Back to Journals » Cancer Management and Research » Volume 12
Investigating the Effects of RBBP6 Gene Expression on Telomerase Activity in Cervical Cancer Cells
Authors Mosweu M , Motadi L , Moela P
Received 20 June 2020
Accepted for publication 3 September 2020
Published 29 October 2020 Volume 2020:12 Pages 10725—10734
DOI https://doi.org/10.2147/CMAR.S261576
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Mpho Mosweu,1,* Lesetja Motadi,2,* Pontsho Moela1,*
1University of Pretoria, Faculty of Natural and Agricultural Sciences, Department of Biochemistry, Hatfield, Pretoria 0002, South Africa; 2University of Johannesburg, Faculty of Science, Department of Biochemistry, Auckland Park Campus, Auckland Park, Johannesburg 2050, South Africa
*These authors contributed equally to this work
Correspondence: Lesetja Motadi Department of Biochemistry Faculty of Science, C2 Lab421, Kingsway Campus, P.O. Box 524, Auckland Park, Johannesburg 2006, South Africa
Email [email protected]
Background: RBBP6 is one of the genes identified as a proliferative gene that plays a role in cancer development, On the other hand both RBBP6 and telomerase activity have been shown to be increase in various cancers. E6 protein of HPV and RBBP6 is known to enhance the progression of cancer cells by interacting with p53 and presenting it to be ubiquitinated by the proteasome thereby promoting cell proliferation and preventing apoptosis. Studies also show that HPV E6 protein can increase telomerase activity by activating the expression of human telomerase reverse transcriptase (hTERT), thus enabling the immortalization of the cells. With RBBP6 and hTERT showing a similar profile in cancer cells, we seek to investigate any possible effect of RBBP6 on telomerase activity.
Results: Using real-time qPCR and TRAPeze RT Telomerase detection kit (Merc) respectively. We used cervical cancer cell lines in which CaSki cell showed the reduction of hTERT expression and reduction in telomerase activity significantly in RBBP6-knockdown cells. While no significant changes were observed in HeLa cells. Real-time growth assay revealed a significant drop in cell growth in co-silenced RBBP6 and hTERT cells substantiating our observation that RBBP6 might be playing a role in cell proliferation.
Conclusion: Taken all together, the observed effect of RBBP6 gene silencing on telomerase activity in cervical cancer is cell line dependent.
Keywords: telomerase activity, RBBP6, hTERT, cervical cancer, cell proliferation
Introduction
Cancer is a disease that is identified by an uncontrolled and abnormal division of cells and the spread of malignant tumour cells to the other organs of an organism.1 Cancer is a major health problem that ranks as the second most common cause of death in the world after cardiovascular disease.2 According to the global cancer incidence, mortality, and prevalence, there were 18.1 million incident cancer cases and 9.6 million deaths in 2018 worldwide.3 The most common cancer cases and deaths worldwide among males and females combined are lungs, followed by breast cancer, colorectum and prostate cancer.3,4 The most common cancer sites among female also diagnosed worldwide is breast cancer, colorectum, and lung. However, the leading cause of cancer deaths reported is breast, lung, and colorectum.5 On the contrary, in developing countries the leading cancer mortality among females, it is breast cancer followed by cervical cancer.5,6
It has been predicted that the burden of the disease has shifted and will continue to shift from developed countries to developing countries, in which the latter accounts for more than 57% of the new cases of cancer and 65% of cancer death worldwide.7 These developing countries mainly consist of Sub-Saharan Africa, Latin America, and South-Eastern Asia.4 Cervical cancer is the second most commonly diagnosed cancer and 90% of cervical cancer deaths have occurred in the developing parts of the world. For example, there were an estimated 570 000 new cervical cancer cases and 311 000 deaths worldwide in 2018.4 Furthermore, cervical cancer has been reported to account for more than 60% of gynecological cancer cases diagnosed in developing countries.7
In Africa, it is estimated that on an annual basis, 78,897 women are diagnosed with cervical cancer and 78% will die from the disease if left untreated.7 Current data on cervical cancer incidence in South Africa is non-existed due to the lack of maintenance of the pathology based cancer registry.8 However, according to Jordaan et al 2017, in South Africa an average of 7735 new cases are diagnosed per annum and about 4248 women die from this disease annually, therefore cervical cancer is a serious threat to South African females.8 Although cervical cancer is a highly preventable disease, alarming mortality rates continue to be reported, as a result of factors such as late diagnosis, lack of organized screening programs, lack of highly skilled medical personnel, and improper infrastructure.8 In this study, we explore the relationship of RBBP6 and telomerase activity in cervical cancer.
