Back to Journals » Research and Reports in Urology » Volume 15
Investigating the Impact of Kratom (Mitragyna speciosa) Use Upon Male Sexual Health
Authors Deebel NA, Scarberry K, O'Connor CA, Dutta R, Matz E, Hanlon CA, Terlecki RP
Received 23 September 2022
Accepted for publication 20 January 2023
Published 10 February 2023 Volume 2023:15 Pages 69—76
DOI https://doi.org/10.2147/RRU.S390094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Nicholas A Deebel,1 Kyle Scarberry,2 Collette A O’Connor,1 Rahul Dutta,1 Ethan Matz,1 Colleen A Hanlon,3 Ryan P Terlecki1
1Department of Urology, Atrium Health Wake Forest Baptist Medical Center, Winston-Salem, NC, USA; 2Department of Urology, University Hospitals Cleveland Medical Center, Cleveland, OH, USA; 3Department of Cancer Biology, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Correspondence: Ryan P Terlecki, Department of Urology, Atrium Health Wake Forest Baptist Medical Center, 1 Medical Center Blvd, Winston-Salem, NC, 27157, Tel +1 336 716 4131, Fax +1 336 716 9042, Email [email protected]
Purpose: Kratom (Mitragyna speciosa) exhibits μ-receptor agonism and is used as an opioid substitute. While opioids are known to inhibit sexual behavior, less is known regarding kratom. We conducted a pilot study to assess the subjective impact of kratom upon male sexual health including erectile and ejaculatory function.
Patients and Methods: Twitter and Reddit (r/Kratom) were accessed to disseminate our survey featuring validated instruments (the International Index of Erectile Function, IIEF, and the premature ejaculation diagnostic tool, PEDT). Sexual health prior to and after 4 weeks of kratom use was assessed.
Results: Most males surveyed (n = 165) were 18– 40 years old (84.9%), with 95.8% of respondents using it at least weekly and 82.4% using kratom for ≥ 1 year. Reasons for use included treating pain (39.4%), and mental health conditions (63.6%). Kratom was associated with a positive (37.7%) and negative (20.5%) impact on sexual health. Kratom subjectively increased time to ejaculation in 104 (66.6%) patients, perceived as positive by 62 (59.6%). Seventy-eight patients answered questions about premature ejaculation. The median (with interquartile range, IQR, following;) pre-kratom and kratom use scores were 13.0; 8.0 and 6.5; 5.0, respectively (p < 0.001). Ejaculation before 5 minutes improved after kratom (51.3% vs 12.8%) (p < 0.0001). Following kratom use, patients reported lack of frustration with ejaculation prior to desire (21.8% vs 61.5%) (p < 0.001). The erectile function domain of the IIEF was statistically significantly different however – clinically similar pre-kratom use (29.0; 5.75) versus 27.0; 6.75 during kratom use (p = 0.037).
Conclusion: Clinicians treating male sexual health should be aware of kratom and its potential effect on ejaculatory and erectile function.
Keywords: kratom, Mitragyna speciosa, premature ejaculation, analgesia, sexual function
Introduction
Kratom (Mitragyna speciosa) is a plant native to regions of Southeast Asia such as Thailand, Indonesia and Malaysia.1 Its leaves can be ingested, smoked directly, boiled in water to be ingested as a decoction, or ground into a powder which can then be used to make tea.2 Historically, it has been used in Southeast Asia for the treatment of acute and chronic pain, fatigue, as well as an opioid substitute in the treatment of opiate addiction and dependency.2,3 More recently, kratom use has also gained traction in the western world for its proposed analgesic properties amidst the ongoing opioid epidemic heavily affecting the United States (US).4
Currently, over 40 compounds have been identified in kratom. Of these, four psychoactive alkaloids have been recognized as pharmacologically active: mitragynine, 7-hydroxymitragynine, speciociliatine, and corynantheidine.2 Mitragynine and 7-hydroxymitragynine have been shown to act as partial μ-receptor agonists and competitive antagonists at δ-receptors with minimal κ-receptor activity.2,5 While mitragynine is the most represented psychoactive alkaloid in kratom, its oxidized form, 7-hydroxymitragynine is 46 times more potent than the former and 13 times more potent than morphine.6,7 While 7-hydroxymitragynine may represent only 0.02% of a given kratom sample, recent work has shown that mitragynine is processed hepatically by the CYP3A4 enzyme to form the more potent 7-hydroxymitragynine variant.2,8 Preliminary evidence shows mitragynine has affinity for the α-2 receptor (postulated to modulate alternative pain pathways) as well as 5-HT serotonin receptors, D2 dopamine receptors and A2A adenosine receptors (yet unknown physiological significance).2,9
