Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Investigation into the Effectiveness of Combining Transcranial Direct Current Stimulation and Transcutaneous Electrical Nerve Stimulation as Treatment Options for Poststroke Shoulder Pain by Utilizing Functional Near-Infrared Spectroscopy
Authors Li Y, Yan ZP, Zhang NN, Ni J, Wang ZY
Received 21 July 2023
Accepted for publication 18 October 2023
Published 9 November 2023 Volume 2023:19 Pages 875—887
DOI https://doi.org/10.2147/TCRM.S431816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yu Li,1 Zhi-Peng Yan,1 Nan-Nan Zhang,1 Jun Ni,1,2 Zhi-Yong Wang1
1Department of Rehabilitation Medicine, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, 350212, People’s Republic of China; 2Department of Rehabilitation Medicine, the First Affiliated Hospital, Fujian Medical University, Fuzhou, 350005, People’s Republic of China
Correspondence: Zhi-Yong Wang, Department of Rehabilitation Medicine, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital of Fujian Medical University, 999 Huashan Road, Fuzhou, 350212, People’s Republic of China, Tel +86-13635298857, Email [email protected]
Objective: The aim of this study is to explore the therapeutic effects of transcranial direct current stimulation (tDCS) and transcutaneous electrical nerve stimulation (TENS) on post stroke shoulder pain (PSSP).
Methods: We enrolled 13 individuals in this study who underwent three different treatments in a random sequence: active tDCS+active TENS, active tDCS+sham TENS, and sham tDCS+active TENS. Each treatment was administered once, with a 3-day washout period between interventions. A blinded rater assessed the visual analog scale (VAS) scores, fNIRS readings, and sensory and pain tolerance thresholds of the participants before and after the stimulation.
Results: All three treatment methods can significantly alleviate PSSP (p< 0.05). Compared with using tDCS alone, tDCS+TENS can significantly improve pain, with a statistically significant difference (p< 0.05). In the 2KHz PTT task, the three treatment methods showed significant differences (p< 0.05) in the mean oxygenated hemoglobin (HbO) levels in the false premotor cortex (PMC)/auxiliary motor area (SMA) before and after intervention.
Conclusion: The combination of tDCS+TENS can increase the pain-relieving impact on PSSP when compared to using tDCS alone. TENS may contribute an additional effect on the inhibitory systems influenced by tDCS that help reduce pain.
Clinical Registration Number: Registration website: https://www.chictr.org.cn. Registration date: 2022-02-25. Registration number: ChiCTR2200056970.
Keywords: functional near-infrared spectroscopy, pain tolerance threshold, post-stroke shoulder pain, transcranial direct current stimulation, transcutaneous electrical nerve stimulation
Introduction
Stroke, the third leading cause of death globally, imposes a significant burden on healthcare systems.1 Individuals who have survived a stroke commonly endure poststroke shoulder pain (PSSP), with incidence rates varying around 30% and prevalence ranging from 4% to 75%.2 Typically, PSSP emerges within two to three months after the stroke event.3 Despite efforts, the underlying mechanisms of PSSP remain incompletely understood. However, it appears that both peripheral and central sensitization play a role in the chronic maintenance of PSSP, leading to symptoms like allodynia, hyperalgesia, central hypersensitivity, and altered cortical somatosensory processing.4,5 PSSP can adversely affect the quality of life of patients, prolong their hospitalization, and worsen their overall recovery, thereby increasing the burden on their families and communities.6,7 Consequently, appropriate treatment of PSSP is crucial. Transcutaneous nerve stimulation (TENS), a form of peripheral nerve stimulation, is frequently employed as a pain relief method for PSSP.8 TENS can be applied in numerous clinical scenarios to alleviate pain, offering an alternative to pharmaceutical intervention.9 According to existing evidence, TENS demonstrates effectiveness in managing both acute and chronic pain conditions, although its long-term impact remains uncertain due to limited data quality.10 Transcranial direct current stimulation (tDCS) is a noninvasive method of neuromodulation that has attracted significant attention for its potential in treating poststroke pain.11,12 Its effects on the nervous system have shown promise in managing chronic and central nervous pain.13,14 However, the application of tDCS for PSSP has not been extensively studied. Previous research by Boggio et al indicated that a single intervention of tDCS combined with TENS provided better immediate pain relief compared to tDCS alone in patients with localized neurogenic arm pain. However, the study only used the visual analog scale to assess pain levels and did not investigate the underlying mechanism for pain reduction.15 Another study by Houde et al focused on a patient with complex regional pain syndrome who experienced persistent pain. The combined use of tDCS and TENS resulted in a greater reduction in pain intensity, as measured by numeric rating scales, compared to tDCS alone.16 Based on these findings, it is suggested that the concurrent application of TENS and tDCS could be an effective therapeutic strategy for alleviating PSSP. However, further clarification of the treatment’s efficacy and the underlying mechanism of pain relief is necessary through randomized controlled clinical trials. Functional near-infrared spectroscopy (fNIRS) is a noninvasive technique that utilizes near-infrared light absorption to continuously monitor hemodynamic brain signals. This advanced optical technology provides real-time insights into alterations in regional cerebral blood flow.17 By accurately measuring changes in the levels of Oxy-Hb and Deoxy-Hb in various regions of the cerebral cortex, fNIRS has been employed to assess brain functionality after tDCS for CPSP.18 Additionally, it enables the observation of the cortex’s response to noxious stimuli.19 Hence, we aimed to examine the healing impact of combining tDCS and TENS on PSSP. Additionally, we employed fNIRS to investigate the central analgesic mechanism of tDCS and TENS when utilized together in patients with PSSP.