Methodology
Normal cell lines and different tumorigenic cell lines were used in this study as the main source of mRNA and protein. MRC-5 lung fibroblasts a normal cell line. Ca-Ski is a human epidermoid-derived cervical cancer line, which contains HPV 16. Hela is an adenocarcinoma-derived cell line, which contains HPV 18. These cell lines were purchased from the National Institute of Biomedical Innovation, Health, and Nutrition (Japan). A normal cancer line MRC-5 and cervical cancer cell lines such as Ca-Ski and HeLa were grown in Dulbecco’s modified medium (DMEM). To make the growth medium complete the following components were added to a final concentration: 10% fetal bovine serum (FBS), 1% antibiotic (penicillin/streptomycin), and 1% fungizone. The medium was changed two times per week by discarding the old media, washing the cells with 1X PBS, and replace it with fresh media. The cells were maintained at 37°C in a 5% CO2 incubator. Monolayer cells were used when they reach 70–90% confluency.
RNA Interference
Cells were seeded to be 70–90% confluent at the time of transfection. The cancer cell lines (CaSki & HeLa) were transfected with 60 pmol using siRBBP6 or hTERT pre-designed Ambion Silencer with lipofectamine® 3000 transfection agent. The lipofectamine™ 3000 reagents and siRBBP6 or sihTERT were diluted separately in the Opti-MEM medium. Then the siRBBP6 or sihTERT were mixed with lipofectamine™ 3000 reagents in a 1:1 ratio and incubated for 10–15 minutes to form a DNA-lipid complex. The DNA-lipid complex was added onto the cells and the cells were incubated for 48 hours at 37°C incubator in the presence of CO2.
Real-Time qPCR
The following components were added to a total of 20µL reaction volume: Luminaris Color HiGreen qPCR master mix (contains SYBR Green, MgCl2, Taq polymerase, and dNTPs, primers of RBBP6 or hTERT or GAPDH, cDNA template and nuclease-free water). The reaction was mixed thoroughly and dispensed into the PCR tubes. The PCR tubes were placed in the real-time cycler and cycling program was started. The thermal cycling was performed using a three-step protocol: UDG pre-treatment for 2 min at 50°C, initial denaturation for 10 min at 95°C, denaturation for 5 s at 95°C, annealing for the 30s at 55–58°C and extension for 30s at 72°C. For 39 cycles.
RBBP6 Primers
Forward primer: 5ʹ CAGCGACGACTAAAAGAAGAG 3ʹ
Reverse primer: 5ʹ GAGCGGCTGAATGATCGAGA 3ʹ
GAPDH Primers
Forward Primer: 5ʹ CAGCCGCATCTTCTTTTGCG 3ʹ
Reverse Primer: 5ʹ TGGAATTTGCCATGGGTGGA 3ʹ
hTERT Primers
Forward Primer: 5ʹ TGACACCTCACCTCACCCAC 3ʹ
Reverse Primer: 5ʹ CACTGTCTTCCGCAAGTTCAC 3ʹ
Western Blot
Before starting with the procedure, the whole-cell protein was extracted using RIPA buffer. After seventy-two hours of post-transfection, the cells were washed with cold PBS. Cells were lysed with RIPA buffer and collected by scraping. The total protein was separated from cell debris by centrifugation at 14,000 rpm for 15 minutes. The supernatant was transferred to a new microcentrifuge tube and the pellet was discarded. The protein was quantified with the Pierce® BCA Protein Assay Kit. The protein was heated at 95°C for 5 min and the protein was loaded per well for electrophoretic separation in 30% acrylamide-bis gel preparation at 130V for 1.5 hours. The protein was then transferred onto a nitrocellulose membrane using a wet electro-transfer method for 1 hour at 100V. This was followed by blocking the membrane with 5% non-fat milk for an hour. The membrane was then incubated with a primary antibody with agitation overnight. The next day, the blot was washed with PBS and incubated with HRP-linked secondary antibody for an hour. The light signal produced from the secondary antibody was detected and enhanced using the Pierce® ECL Western Blotting Chemiluminescence Substrate and the blots were imaged by the CCD-based ChemiDoc™ MP system.