There is a well-established connection between traditional opioid abuse and men’s sexual health including androgen deficiency (opioid-induced androgen deficiency; OPIAD).10 Studies assessing the prevalence of OPIAD have shown that 90% of the symptomatic men and 53% of the asymptomatic men on chronic opioid therapy exhibit OPIAD.10–12 Opioids interfere with the pulsatile release of gonadotropin-releasing hormone (GnRH) as well as the hypothalamic-adrenal axis through the reduction in secretion of dehydroepiandrosterone sulfate (DHEAS).10 Chronic opioid use has also been associated with fertility impairment including decreased sperm concentration and increased DNA fragmentation.13 Additionally, the synthetic opioid tramadol carries the side effect of delayed ejaculation and has been utilized as a therapy for premature ejaculation (PE) in sexual health clinics.14,15
Several studies have attempted to characterize the prevalence of kratom use in the US. A survey by Smith et al 2017 found a reported lifetime use in 21% of the respondents. However, this data was drawn from a population receiving treatment for substance abuse.16 Selection bias may affect studies involving recruitment through the American Kratom Association webpage.3,17 Studies involving the greater US population estimate the prevalence of kratom use as 0.8–6.1%.4,18 However, as pointed out by Grundmann et al, given the level of self-medication among patients, the actual prevalence is likely higher.19
Many patients report using kratom for dependency related to prescription or illicit drugs, self-management of acute or chronic pain, anxiety, depression, or Post-Traumatic Stress Disorder (PTSD).3 Perceived benefits among surveyed patients included increased energy, elevated mood, increased focus, and reduction of anxiety and PTSD symptoms.3 Since kratom is legal in all but 6 US states, many people use this agent to obtain an opioid-like high.2 However, side effects and adverse events have been reported.
There are extremely limited data related to the effect of kratom on men’s sexual health. Several anecdotal reports from Thailand and Malaysia detail the use of kratom for male enhancement, but detailed information is lacking.20–23 A single-case report describes a rise in prolactin and secondary hypogonadism in a 42-year-old man.24 Finally, a survey-based study examining long-term, kratom users in Penang, Malaysia, showed increased energy during sex, delay in time to ejaculation, increased sexual desire, and increased ability to maintain an erection.25 However, there remains a paucity of detail surrounding kratom’s specific impact on male sexual health. For this study, we sought to query users about their sexual health at baseline and following initiation of kratom therapy in both a qualitative and quantitative fashion from a western medicine perspective.
Materials and Methods
Patient Selection
This pilot study was conducted over the course of 10 weeks (2019) through targeted crowdsourcing methods wherein the study was described in posts in Reddit (r/Kratom) forum and several Twitter-based Kratom groups which were chosen at the discretion of the investigators. The consent form for participation as well as the included surveys were pinned to the website for user access. The survey was available for a total of 10 weeks. Participants eligible for inclusion were adults with greater than or equal to 4 weeks of kratom use. Exclusion criteria included age under 18, non-english speaking status, and last kratom dose over a year ago.
A total of 263 participants from the United States filled out the consent form, 165 (62.7%) of those met the eligibility criteria, 165 completed the baseline assessments, 156 completed sexual health screening surveys, and 76 and 78 patients completed the follow-up assessments (the International Index of Erectile Function, IIEF, and the premature ejaculation diagnostic tool, PEDT, respectively).
Questionnaire Use
The present study complied with the Declaration of Helsinki. The protocol was reviewed and approved by the Institutional Review Board of Wake Forest Baptist Medical Center (approval No. 00060915). Informed consent was obtained digitally by all subjects prior to participation. The primary outcomes of the study were to assess erectile function and ejaculatory function as it relates to kratom use. Participants were prompted to provide demographic information (age, ethnicity, sexual orientation, marital status) and to complete a series of questions related to kratom. Participants were also asked to identify their typical dose and frequency of kratom use (<1g, 1–3g, 3–5g, >5g) (less than weekly, less than once a day but at least weekly, once daily, twice daily, three or more times daily). Additionally, a questionnaire regarding each participant’s perceived effect of the agent on their sexual health was completed. Response to these questions (reporting perceived change in sexual health) prompted the participant to complete the previously validated IIEF and PEDT questionnaires.26 The sexual health surveys were completed before and after 4 weeks of kratom consumption. While participants were not guaranteed compensation for their participation, completing the surveys made them eligible for one of three $50.00 gift cards. Winners for the gift card were chosen with the use of a random number generator. The survey was administered through a secure, Redcap database and given the cross-sectional nature of the project ongoing communication with the participants was not required for this study.