Methods
Trial Registration and Ethics
Each study participant signed the informed consent form after receiving comprehensive information. The research plan received approval from the ethics committee of the First Affiliated Hospital of Fujian Medical University under the reference number MRCTA, ECFAH of FMU [2020] 269. Furthermore, the trial was registered with the Chinese Clinical Trials Registry (Registration website: https://www.chictr.org.cn, Registration date: 2022-02-25, Registration number: ChiCTR2200056970).
Participants
Between March and October 2022, a total of 17 patients met the inclusion criteria, of which 3 patients were excluded because they were discharged from the hospital and could not participate in the study, and 1 patient was excluded because of unstable condition. Finally, 13 PSSP patients were enrolled in the experiment. The research was conducted at the Binhai Campus of the First Affiliated Hospital, Fujian Medical University, located in Fuzhou, China. Inclusion criteria: (1) having a confirmed diagnosis of stroke; (2) being between the ages of 18 and 80; (3) experiencing shoulder pain with a score > 2 on the VAS scale (scores ≥ 3); (4) scoring > 20 on the mini-mental state examination, and (5) providing signed informed consent forms.
Exclusion criteria: (1) history of head injury; (2) severe sensory disturbances; (3) scapulohumeral periarthritis; (4) diagnosed with a serious mental illness, drug abuse, or alcoholism; (5) contraindications to noninvasive brain stimulation; (6) ongoing participation in other clinical studies; (7) complicated with diabetes mellitus. Additionally, patients were asked to discontinue analgesic medication 24 hours before receiving stimulation and to refrain from taking any medication throughout the duration of the study.
Intervention
Our research involved a clinical trial that followed a randomized, double-blinded, cross-controlled design. In this trial, all patients underwent three different treatments: active tDCS combined with active TENS (tDCS+TENS), active tDCS combined with sham TENS (tDCS), and sham tDCS combined with active TENS (TENS). The order of these treatments was determined randomly using a computer-generated list. Each treatment was administered once, and there was a 3-day washout period between treatments (as shown in Figure 1). A blinded rater conducted evaluations both before and after each treatment.
Transcutaneous Electrical Nerve Stimulation
The Tensmed S84 device (Enraf-Nonius Co., Ltd. in Rotterdam, Netherlands) was utilized for stimulation. The patients were placed in a comfortable reclined position. Subsequently, we positioned two rubber electrodes, each measuring 5×5 cm in size, around the most painful area of the affected shoulder. These electrodes were spaced approximately 5 cm apart, centered over the point of pain, and set to a level 10% below the patient’s motor threshold. We employed the TENS analgesia paradigm from the system library for a duration of 20 minutes. The TENS device remained active during actual stimulation, whereas it was deactivated during sham stimulation.
