < 0.01 and P < 0.05 at concentrations of 250 and 500 mg/kg, respectively) and protection against erosion and ulcer formation (P < 0.05). The representative images and the details of the rectal histology of different groups of animals are shown in (Table 5, Figure 8).
In animals treated with acetic acid, there was a significant increase in serum levels of IL-6 and TNF-α (P < 0.001 and P < 0.01, respectively) compared with that in the control animals. Treatment with 250 and 500 mg/kg doses of HPLF-111624 brought about a mild decrease in TNF-α level and a significant decrease in IL-6 (P < 0.05) at 250 mg/kg dose (Table 5).
Evaluation of HPLF-111624 in an Experimental Model of Anal Fissure
There was no significant change in the body weights of animals was observed in the groups throughout the experimental period. A weight of 100 g showed a clear slit formation in the rectal region of the pathological control animals compared with that in the control animals. Treatment with HPLF-111624 at 250 mg/kg and 500 mg/kg brought about mild-to-moderate healing in the fissure compared with that in the pathological control animals (Figure 9).
Control animals exhibited normal histoarchitecture of the rectum. The histopathological examination of the rectal sections of animals induced with 100 g weight showed a significant increase (P < 0.001) with clear ulceration and erosion with inflammatory cell infiltration when compared with control animals. Treatment with HPLF-111624 at 250 and 500 mg/kg doses showed a significant decrease in inflammatory cell infiltration (P < 0.05) and a decrease in ulcer formation at both doses with a significant decrease (P < 0.05) at 250 mg/kg in the rectal sections when compared with pathological control animals rectal sections. Although both doses of HPLF-111624 brought about a significant protection of the rectal histoarchitecture, the effect was not dose dependent (Table 6, Figure 10).
 |
Table 6 Effect of HPLF-111624 on Histology Scoring in Anal Fissure Model |
Discussion
Anorectal conditions like UP and AF are affecting millions of individuals worldwide. Although the incidence of these conditions is high, the available treatment options are limited and are accompanied by side effects. The limitation of animal models for testing drugs intended to treat UP and AF hindered the development of new and effective approaches. With this perspective, the study was divided into 2 sections—the first part was to modify and standardize an appropriate and consistent animal model for both disease conditions and the second part was to evaluate the polyherbal formulation HPLF-111624 in the standardized animal model.
The signs, symptoms, etiology, histopathology, and pathophysiological features of the disease were considered before developing the animal models. In the case of UP, we considered the etiology and pathophysiology of the disease,4 whereas histopathological evaluation was the key parameter in the case of AF.14,15
UP is an idiopathic mucosal inflammatory disease restricted to the rectal region. The symptoms include abdominal pain, especially in the lower part, bowel clearance, stools with blood or mucus passage through the rectum, or mucus mixed with stool. Furthermore, its histopathology is characterized by nonspecific findings of mucosal inflammation, crypt abscesses or degeneration, and reduced numbers of goblet cells.4
Body weight change or weight loss and changes in hematological parameters play a significant role in these disease conditions, thus assessment of both weight loss and hematological parameters is important.19 The wet weight of the inflamed rectal tissue is considered a reliable and sensitive indicator of the severity and extent of the inflammatory response.20
To achieve the above criteria, we used acetic acid as the phlogistic agent to induce UP. Acetic acid triggers an acute inflammatory response following injury, accompanied by extensive hemorrhage, the release of mediators, and formation of lesions. The protonated form of the acid liberates protons within the intracellular space, causing massive intracellular acidification, which results in extensive epithelial damage. Furthermore, it also causes infiltration of WBCs to the damaged area, along with the release of inflammatory mediators such as cytokines and arachidonic acid metabolites and reactive oxygen species, leading to oxidative damage.21
Consistent with this understanding of acetic acid’s mechanism of action, rats treated with 5% acetic acid showed a marked decrease in body weight, a significant increase in fecal consistency score, rectal weight, and gross lesion score, along with severe tissue ulceration, necrosis, and inflammatory cell infiltration, which are the etiological factors that characterize UP. Hence, the same concentration of acetic acid was used for further evaluation studies.
Several researchers used physical injury techniques to induce diseases such as abrasion wounds22 and oral mucositis.16 Hence, we also used the physical injury method to induce AF with the help of electro nickel–plated wires suspended with different weights.