Statistical Analysis
In order to analyze the primary outcomes (IIEF and PEDT), participants with incomplete demographic, sexual health, kratom use, IIEF or PEDT surveys were excluded. Data were assessed for Gaussian distribution using the Shapiro–Wilk normality test. Given the lack of Gaussian distribution (p > 0.05), the comparison of paired data was performed using the Wilcoxon matched-pairs signed rank test and unpaired data with the Mann–Whitney test. In order to perform a one-way ANOVA test to ascertain the differences in kratom frequency or dose and the primary outcomes (IIEF and PEDT scores) the Kruskal–Wallis test was performed. The Chi-square test was used to analyze frequencies of categorical variables (Prism 8, GraphPad).
Results
Patient Demographics and Kratom Use
Following a 10-week recruitment period, 165 males participated in the initial phases of our surveys. Male participants were typically 18–40 years old (84.9%) with less than 1% over the age of 61 (0.6%). The predominant ethnicity and sexual orientation were white (non-Hispanic) 86.7% and heterosexual (89.1%), respectively. Overall, 82.4% of the participants (n = 134) reported using kratom for 1 or more years. There was a wide range of kratom dosage per administration (Figure 1); however, the most common was 3–5 grams (40.6%, n = 67). Similarly, there was variation in frequency of use (Figure 1), with the most common being ≥3 times per day (39.4%, n = 65). When queried about motivation for use, the most common reason was for treatment of a mental health condition (63.6%) (Table 1).
 |
Table 1 Distribution of Reported Reasons for Using Kratom (Some Respondents Reported Multiple Reasons for Kratom Consumption) |
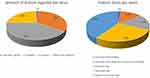 |
Figure 1 Patterns of kratom use amongst study participants as it relates to typical dose (A) and number of doses per week (B). |
Impact of Kratom Use on Sexual Health (IIEF and PEDT)
Prior to obtaining objective measures on the impact of kratom on sexual health, respondents were queried about perceived effects. In total, 156 participants completed a subjective sexual health survey. The effect of kratom on overall sexual health was viewed as positive for 37.7% (n=59) and negative for 20.5% (n=32) (p = 0.0008). The effect of kratom on enjoyment of sexual activity was viewed as positive for 42.9% (n=67) and negative for 15.4% (n=24) (p = 0.0001). The ability to obtain/maintain an erection was subjectively impaired in 23.1% (n=36) while improved in 23.7% (n=37) of the participants (p > 0.05). Kratom use reportedly increased time to ejaculation in 104 (66.6%) patients, which was perceived as a positive effect by 62 (59.6%). Finally, kratom use lead to a decrease in sexual desire for 37.8% (n=59) yet an increase for 37.2% (n=58) of the respondents (p > 0.05). Only three respondents (1.9%) reported a diagnosis of low testosterone following the use of kratom.
Following the subjective assessment, the IIEF questionnaire was administered. There was a statistically significant decline in the orgasmic function and sexual desire domain following 4 weeks of kratom use (Table 2). There was a statistically significant increase in the overall satisfaction domain with kratom use. The sexual desire domain of the patients who subjectively reported trouble with libido was not dose dependent (p = 0.7). Finally, the erectile function domain was statistically significantly different however – clinically similar pre-kratom use (29.0; 5.75) versus 27.0; 6.75 during kratom use (p = 0.037). When stratified by patients who self-reported impairment versus improvement in erection with kratom use, there was a significant difference (24.0; 7.0 versus 28.0; 4.5 respectively) (p = 0.0005). The erectile function domain of the patients who subjectively reported impairment in their erectile function was not dose dependent (p = 0.7).
 |
Table 2 Patterns in International Index of Erectile Function Prior to and with Kratom Use (Median; IQR) |
Among the 78 patients who completed the PEDT, median (with interquartile range, IQR, following;) pre-kratom and kratom use scores were 13.0; 8.0 and 6.5; 5.0, respectively (p < 0.001). Ejaculation prior to 5 minutes was improved after kratom use (n=40; 51.3% vs n=10; 12.8%) (p < 0.0001) (Figure 2). Following kratom use, more patients reported lack of frustration with ejaculation prior to desire n=17 (21.8%) vs n=48 (61.5%) (p < 0.001). There was not a significant difference in PEDT scores in kratom users based on dose (p = 0.8).