Transcranial Direct Current Stimulation
A pair of surface sponge electrodes (35 cm × 2) soaked in a saline solution with a concentration of 0.9% were employed in the study. An electrical current of 2 mA, provided by a cranial electrotherapy stimulator (EM8060, E&M Medical Technology Co., Ltd., Wuhan, China), was administered continuously for a duration of 20 minutes. The positioning of the electrodes followed the 10–20 system, with the anode electrode placed on the hemisphere contralateral to the affected shoulder, specifically over M1 (C3 or C4). The cathode electrode was positioned over the ipsilateral supraorbital area. To perform the sham stimulation, the electrodes were placed in the identical positions as for the anodal M1 stimulation, but the stimulator was deactivated after 30 seconds. Outcome Measures.
Visual Analogue Scale
The main result was assessed using the visual analogue scale (VAS), a measurement tool consisting of a 10 cm horizontal line. On this scale, a rating of 0 represents the absence of pain, ratings of 1–3 indicate mild pain, ratings of 4–6 indicate moderate pain that disrupts sleep, and ratings of 7–10 indicate severe pain that makes falling asleep difficult. The VAS ruler was utilized to determine the highest level of pain reported by patients while their shoulder joint was moved passively.
Sensory and Pain Threshold Assessments
The Neurometer®CPT device (Neurotron Company, USA) was utilized to evaluate the measurement of sensory responses (Figure 2A). To minimize any disruptions, the patient was positioned comfortably. Two circular electrodes, measuring 1 cm in diameter and made of gold-plated material, were securely placed in the middle of the deltoid pectoralis sulcus on the affected shoulder. The assessment was carried out in a fully automatic mode (Figure 2B).
 |
Figure 2 (A) Neurometer CPT/C sensory neuroquantitative assay. (B) The electrode is placed on the affected shoulder. |
The Neurometer detector emits electrical stimulation at three different frequencies (2000 Hz, 250 Hz, and 5 Hz) to examine the specific location. Meanwhile, the detector randomly generates “true” and “false” stimuli, and the patients were instructed to differentiate between them to determine the CPT. The PTT (Pain Tolerance Threshold) was determined subsequently, following the CPT. Pain is induced by gradually increasing the intensity of stimulation beyond the painless CPT value. PTT is defined as the highest level of nerve-selective electrical stimulation that the participants can tolerate.
Functional Near-Infrared Spectroscopy Neuroimaging
We utilized an fNIRS system (BS-3000, Zilian Hongkang Technology Co., Ltd., Wuhan, China) operating at wavelengths of 695 and 830 nm. This system was employed to simultaneously capture signals indicating changes in the concentration of cerebral hemoglobin during the evaluation of PTT (Figure 3A). Our setup included a total of 51 channels, comprising 16 light sources and 16 detectors, evenly spaced 3 cm apart. The sampling rate was set at 20 Hz, with channel 29 serving as the central midline reference point based on the EEG-10-20 system. To minimize positional variations, we secured the cap in place using a chin strap. The cap covered primarily the somatosensory association cortex (SAC), the somatosensory cortex (SMC), the primary motor cortex (M1), the premotor cortex (PMC)/supplementary motor area (SMA), the Broca area, and several other regions (Figure 3B). Before recording the data, we conducted a quality check on the NIR gain to ensure the proper acquisition of data. The process of conducting the fNIRS test involved several steps. Firstly, the patients’ information was entered. Then, fNIRS data was collected. Next, a task plan was selected, which consisted of three tasks: task one with 2 KHz stimulation, task two with 250 Hz stimulation, and task three with 5 Hz stimulation. Each task had a duration of 50 seconds, preceded by a 50-second rest period before task 1 and followed by a 50-second rest period after task 3. There was a 50-second interval between each task, and the total duration of the test was 350 seconds. The cap was recalibrated after each patient wore it. Subsequently, the test was conducted, and upon completion, a report was generated (Figure 3C).
 |
Figure 3 (A) Scene of fNIRS testing. (B) Brain localization schema of channels. (C) fNIRS testing procedure. Abbreviation: PTT, pain tolerance threshold. |
Statistical Analysis
Data analysis was performed using version 26.0 of the SPSS statistical software package. Given the limited size of the sample, the Shapiro–Wilk test was utilized to assess normality. In this study, variables such as Age, Duration of Disease, Pain Duration, VAS, CPT, and PTT are presented as mean ± standard deviation, and comparisons within and between the two groups were conducted using paired t-tests and independent sample t-tests, respectively. To evaluate the three groups, a one-way ANOVA was employed, and Bonferroni correction was applied to account for multiple comparisons. Categorical variables were examined using the chi-squared test and Fisher’s exact test to identify discrepancies between groups. A p < 0.05 denoted statistical significance for observed differences. The fNIRS data was analyzed using NIRS-SPM and Homer2 software. To eliminate noise from heartbeats, respiration, and ambient light, we applied a low-pass filter (rejected channels with a coefficient of variation > 15%). The light intensity was converted to optical density, and for patients with left hemisphere lesions, the scalp channel position of the fNIRS data was reversed along the midsagittal plane to the right hemisphere. The concentrations of HbO2 and deoxygenated hemoglobin (HbR) were calculated based on the Modified Beer-Lambert law (MBLL) to create and analyze a brain function activation diagram. For within-group comparisons, a paired t-test was conducted, while differences among the three groups were assessed using a one-way ANOVA. Statistical significance was determined at P < 0.05.