Telomerase Activity Assay
Telomerase was extracted from the tumorigenic and non-tumorigenic cells by lysing the cells with the CHAPS lysis buffer. Protein extracts were measured using a NanoDropTM 1000 Spectrophotometer (Thermo Scientific). Protein concentrations of samples were normalized to 500 ng/µL. A master mix was prepared by mixing the following reagents: 5X TRAPEZE® RT reaction mix, Taq polymerase, and nuclease-free water. The reaction mix was aliquoted into respective PCR tubes and the different cell extract was added accordingly. The thermocycler was initiated with the following conditions: telomerase extension for 30 minutes at 30°C for 1 cycle, PCR amplification initial denaturation for 2 minutes at 95°C for 1 cycle, for 45 cycles: denaturation at 95°C for 15s, annealing at 59°C for the 60s and extension for 10s at 45°C. The data were analyzed with BioRad CFX Maestro software version 1.1. Telomerase activity values were extrapolated from the standard curve of TSR8 control generated by 1:10 serial dilutions (20–0.2 amoles).
xCELLigence Assay
Before the start of the experiment, the xCELLigence instrument was placed in a 37°C incubator than a volume of 100 µL of antibiotic-free culture medium was added onto the 16-well E-Plate and the plate was placed on the xCELLigence instrument to record the background reading. 1.5 x 104 cells were seeded onto the well plates and the E-plate was placed back to the current flow of the instrument. The next day, cells were transfected with both siRBBP6 and sihTERT and cell growth was monitored after 48 hours. Cell index values were recorded at 15 minutes’ interval sweeps until the end of the experiment. The xCELLigence parameters were as follows: Step 1: 1 sweep, 1 minute, 00:00:01 total time to measure background. Step 2: 100 sweeps, 15 minutes’ interval, 24:45: 01 total time to measure cell impedance. Step 3: 25 sweeps, 15 minutes’ interval, 30:45:01 total time to add siRNA, and stop after 6 hours. Step 4: 193 sweeps, 15 minutes’ interval, 79:00:00 total time to monitor cell.
Results
The primary hypothesis of this study is that there is a possible correlation between RBBP6 expression and telomerase activity in cervical cancer. Both RBBP6 and telomerase are highly expressed in cervical cancer. RBBP6 promotes cancer progression through cell cycle acceleration and apoptosis reduction. Human telomerase reverse transcriptase (hTERT), an essential subunit responsible for the main function of telomerase, is overexpressed in ~90% of cervical cancer cases. Increased expression of hTERT and high telomerase activity strongly associated with advanced cancer. Given that RBBP6 and telomerase both play a role in cervical cancer progression, we seek to probe a possible relation between RBBP6 and telomerase activity. To exploit the relationship, RBBP6 was knocked down in cervical cancer cells using RNA interference, followed by analysis of hTERT expression at both RNA (qPCR) and protein level (Western blotting). Furthermore, telomerase activity was analysed in RBBP6-knockdown cells using the TRAPeze RT Telomerase detection kit (Merc). Lastly, cell proliferation in response to RBBP6/hTERT co-silencing was monitored in real-time using the xCELLigence system.