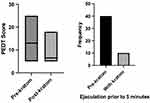 |
Figure 2 Effect of kratom consumption on ejaculatory function as measured by the median PEDT score and self-reported change in ejaculation prior to 5 minutes. |
Kratom users were unlikely to report concerns regarding fertility (n=1, 6.1%), testosterone levels (n=25, 15.2%), libido (n=38, 23.0%), or ED (n=30, 18.2%). Users rarely stopped kratom due to concerns regarding delayed ejaculation (n=5, 10%), low libido (n=8, 7.5%), or ED (n=6, 7.9%).
Discussion
To the best of our knowledge, this is the first study to utilize the IIEF and the PEDT questionnaire to examine the objective impact of kratom use on male sexual health. Our preliminary data suggest kratom use is associated with mixed perceptions as to the impact upon overall sexual health and desire among patients. Two-thirds of participants reported that kratom use increased time to ejaculation. This was substantiated by an improvement in PEDT scores before and after kratom use. Based on the work of Symonds et al 2008, PEDT scores are classified as follows: ≤8 no premature ejaculation (PE), 9–10 probable PE, and ≥11 as PE.26 Therefore, on average, our participants had baseline PEDT scores indicative of PE, and kratom use scores indicative of improvement to the point of resolution. This is consistent with the work of Singh et al who demonstrated improvement in the duration of erection and delay to ejaculation based on the subjective reports of participants consuming kratom.25
While there are a myriad of proposed treatments for PE, one of these includes use of on-demand tramadol (off-label). According to the work of Frink et al, the major metabolite of tramadol, O-desmethyltramadol (M1), has a 200-fold affinity for the μ-opioid receptor.27 However, it is hypothesized that tramadol ameliorates PE through CNS modulation, possibly by inhibiting reuptake of serotonin and norepinephrine.27,28 Despite the paucity of quality evidence for tramadol’s efficacy in treating PE, data suggest it offers a mild improvement in Intravaginal Ejaculation Latency Time (IELT). A blinded study by Bar-Or et al compared tramadol (62 mg or 89 mg Oral Disintegrating Tablet, ODT) to placebo and found an IELT increase of 1.2 minutes (2.4-fold) and 1.5 minutes (2.5-fold) associated with tramadol 62mg and 89 mg ODT respectively.29 The authors speculated this was likely due to weak inhibition of serotonin reuptake. Similarly, kratom has affinity for the 5-HT2C and 5-HT7 receptors.2 Despite the unknown clinical significance of this, it is possible that kratom could affect IELT via a mechanism similar to tramadol and the far more potent paroxetine.
While only 4.8% of the participants reported using kratom to primarily address their sexual health, our study implies that the perception of sexual health effects attributed to kratom use is impressive. While respondents reported kratom having a positive impact on their sexual health (37.7%) and enjoyment of sexual activity (42.9%), there was also a significant proportion (20.5% and 15.4%) who reported a negative effect. Analysis of objective data including the orgasmic function, sexual desire, erectile dysfunction, and overall satisfaction domains of the IIEF showed significant differences before and after initiating kratom consumption. However, it is important to acknowledge that despite statistical significance, differences within each domain were no more than 1.2 points and are unlikely to reflect clinical significance. Similarly, while there was a statistically significant difference in the erectile function domain after kratom initiation, the 0.7-point difference in the means is unlikely to be clinically significant change. Overall, a substantial proportion of participants viewed kratom as a positive influence on their sexual health, noting a delay in ejaculation which was objectively supported by decreased PEDT scores. To date, there is only one other study in the literature that surveys kratom users about its impact on male sexual health.25 Singh et al surveyed 92 long-term kratom users in Penang, Malaysia, using the Malay version Brief Male Sexual Function Inventory (Mal-BMSFI) and showed that the average Mal-BMSFI score was 33.9 (out of a total of 45). While this study did not capture subjective or objective sexual health metrics prior to initiation of kratom use, 85% of the participants reported subjective improvement in sexual performance with kratom consumption.