Results
Comparison of Patient Baseline Data
Out of the 13 participants experiencing hemiplegic shoulder pain who were part of the study, 4 chose to discontinue their participation as they were discharged and unable to attend the second and third treatment sessions. Eventually, the analysis focused on 11 patients from both the TENS and tDCS+TENS groups, as well as 9 patients from the tDCS group. Table 1 provides information on the participants’ demographic and baseline characteristics. The findings indicated that there were no significant differences among the three treatment groups in terms of age, gender, hemiplegic side, disease progression, duration of shoulder pain, stroke type, VAS, CPT, and PTT (P >0.05).
 |
Table 1 Demographics and Baseline Evaluations |
Comparison of Three Treatment Methods for Pain Improvement
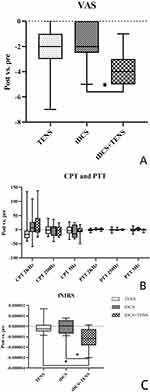
Table 2 presents the pain levels experienced during passive movement, as assessed using the VAS, before and after the intervention across the three groups. A significant improvement was observed in all three groups when comparing the pain levels before and after the intervention (TENS P = 0.004, 95% CI: −3.62~−0.93; tDCS p = 0.017, 95% CI: −2.94~−0.39; tDCS+TENS P < 0.001, 95% CI: −4.78~−3.04). Following the intervention, there was a statistically significant difference between the tDCS+TENS group and the tDCS group (P = 0.011). However, no significant difference was found between the tDCS+TENS group and the TENS group (P = 0.197), or between the tDCS group and the TENS group (P = 0.559).(Figure 4A).
 |
Table 2 Visual Analogue Scale Values Before and After Intervention in the Three Groups (Intra-Group and Inter-Group Differences) |
Comparison of CPT and PTT Before and After Three Treatment Methods
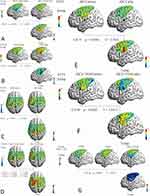
Table 3 displays the CPT and PTT changes prior to and after the intervention. There were no significant variations observed in any of the test stimulations, either within the same group (P > 0.05) or between different groups (CPT-2K Hz: F = 0.26, P = 0.775; CPT-250 Hz: F = 0.43, P = 0.653; CPT-5 Hz: F = 0.99, P = 0.385; PTT-2K Hz: F = 1.15, P = 0.333; PTT-250 Hz: F = 0.37, P = 0.692; PTT-5 Hz: F = 0.33, P = 0.719). Furthermore, there was no statistical significance found in CPT and PTT changes between the pre- and post-stimulation periods (CPT-2K Hz: F = 0.82, P = 0.451; CPT-250 Hz: F = 0.12, P = 0.886; CPT-5 Hz: F = 0.22, P = 0.801; PTT-2K Hz: F = 1.48, P = 0.244; PTT-250 Hz: F = 0.32, P = 0.731; PTT-5 Hz: F = 1.24, P = 0.305) (Figure 4B). A notable distinction was observed in the average level of HbOμm in the opposite PMC/SMA (channel 38: T = 2.693, P = 0.043) following stimulation during the PPT 2K Hz task, when comparing the baseline measurement with the post-intervention measurement after applying tDCS+TENS (Figure 5A). There were no significant differences in the average HbOμm in either the tDCS group or the TENS group before and after the intervention. However, during the PPT 250 Hz task, a significant difference in average HbOμm was found in the opposite SMC (channel 21: T = −2.756, P = 0.028) when comparing the post-TENS measurement with the baseline (Figure 5B). Additionally, a significant difference in average HbOμm was observed in the opposite SMC (channel 05: T = 3.128, P = 0.017) in the tDCS group (Figure 5C). The tDCS+TENS group showed statistically different average HbOμm in the opposite PMC/SMA (channel 18: T = 3.230, P = 0.010; channel 31: T = 2.859, P = 0.029; channel 34: T = 2.604, P = 0.031) (Figure 5D). During the PPT 5 Hz task immediately after stimulation, there was a significant difference in average HbOμm in the opposite SMC of the tDCS group (channel 37: T = 2.544, P = 0.038) (Figure 5E), and in the opposite PFC (prefrontal cortex) of the tDCS+TENS group (channel 49: T = 3.652, P = 0.006) (Figure 5F) between pre- and post-intervention measurements. However, there was no statistically significant difference in the TENS group.