Histopathological evaluation plays a critical role in developing animal models. There is a dearth of scientific literature describing the histopathology of AF. We relied on insights from 2 studies14,15 to define the criteria for the evaluation of AF. Both these studies demonstrate a chronic inflammatory process with acute activity and inflammatory cryptitis in the disease mechanism of AF. Our preliminary results showed that electro nickel–plated wire suspended to a 100 g weight produced a marked difference in the histopathological features of the rectal sections with a significant increase in erosion and ulcer formation and inflammatory cell infiltration; hence, the same method was used in the evaluation of HPLF-111624.
After the model development process, we evaluated the efficacy of HPLF-111624 (at 250 and 500 mg/kg doses) in both disease conditions using these standardized models. The therapeutic doses were selected based on the LD50 value (data not shown). Treatment with HPLF-111624 showed a marked decrease in UP. The polyherbal formulation improved the body weight and brought about a decrease in the fecal consistency score. The improvement in body weight maybe because of the decrease in diarrhea or fecal consistency score and a reduction in inflammation, which prevents excessive nutrient loss that occurs in diarrhea.19
Treatment with HPLF-111624 decreased the relative rectal weight, gross lesion and histopathological score in the UP model. The treatment also showed a significant decrease in the inflammatory markers, which maybe because of the potent anti-inflammatory activity of the herbs present in the formulation such as Azadirachta indica A. Juss, Phyllanthus emblica L., Terminalia chebula Retz., Berberis aristata DC., and Bauhinia variegata L.23–27 In case of AF, treatment with HPLF-111624 significantly decreased the inflammatory cell infiltration and ulcer formation. This can be attributed to the anti-inflammatory and wound healing activity of the herbal constituents of the formulation.23–27
Conclusion
The experimental models of UP and AF were successfully modified and standardized to mimic the human pathological conditions of UP and AF. As there are no full-proof experimental models available, this research may be useful to the researchers/scientists working in this area to screen and evaluate drugs or herbal actives intended for these conditions. Besides the experimental models, the satisfied treatment for these conditions is not available. This study demonstrated the beneficial effects of the polyherbal formulation HPLF-111624 in UP and AF. However, the precise molecular mechanism of HPLF-111624 in UP and AF needs to be further explored.
Acknowledgment
The authors are thankful to Dr.Hariprasad VR, Natural Product Innovation department and Scientific publication department for their support and M/S Himalaya Wellness Company, Makali, Bangalore, India, for providing the necessary facilities to carry out the research work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors are employees of Himalaya Wellness Company, Bengaluru, Karnataka, India. The authors declare no other conflicts of interest in this work.
References
1. Foxx-Orenstein AE, Umar SB, Crowell MD. Common anorectal disorders. Gastroenterol Hepatol. 2014;10(5):294–301.
2. Patcharatrakul T, Rao SSC. Update on the pathophysiology and management of anorectal disorders. Gut Liver. 2018;12(4):375–384. doi:10.5009/gnl17172
3. Wu XR, Liu XL, Katz S, Shen B. Pathogenesis, diagnosis, and management of ulcerative proctitis, chronic radiation proctopathy, and diversion proctitis. Inflamm Bowel Dis. 2015;21(3):703–715. doi:10.1097/MIB.0000000000000227
4. Whitlow CB. Ulcerative Proctitis. Clin Colon Rectal Surg. 2004;17(1):21–27. doi:10.1055/s-2004-823067
5. Hanauer SB, Present DH, Rubin DT. Emerging issues in ulcerative colitis and ulcerative proctitis: individualizing treatment to maximize outcomes. Gastroenterol Hepatol. 2009;5(15):1–16.
6. Mapel DW, Schum M, Von Worley A. The epidemiology and treatment of anal fissures in a population-based cohort. BMC Gastroenterol. 2014;14(1):2–8. doi:10.1186/1471-230X-14-2
7. Fahadullah M, Peirce C. Fissure-In-ANO. In: Proctological Diseases in Surgical Practice. InTech; 2018.
8. Tranqui P, Trottier DC, Victor C, Freeman JB. Nonsurgical treatment of chronic anal fissure: nitroglycerin and dilatation versus nifedipine and botulinum toxin. Can J Surg. 2006;49(1):41–45.