We should not ignore that some participants associated their kratom use with an impaired ability to maintain an erection (23.1%) and decreased sexual desire (37.8%). Similarly, Singh et al reported that 18/92 patients in their cohort were stratified into a “lower sexual performance cluster” and had a significantly decreased Mal-BMSFI of 22.2 with decreased erectile metrics compared to the “higher sexual performance cluster.”25 A separate qualitative interview of 34 kratom users demonstrated significant heterogeneity in the perception of the related effect on sexual drive.23 These findings are in line with known side effects of other therapeutic options for PE, including SSRIs and on-demand tramadol. However, there may be significant confounders present in this study given the prevalence of mental health disorders and likely concomitant pharmacologic treatment of these conditions; all of which are also known to affect men’s sexual health. Therefore, further work is needed to investigate kratom’s sexual side effect profile in a more controlled nature. Doing so will help clinicians more adequately counsel patients about kratom use.
Despite the novelty of this study, several limitations exist. This project was of a cross-sectional nature, subjecting it to several types of bias. Participants completed the “pre-kratom” survey in retrospect, introducing recall bias. Additionally, the opportunity to participate in this study was presented through r/Kratom and Twitter advocacy groups. Recruited participants may have been reticent to report negative implications of kratom use. It is unknown whether individuals recruited through these platforms accurately represent the greater (and larger) population of kratom users, introducing a selection and possible response bias. This seems compounded by the complicated political climate surrounding kratom use, with numerous prior attempts to classify it as a Schedule I drug. Finally, the reported metrics are largely subjective and show some heterogeneity with regard to the impact on sexual health. Given the lack of statistical and clinical significance among many of the IIEF parameters, greater clarity could be obtained with a prospective study. Despite the evident methodological limitations in this work, men’s health as it relates to the ongoing opioid epidemic is often overlooked. This work serves to highlight the potential sexual health ramifications of kratom and call for more future methodologically sophisticated, prospective studies.
Further work is needed to objectively assess kratom’s potential therapeutic role in sexual health, which would necessitate an understanding of both efficacy and safety. In a review of 935 instances of kratom use, Eggleston et al noted adverse events to include “agitation, tachycardia, drowsiness, hallucination, respiratory depression, coma, and cardiac or respiratory arrest”. Kratom was listed as the isolated cause of death in two patients.30
Conclusion
Male users of kratom were found to report varied effects upon sexual health, with a small proportion admittedly using the drug for that purpose. Kratom appears to decrease PEDT scores, which may indicate a potential target of study for treatment of PE. While this study is not meant to support kratom use to treat PE, it should serve as a notice to physicians who treat men for sexual health in order to maintain awareness of this increasingly popular agent.
Acknowledgments
The authors would like to thank Matthew Cowper, MD, for his technical assistance at the inception of this project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa Korth). Addiction. 2008;103(6):1048–1050. doi:10.1111/j.1360-0443.2008.02209.x
2. Eastlack SC, Cornett EM, Kaye AD. Kratom-pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9(1):55–69. doi:10.1007/s40122-020-00151-x
3. Grundmann O. Patterns of Kratom use and health impact in the US-Results from an online survey. Drug Alcohol Depend. 2017;176:63–70. doi:10.1016/j.drugalcdep.2017.03.007
4. Covvey JR, Vogel SM, Peckham AM, Evoy KE. Prevalence and characteristics of self-reported kratom use in a representative US general population sample. J Addict Dis. 2020;38(4):506–513. doi:10.1080/10550887.2020.1788914
5. Kruegel AC, Gassaway MM, Kapoor A, et al. Synthetic and receptor signaling explorations of the Mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. J Am Chem Soc. 2016;138(21):6754–6764. doi:10.1021/jacs.6b00360
6. Yamamoto LT, Horie S, Takayama H, et al. Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa. Gen Pharmacol. 1999;33(1):73–81. doi:10.1016/S0306-3623(98)00265-1