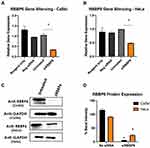
Confirmation of siRNA-Mediated RBBP6 Knockdown
Before conducting our investigation on whether there is a possible correlation between RBBP6 and telomerase in cervical cancer progression, we first had to verify if silencing was successful. RBBP6 silencing was confirmed at both RNA and protein levels using real-time quantitative PCR and Western blotting. RBBP6 gene silencing was significantly high (P < 0.05) in both CaSki and HeLa cells, with mRNA relative ratios of 0.3 and 0.5, respectively (Figure 1A and B). Quantification at the protein level using Western blotting further confirmed the observed gene-silencing (Figure 1C). In the CaSki cell line, RBBP6 protein expression was knocked down almost completely, as can be seen by the faded band intensity (~ 5% band intensity) in transfected cells compared to non-transfected cells (Figure 1C and D). A similar trend was observed in the HeLa cell line where ~ 25% band intensity was recorded in cells transfected with siRBBP6 (Figure 1D).
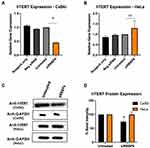
hTERT Gene Expression in RBBP6-Knockdown Cells
In this section, we examined the effects of RBBP6 knockdown on hTERT expression in cervical cancer cell lines. To examine a possible relationship between RBBP6 and hTERT in cervical cancer, cells were transfected with siRBBP6 for 48 and 72 hours followed by measurement of hTERT expression at mRNA and protein level, respectively. In CaSki cell line, hTERT mRNA expression was significantly (P < 0.05) decreased (0.4) relative to untreated cells (Figure 2A). On the contrary, in HeLa cells, there was a non-significant (P > 0.05) increase (1.3) in hTERT expression (Figure 2B). At the protein level, the effect of RBBP6 silencing on hTERT in CaSki cells conformed to that observed at the RNA level, although the decrease in hTERT protein expression was minimal, as shown by the band intensity (Figure 2C and D). Interestingly, there was no change in hTERT protein expression in HeLa cells compared to untreated cells (Figure 2B).
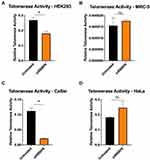
Telomerase Activity in RBBP6-Knockdown Cells
In this section, the detection and quantification of telomerase activity in response to RBBP6 gene silencing was investigated in CaSki and HeLa cell lines using the TRAPeze RT Telomerase detection kit (Merc). HEK293 was used as a telomerase-positive cell line and MRC-5 as a telomerase-negative cell line. Cells were transfected with siRBBP6 for 72 hours before obtaining whole-cell extracts, which were then subjected to a two-enzyme fluorometric quantitative RT-PCR. A statistically significant reduction in telomerase activity was observed in RBBP6-knockdown CaSki and the positive control HEK293 cells (Figure 3A and C). However, there was a non-significant change in telomerase activity in HeLa cells (Figure 3D). Telomerase activity in MRC-5 was almost undetectable as expected from a negative control cell line (Figure 3B).
Cell Proliferation in Response to RBBP6 and hTERT Co-Silencing
Previous studies have shown that the proliferation of cervical cancer cells is directly proportional to RBBP6 expression, where RBBP6 overexpression results in increased cell proliferation and knockdown reduces cell growth (Moela et al 2016, Lekganyane et al 2018). It is also a known fact that hTERT expression strongly associates with advanced cancer. Having observed the effect of RBBP6 knockdown on hTERT expression and telomerase activity, we were therefore interested in analysing cell proliferation in cells deficient of both RBBP6 and hTERT as this will substantiate our current observations. Cell growth was monitored for a period of ~ 72 hours, with transfection of siRBBP6 and sihTERT after 24 hours of cell growth using the xCELLigence system. This system uses a non-invasive electrical impedance or cell index (CI) as a measure of cell growth.
As shown in Figures 4 and 5 below, there was a reduction in cell growth in response to either siRBBP6 or sihTERT in both HeLa and CaSki cells. Co-transfection with sihTERT and siRBBP6 further reduced cell growth in HeLa cells (Figure 4) and resulted in the same level of reduction as individual transfections in CaSki cells. Untreated cells and control cells treated with transfection reagent only or negative siRNA showed a steadily increasing growth rate that plateaued at ~ 60 hours in HeLa and 53 hours in CaSki. The type of inhibition was associated with cell cycle arrest at M-phase due to a lack of telomerase activity.