9. Derakhshan AR. Natural treatments for fissure in ano used by traditional Persian scholars, Razi (Rhazes) and Ibn Sina (Avicenna). J Evidence-Based Complement Altern Med. 2017;22(2):324–333.
10. Yavuz E, Ercan G, Karagulle OO, et al. Evaluation of prophylactic and therapeutic effects of sildenafil on acute radiation proctitis in rats. Acta Cir Bras. 2018;33(4):362–374. doi:10.1590/s0102-865020180040000008
11. Doi H, Kamikonya N, Takada Y, et al. Low-dose aspirin therapy does not increase the severity of acute radiation proctitis. World J Oncol. 2012;3(4):173–181. doi:10.4021/wjon559w
12. Ashcraft KA, Miles D, Sunday ME, et al. Development and preliminary evaluation of a murine model of chronic radiation-induced proctitis. Int J Radiat Oncol Biol Phys. 2018;101(5):1194–1201. doi:10.1016/j.ijrobp.2018.04.061
13. Adegoke GA, Onasanwo SA, Eyarefe OD, Olaleye SB. Ameliorative effects of Musa sapientum peel extract on acetic acid-induced colitis in rats. J Basic Appl Zool. 2016;77:49–55.
14. Brown AC, Sumfest JM, Rozwadowski JV. Histopathology of the internal anal sphincter in chronic anal fissure. Dis Colon Rectum. 1989;32(8):680–683. doi:10.1007/BF02555773
15. Whitney ET. Fissure-in-ano. Am J Surg. 1943;59(1):9–17.
16. Lee HR, Yoo N, Kim JH, et al. The therapeutic effect of PLAG against oral mucositis in hamster and mouse model. Front Oncol. 2016;6:1–8. doi:10.3389/fonc.2016.00001
17. Azeemuddin M, Viswanatha GL, Rafiq M, et al. An improved experimental model of hemorrhoids in rats: evaluation of antihemorrhoidal activity of an herbal formulation. ISRN Pharmacol. 2014;2014:1–7.
18. Agarwal SP, Khanna R, Karmarkar R, Anwer MK, Khar RK. Shilajit: a review. Phyther Res. 2007;21(5):401–405.
19. Olamilosoye KP, Akomolafe RO, Akinsomisoye OS, Adefisayo MA, Alabi QK. The aqueous extract of Ocimum gratissimum leaves ameliorates acetic acid-induced colitis via improving antioxidant status and hematological parameters in male Wistar rats. Egypt J Basic Appl Sci. 2018;5(3):220–227.
20. Rachmilewitz D, Simon PL, Schwartz LW, Griswold DE, Fondacaro JD, Wasserman MA. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989;97(2):326–337. doi:10.1016/0016-5085(89)90068-1
21. Praveen kumar P, Santhosh G, Sri Chandana M, Prasad K, Raghu prasad M, Khasim S. Protective effect of A2B receptor antagonist (TRP 2) on acetic acid induced ulcerative colitis in rats: in vitro, in vivo and in silico methods. Indian J Physiol Pharmacol. 2018;62(3):327–338.
22. Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. Animal models of external traumatic wound infections. Virulence. 2011;2(4):296–315.
23. Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (Neem). J Oleo Sci. 2009;58(11):581–594. doi:10.5650/jos.58.581
24. Asmawi MZ, Kankaanranta H, Moilanen E, Vapaatalo H. Anti-inflammatory activities of Emblica officinalis Gaertn leaf extracts. J Pharm Pharmacol. 2013;5724(2001):581–584.
25. Sireeratawong S, Jaijoy K, Khonsung P, Soonthornchareonnon N. Analgesic and anti-inflammatory activities of the water extract from Terminalia chebula Retz. African J Tradit Complement Altern Med. 2015;11(6):77.
26. Gupta SK, Agarwal R, Srivastava S, et al. The anti-inflammatory effects of Curcuma longa and Berberis aristata in endotoxin-induced uveitis in rabbits. Investig Ophthalmol Vis Sci. 2008;49(9):4036–4040.
27. Saha S. In vivo study for anti-inflammatory activity of Bauhinia variegata L. leaves. Pharm Crop. 2011;2(1):70–73.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.