 |
Table 3 Sensory and Pain Thresholds Measurements Before and After Intervention in the Three Groups (Intra-Group and Inter-Group Differences) |
Comparison of fNIRS Results Among Three Treatment Methods
Comparing the difference in average HbO levels before and after the intervention, a significant difference was found in the opposite PMC/SMA during the 2K Hz PPT task in all three groups (channel 38: F = 4.196, P = 0.030; tDCS+TENS vs TENS: P = 0.043, 95% CI: 3.310e-08~ 2.248e-06; tDCS+TENS vs TDCS: P = 0.048, 95% CI: −2.171e-06~-9.400e-09) (Figures 4C and 5G). There were no significant differences in the average HbO levels before and after the intervention among the three groups during the 250 Hz and 5 Hz PPT tasks.
Adverse Effects
During the initial tDCS session, one participant encountered a slight headache, while no significant harmful outcomes were observed across the three groups.
Discussion
Pain is a major public health challenge in modern society. It is estimated that the prevalence of chronic pain is as high as 20%-50%,15,16 causing economic losses of billions of RMB in China every year.17,18 Electrical stimulation nerve regulation technology is a non-pharmaceutical pain treatment method with great potential. This method has been applied in the treatment of clinical pain.
TENS applies electrical stimulation through electrodes placed on the surface of the skin to produce a pain relief effect in individuals. TENS has a good alleviating effect on some clinical chronic pains.19,20 The analgesic effect of TENS can be explained by the gate control theory.9 This theory suggests that the transmission of pain information depends on the relative activation of the coarse fibers (Aβ fibers) that transmit touch and pressure sensation and the fine fibers (Aδ and C fibers) that transmit pain and warmth sensation. Aβ fibers tend to activate the substantia gelatinosa (SG) cells of the spinal dorsal horn first, thereby inhibiting the activation of secondary neurons T cells and preventing the transmission of pain information. Aδ and C fibers tend to inhibit SG cells, thereby activating secondary neurons T cells and promoting the transmission of pain information. TENS is usually strong but not painful, and it hardly activates Aδ and C fibers, but it can activate Aβ fibers,21 thereby inhibiting the transmission of pain information and producing a pain relief effect.
tDCS uses two or more electrodes to apply direct current stimulation to specific brain regions, thereby altering their excitability and regulating the activity of the corresponding areas, which has a pain relief effect.22,23 Currently, there are numerous studies on the use of tDCS to alleviate both acute and chronic pain. Although some studies have questioned the analgesic effect of tDCS,24,25 most results still support the significant analgesic effect of tDCS on both acute and chronic pain.26,27 Typically, the mechanism of tDCS is that the current alters the excitability of nerve cells when it passes through brain tissue.22,28 The current intensity used in tDCS is usually weak and does not usually cause action potentials,29 but only changes the resting membrane potential of nerve cells, thereby regulating their excitability.22,28 The change in membrane potential is the physiological basis for the immediate regulatory effect of tDCS.30 DCS not only affects the activity of specific regions but also has a broader impact on brain networks.13,31 These effects can change the connections and functions between different regions in the brain.32,33
Although there are currently many studies on the above two analgesic methods in clinical practice,32,33 there is still a lack of research on their application in the treatment of pain in PSSP patients. PSSP is the result of multiple factors.34,35 Its causes are complex, and there is currently no good treatment method in clinical practice. Based on this, this article explores the application value of TENS and tDCS in the treatment of PSSP.