Discussion
Cervical cancer, especially in developing countries like South Africa, continues to defy medical science and be a burden to the overstretched health care systems. In addition to this, the high rate of HIV/AIDS infection compromises the immune system of many and results in infectious diseases such as HPV to take advantage resulting in cervical cancer development. With the current vaccine available for young adolescent women, those who are already sexually active remain at the risk of developing cervical cancer with no cure insight mostly due to late detection. In this study, we exploited the co-treatment of two genes that might be playing a big role in cancer development in general. One is RBBP6, which according to several researchers, binds to Tp53 and leads to its degradation thereby leading to cancer development and progression. The other is telomerase activity, which is highly expressed in several cancer also due to continuous cell proliferation.
In this study, we investigated the mechanism of action of RBBP6 in promoting cancer cell proliferation by analysing a possible relationship between RBBP6 and telomerase activity in cervical cancer cells. Our current findings revealed early insights into a possible relationship between RBBP6 expression and telomerase activity in cervical cancer. We have demonstrated for the first time that successful RBBP6 silencing at mRNA and protein level decreases hTERT expression with a subsequent reduction in telomerase activity at least in the squamous cell carcinoma (SCC)-derived CaSki cell line. This is consistent with findings that hTERT mRNA expression is proportional to telomerase activity.9–11 In HeLa cells however, this was not the case as seen by the non-significant change in hTERT expression and telomerase activity following knockdown of RBBP6.
One of the experiments we performed to establish the relationship between RBBP6 and telomerase activity was to silence RBBP6 and then measure telomerase activity. In brief, Telomerase is the enzyme responsible to maintain the length of the telomeres during cell division. As we reported in many studies, Cancer is characterised by cell proliferation, which then will require increased telomerase activity. Several studies have shown that cell lines, immortalized spontaneously or after transformation by oncogenic viruses, such as simian virus 40 or human papillomavirus types 16 or 18, are usually telomerase-positive. Whereas many normal somatic cells were telomerase-negative. These results were also reflected in our study that involved RBBP6 silencing.
Figure 3 indicates that telomerase activity in untreated CaSki (C) is lower than that of untreated HeLa (D). Therefore, one might argue that the observed differential response of the two cell lines to RBBP6 silencing is as a result of their distinctive telomerase activity. However, similar results were obtained under different siRBBP6 transfection conditions. These observations, therefore, suggest different mechanisms of RBBP6-mediated telomerase activity in HeLa and CaSki cells. Nonetheless, we cannot rule out the possibility that histological differences between the two cell lines might be an explanation for the differential effects of RBBP6 silencing on telomerase activity. This is in agreement with previous findings where adenocarcinomas in advanced cervical cancer had an overall poor response rate to treatment compared to SCC tumours.9,12,13 From these preliminary results, it was clear that RBBP6 might affect telomerase activity either by reducing cell proliferation or inactivating the enzyme itself.
Over and above, it is important to note that telomerase activity in cervical cancer is greatly influenced by HPV 16/18 E6 oncoprotein. A recent study by [14] on hTERT expression in HPV 16/18-positive cervical cancer cell lines focused mainly on the consequences of HPV16/18 E6 knockdown on hTERT mRNA levels and hTERT promoter DNA methylation in CaSki, HeLa and SiHa cells. Their findings were that hTERT expression in response to HPV 16/18 E6 silencing was highest in HeLa cells with relatively less dense methylation around the transcription start site. On the contrary, hTERT expression was the lowest in E6 knocked-down CaSki cells, with relatively denser methylation around the transcription start site. This differential response to transfection seen in HeLa and CaSki cells is consistent with our findings. They speculate that the differential response may be due to the involvement of a large variety of transcription factors that interact with the hTERT promoter to determine the activity of hTERT.14