7. Matsumoto K, Horie S, Ishikawa H, et al. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2004;74(17):2143–2155. doi:10.1016/j.lfs.2003.09.054
8. Kruegel AC, Uprety R, Grinnell SG, et al. 7-hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci. 2019;5(6):992–1001. doi:10.1021/acscentsci.9b00141
9. Matsumoto K, Horie S, Takayama H, et al. Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2005;78(1):2–7. doi:10.1016/j.lfs.2004.10.086
10. Hsieh A, DiGiorgio L, Fakunle M, Sadeghi-Nejad H. Management strategies in opioid abuse and sexual dysfunction: a review of opioid-induced androgen deficiency. Sex Med Rev. 2018;6(4):618–623. doi:10.1016/j.sxmr.2018.04.003
11. Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100(4):851–858. doi:10.1002/cncr.20028
12. Rubinstein AL, Carpenter DM, Minkoff JR. Hypogonadism in men with chronic pain linked to the use of long-acting rather than short-acting opioids. Clin J Pain. 2013;29(10):840–845. doi:10.1097/AJP.0b013e31827c7b5d
13. Safarinejad MR, Asgari SA, Farshi A, et al. The effects of opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity. Reprod Toxicol. 2013;36:18–23. doi:10.1016/j.reprotox.2012.11.010
14. Martyn-St James M, Cooper K, Kaltenthaler E, et al. Tramadol for premature ejaculation: a systematic review and meta-analysis. BMC Urol. 2015;15:6. doi:10.1186/1471-2490-15-6
15. Ciocanel O, Power K, Eriksen A. Interventions to treat erectile dysfunction and premature ejaculation: an overview of systematic reviews. Sex Med. 2019;7(3):251–269. doi:10.1016/j.esxm.2019.06.001
16. Smith KE, Lawson T. Prevalence and motivations for kratom use in a sample of substance users enrolled in a residential treatment program. Drug Alcohol Depend. 2017;180:340–348. doi:10.1016/j.drugalcdep.2017.08.034
17. Nicewonder JA, Buros AF, Veltri CA, Grundmann O. Distinct kratom user populations across the United States: a regional analysis based on an online survey. Hum Psychopharmacol. 2019;34(5):e2709. doi:10.1002/hup.2709
18. Schimmel J, Amioka E, Rockhill K, et al. Prevalence and description of kratom (Mitragyna speciosa) use in the United States: a cross-sectional study. Addiction. 2020; 116(1):176–181.
19. Grundmann O, Babin JK, Henningfield JE, et al. Kratom use in the United States: a diverse and complex profile. Addiction. 2020;116:202–203. doi:10.1111/add.15173
20. Vicknasingam B, Narayanan S, Beng GT, Mansor SM. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of Peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283–288. doi:10.1016/j.drugpo.2009.12.003
21. Ahmad K, Aziz Z. Mitragyna speciosa use in the northern states of Malaysia: a cross-sectional study. J Ethnopharmacol. 2012;141(1):446–450. doi:10.1016/j.jep.2012.03.009
22. Veltri C, Grundmann O. Current perspectives on the impact of Kratom use. Subst Abuse Rehabil. 2019;10:23–31. doi:10.2147/SAR.S164261
23. Saingam D, Assanangkornchai S, Geater AF, Balthip Q. Pattern and consequences of kratom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: a qualitative study. Int J Drug Policy. 2013;24(4):351–358. doi:10.1016/j.drugpo.2012.09.004
24. LaBryer L, Sharma R, Chaudhari KS, Talsania M, Scofield RH. Kratom, an emerging drug of abuse, Raises prolactin and causes secondary hypogonadism: case report. J Investig Med High Impact Case Rep. 2018;6:2324709618765022. doi:10.1177/2324709618765022
25. Singh DGO, Murugaiyah V, Rahim AB, Balasingam V. Improved sexual functioning of long-term daily users of Mitragyna speciosa (Korth). J Herb Med. 2019;19:100293.
26. Symonds T, Perelman MA, Althof S, et al. Development and validation of a premature ejaculation diagnostic tool. Eur Urol. 2007;52(2):565–573. doi:10.1016/j.eururo.2007.01.028
27. Frink MC, Hennies HH, Englberger W, Haurand M, Wilffert B. Influence of tramadol on neurotransmitter systems of the rat brain. Arzneimittelforschung. 1996;46(11):1029–1036.
28. Szkutnik-Fiedler D, Kus K, Balcerkiewicz M, et al. Concomitant use of tramadol and venlafaxine - evaluation of antidepressant-like activity and other behavioral effects in rats. Pharmacol Rep. 2012;64(6):1350–1358. doi:10.1016/S1734-1140(12)70932-5
29. Bar-Or D, Salottolo KM, Orlando A, Winkler JV, Tramadol OD. A randomized double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. Eur Urol. 2012;61(4):736–743. doi:10.1016/j.eururo.2011.08.039
30. Eggleston W, Stoppacher R, Suen K, Marraffa JM, Nelson LS. Kratom Use and Toxicities in the United States. Pharmacotherapy. 2019;39(7):775–777. doi:10.1002/phar.2280
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