In this study, VAS assessment results showed that both tDCS and TENS had analgesic effects on PSSP. We further explored the mechanism using fNIRS, and observed that when TENS or tDCS was applied alone, the HbO level mainly changed in the SMC region. We further evaluated the analgesic effect of tDCS and TENS combined use on PSSP using VAS. The results showed that compared with tDCS alone, the combination of tDCS and TENS had better analgesic effects on PSSP. In addition, compared with the effects of tDCS or TENS alone, the combination of tDCS and TENS could regulate the sensitivity of Aβ fibers by effectively stimulating large-diameter afferent Aβ fibers, thereby reducing pain sensation. Furthermore, compared with tDCS or TENS alone, TENS enhanced the effect of tDCS on the excitability of PMC/SMA. Specifically, after tDCS+TENS intervention, the activity of the unaffected hemisphere decreased, indicating that tDCS can potentially balance the activity of both hemispheres. This finding supports the view that tDCS can regulate target functional networks through neuroplasticity, consistent with previous reports.36,37 Using fNIRS to observe the mechanism of combined treatment, we noticed that HbO signal changes not only occurred at the directly stimulated site but also occurred in other brain regions such as the PFC. These findings suggest that the tDCS+TENS method may activate a wide range of neural and/or vascular networks, indicating that combined central and peripheral electrical stimulation may benefit the pain system by promoting neuroplasticity in specific functional networks.
The imbalance between the cerebral hemispheres after a stroke may be the basis for compensating for loss of function (including regulating pain transmission) after a stroke.38 Previous studies have shown that in a patient with chronic painful stroke who received 10 active tDCS stimuli, the imbalance of motor activity between the left and right hemispheres was improved.37 The results of this study indicate that the combination of tDCS+TENS can offset the impact of stroke on cross inhibition by restoring balance between the two hemispheres. After the combined use of tDCS and TENS intervention, we observed a decrease in excitability in the PMC/SMA region on the healthy side. Although there was no significant difference in PTT before and after the combined intervention, brain activation levels decreased after the combined intervention under pain stimuli of similar intensity. This decrease indicates a decrease in central sensitivity, indicating that stroke patients participating in this study had central sensitization in patients with chronic shoulder pain before intervention. In addition, when stimulated by pain, the opposite hemisphere of the affected shoulder exhibits increased excitatory activation. This response may be attributed to long-term dysfunction of the contralateral cortex, which compensates for pain related injuries.
There were some limitations in our study as follows: Firstly, the sample size was small and the observation time was short. Secondly, the assessment of the central system was limited to cortical regions, so we did not analyze the influence of deep brain nuclei on the overall function of the brain network. Thirdly, there was no placebo group included, which made it difficult to exclude the possibility of placebo effects.
Conclusion
Our research results indicate that compared to using tDCS or TENS alone, the combination of tDCS and TENS can amplify the pain relief effect on PSSP. The main mechanism is that tDCS+TENS treatment can significantly reduce the activation of PMC/SMA on the opposite side of the site of action, while Aβ The sensitivity of fibers increases. To verify our results, future investigations should include larger scale high-quality trials.
Abbreviations
PSSP, post stroke shoulder pain; TENS, transcutaneous electrical nerve stimulation; tDCS, transcranial direct current stimulation; fNIRS, functional near-infrared spectroscopy; CRPS, complex regional pain syndrome; CPSP, central poststroke pain; CPT, current perception thresholds; PTT, pain tolerance threshold; VAS, visual analogue scale; SAC, the somatosensory association cortex; SMC, the somatosensory cortex; M1, the primary motor cortex; PMC, the premotor cortex; SMA, the supplementary motor area; PFC, the prefrontal cortex; HbR, deoxygenated hemoglobin; HbO, oxygenated hemoglobin; IHI, interhemispheral inhibition.
Data Sharing Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study protocol was approved by the ethics committee of the first affiliated hospital of Fujian Medical University, No: MRCTA, ECFAH of FMU [2020] 269. A written informed consent was obtained from all participants.
Consent for Publication
Consent for publication was obtained from every individual whose data are included in this manuscript.
Acknowledgments
The authors wish to thank all participants for their willingness and help. We would like to thank the reviewers for their constructive criticism and suggestions.
Funding
This work was supported by the StartupFund for scientific research, Fujian Medical University (2019QH1086, Fuzhou) to Y Li.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Feigin VL, Stark BA, Johnson CO. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi:10.1016/S1474-4422(21)00252-0
2. Zhang Q, Chen D, Shen Y, Bian M, Wang P, Li J. Incidence and prevalence of poststroke shoulder pain among different regions of the world: a systematic review and meta-analysis. Front Neurol. 2021;12:724281. doi:10.3389/fneur.2021.724281
3. Adey-Wakeling Z, Arima H, Crotty M, et al. Incidence and associations of hemiplegic shoulder pain poststroke: prospective population-based study. Arch Phys Med Rehabil. 2015;96(2):241–247.e1. doi:10.1016/j.apmr.2014.09.007
4. Zhan J, Wei X, Tao C, et al. Effectiveness of acupuncture combined with rehabilitation training vs. rehabilitation training alone for post-stroke shoulder pain: a systematic review and meta-analysis of randomized controlled trials. Front Med. 2022;9. doi:10.3389/fmed.2022.947285
5. Deer TR, Eldabe S, Falowski SM, et al. Peripherally induced reconditioning of the central nervous system: a proposed mechanistic theory for sustained relief of chronic pain with percutaneous peripheral nerve stimulation. J Pain Res. 2021;14:721–736. doi:10.2147/JPR.S297091
6. Feng J, Shen C, Zhang D, Yang W, Xu G. Development and validation of a nomogram to predict hemiplegic shoulder pain in patients with stroke: a retrospective cohort study. Arch Rehabil Res Clin Transl. 2022;4(3):100213. doi:10.1016/j.arrct.2022.100213
7. Torres-Parada M, Vivas J, Balboa-Barreiro V, Marey-López J. Post-stroke shoulder pain subtypes classifying criteria: towards a more specific assessment and improved physical therapeutic care. Braz J Phys Ther. 2020;24(2):124–134. doi:10.1016/j.bjpt.2019.02.010
8. Li Z, Alexander SA. Current evidence in the management of poststroke hemiplegic shoulder pain: a review. J Neurosci Nurs. 2015;47(1):10–19. doi:10.1097/JNN.0000000000000109
9. Mokhtari T, Ren Q, Li N, Wang F, Bi Y, Hu L. Transcutaneous electrical nerve stimulation in relieving neuropathic pain: basic mechanisms and clinical applications. Curr Pain Headache Rep. 2020;24(4):1–14.
10. Vance CGT, Dailey DL, Chimenti RL, Van Gorp BJ, Crofford LJ, Sluka KA. Using TENS for pain control: update on the state of the evidence. Medicina. 2022;58(10):1332. doi:10.3390/medicina58101332
11. Yang S, Chang MC. Transcranial direct current stimulation for the management of neuropathic pain: a narrative review. Pain Physician. 2021;24:E771–E781.
12. Molero-Chamizo A, Salas Sánchez Á, Álvarez Batista B, et al. Bilateral motor cortex tDCS effects on post-stroke pain and spasticity: a three cases study. Front Pharmacol. 2021;12:624582. doi:10.3389/fphar.2021.624582
13. Yang QH, Zhang YH, Du SH, Wang YC, Fang Y, Wang XQ. Non-invasive brain stimulation for central neuropathic pain. Front Mol Neurosci. 2022;15:879909. doi:10.3389/fnmol.2022.879909
14. Knotkova H, Hamani C, Sivanesan E, et al. Neuromodulation for chronic pain. Lancet. 2021;397(10289):2111–2124. doi:10.1016/S0140-6736(21)00794-7
15. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi:10.1016/j.ejpain.2005.06.009
16. Chen B, Li L, Donovan C, et al. Prevalence and characteristics of chronic body pain in China: a national study. Springerplus. 2016;5(1):938. doi:10.1186/s40064-016-2581-y
17. Yu S, Liu R, Zhao G, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache J Head Face Pain. 2011;52(4):582–591. doi:10.1111/j.1526-4610.2011.02061.x
18. Zhang F, Xiang W, Li C, Li S. Economic burden of irritable bowel syndrome in China. World J Gastroenterol. 2016;22(47):10450–10460. doi:10.3748/wjg.v22.i47.10450
19. Searle RD, Bennett MI, Johnson MI, Callin S, Radford H. Transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. J Pain Symptom Manage. 2009;37(3):424–428. doi:10.1016/j.jpainsymman.2008.03.017
20. van der Spank JT, Cambier DC, De Paepe HMC, Danneels LAG, Witvrouw EE, Beerens L. Pain relief in labour by transcutaneous electrical nerve stimulation (TENS). ArchGynecol Obstet. 2000;264(3):131–136.
21. Vance C, Dailey D, Rakel B, et al. A novel method to obtain higher intensity TENS stimulation in clinical application. J Pain. 2015;16(4):S93. doi:10.1016/j.jpain.2015.01.388
22. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimulat. 2008;1(3):206–223. doi:10.1016/j.brs.2008.06.004
23. Pinto CB, Costa BT, Duarte D, Fregni F. Transcranial direct current stimulation as a therapeutic tool for chronic pain. J ECT. 2018;34(3):E36–E50. doi:10.1097/YCT.0000000000000518
24. Serrano GB, Rodrigues LP, Schein B, et al. Comparison of hypnotic suggestion and transcranial direct-current stimulation effects on pain perception and the descending pain modulating system: a crossover randomized clinical trial. FrontNeurosci. 2019;13:662.
25. Mariano TY, Burgess FW, Bowker M, et al. Transcranial direct current stimulation for affective symptoms and functioning in chronic low back pain: a pilot double-blinded, randomized, placebo-controlled trial. Pain Med. 2019;20(6):1166–1177. doi:10.1093/pm/pny188
26. Auvichayapat P, Keeratitanont K, Janyachareon T, Auvichayapat N. The effects of transcranial direct current stimulation on metabolite changes at the anterior cingulate cortex in neuropathic pain: a pilot study. J Pain Res. 2018;11:2301–2309. PMID: 30349356; PMCID: PMC6188066. doi:10.2147/JPR.S172920
27. Suchting R, Colpo GD, Rocha NP, Ahn H. The effect of transcranial direct current stimulation on inflammation in older adults with knee osteoarthritis: a Bayesian residual change analysis. Biol Res Nurs. 2020;22(1):57–63. doi:10.1177/1099800419869845
28. To WT, Hart J, Ridder DD, Vanneste S. Considering the influence of stimulation parameters on the effect of conventional and high-definition transcranial direct current stimulation. Expert Rev Med Devices. 2016;13(4):391–404. doi:10.1586/17434440.2016.1153968
29. Bikson M, Inoue M, Akiyama H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro: modulation of neuronal function by electric fields. J Physiol. 2004;557(1):175–190. doi:10.1113/jphysiol.2003.055772
30. Nitsche MA, Kuo MF, Paulus W, Antal A. Transcranialdirect current stimulation: protocols and physiologicalmechanisms of action. In: Knotkova H, Rasche D, editors. Textbook of Neuromodulation. Springer New York Press; 2015:101–111.
31. Stagg CJ, Antal A, Nitsche MA. Physiology of transcranial direct current stimulation. J ect. 2018;34(3):144–152. doi:10.1097/YCT.0000000000000510
32. Cummiford CM, Nascimento TD, Foerster BR, et al. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther. 2016;18(1):40. doi:10.1186/s13075-016-0934-0
33. Lin RL, Douaud G, Filippini N, Okell TW, Stagg CJ, Tracey I. Structural connectivity variances underlie functional and behavioral changes during pain relief induced by neuromodulation. Sci Rep. 2017;7(1):41603. doi:10.1038/srep41603
34. Anwer S, Alghadir A. Incidence, prevalence, and risk factors of hemiplegic shoulder pain: a systematic review. Int J Environ Res Public Health. 2020;7(14):4962. doi:10.3390/ijerph17144962
35. Chen K. Research progress on the pathogenesis and treatment of hemiplegic shoulder pain after stroke. Chin J Brain Dis Rehabil. 2019;9(6)375–379.
36. Kim H, Kim J, Lee G, Lee J, Kim YH. Task-related hemodynamic changes induced by high-definition transcranial direct current stimulation in chronic stroke patients: an uncontrolled pilot fNIRS study. Brain Sci. 2022;13(1):12. doi:10.3390/brainsci13010012
37. Morishita T, Hyakutake K, Saita K, Takahara M, Shiota E, Inoue T. Pain reduction associated with improved functional interhemispheric balance following transcranial direct current stimulation for post-stroke central pain: a case study. J Neurol Sci. 2015;358(1–2):484–485. doi:10.1016/j.jns.2015.08.1551
38. Boddington LJ, Reynolds JNJ. Targeting interhemispheric inhibition with neuromodulation to enhance stroke rehabilitation. Brain Stimul. 2017;10(2):214–222. doi:10.1016/j.brs.2017.01.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.