RBBP6 is not a DNA binding protein, which means possibilities of it regulating hTERT expression through direct interaction with the hTERT promoter are limited. However, there is a shred of vast evidence showing that E3 ligases can bind to and alter the expression of transcription factors on the hTERT promoter. For example, Koivusalo (2006) indicated that E6 oncoprotein, through its E3 ligase activity, alters the expression of a c-Myc transcription factor on the hTERT promoter in cervical cancer cells. We, therefore, hypothesize that through its E3 ligase activity, RBBP6 might be indirectly affecting hTERT expression by interacting with certain activators and/or repressors on the hTERT promoter. This suggestion has merit on the basis that the function of RBBP6 in protein binding and degradation has been strongly elucidated. Chibi et al revealed that RBBP6 binds to and degrades the transcription factor, Y-box-binding protein 1 (YB-1), an essential regulator of cellular propagation and apoptosis.3 Additionally, RBBP6 facilitates MDM2-mediated degradation of p53 through its p53-binding domain and E3 ligase activity.15 The involvement of RBBP6 in p53 degradation has a great potential to result in the inhibition of DNA repair mechanisms and p53-mediated apoptosis, as postulated in previous findings.15–17 Loss of DNA repair and evasion of apoptosis is the primary candidates for cancer development and progression. This functioning of RBBP6 in these cellular processes, therefore, implicates it in cancer cell proliferation. In cervical cancer,18 have shown that RBBP6 is highly expressed in human tissue sections and that cancer cell proliferation is associated with increased expression of RBBP6. Telomerase is also essential for the indefinite proliferation of immortalized cells both in vitro and in vivo.19 As a result, we were interested in analysing the proliferation of cervical cancer cells that are deficient in both RBBP6 and telomerase activity.
CaSki and HeLa cells were subjected to co-silencing of RBBP6 and hTERT and their growth monitored in real-time throughout ~ 72 hours. As expected, this resulted in a reduced growth rate over time in cells deficient of either RBBP6 or hTERT in both CaSki and HeLa. These observations substantiate the fact that both RBBP6 and hTERT are strongly associated with cancer cell proliferation. Furthermore, it was interesting to see that co-silencing elicited a much higher cell growth reduction in HeLa cells, highlighting a possible additive effect of RBBP6 and hTERT on cell growth.20 For the first time, our study has revealed early insights into possible crosstalk between RBBP6 and telomerase in terms of regulating cell proliferation in cervical cancer cells. Because of the elicited further reduction of cancer cell growth in co-silenced treatment, the results further support the relationship that might exist between RBBP6 and telomerase activity. It would be interesting results that show the relation and the molecular mechanism of the two genes in regulating cancer cell inhibition.
In conclusion, we have shown for the first time that there is a possible relationship between RBBP6 expression and telomerase activity in cervical cancer cells. This was seen by a change in hTERT expression following the RBBP6 knockdown. We further indicated that hTERT expression is proportional to telomerase activity in RBBP6-knockdown cells, meaning that RBBP6-mediated change in hTERT expression has consequential effects on telomerase activity. We further illustrated a possible additive effect of both RBBP6 and hTERT on cervical cancer cell proliferation. We, however, draw these conclusions with caution because our study was conducted in only two cervical cancer cell lines, which compromises the robustness of our findings. Therefore, a more intensive study is still required in future to deeply explore interrelations between RBBP6 and telomerase activity. Such prospects should include investigation on whether there is a possible RNA-protein interaction between RBBP6 protein and hTERT mRNA using RNA-Chromatin Immunoprecipitation (ChIP) assay; as well as possible nuclear co-localization of RBBP6 and hTERT. This will validate our speculation around the possibility of RBBP6 interacting with hTERT. Furthermore, understanding the mechanism of cell proliferation in hTERT/RBBP6-deficient cells by exploring the cell cycle is highly important. Lastly, RBBP6 plays a significant role in the apoptosis pathway and it would, therefore, be interesting to analyze the downstream effects of hTERT/RBBP6 co-silencing on cell death.
Acknowledgment
The authors would like to thank the South Africa Medical Research Council for continued support and funding.
Disclosure
Mpho Mosweu reports grants from South African Medical Research Council and National Research Foundation, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Russell PJ. IGenetics: A Molecular Approach. Upper Saddle River, N.J: Pearson Education; 2010.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68(1):7–30.
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
4. Broccoli D, Smogorzewska A, Chong L, de Lange T. Human telomeres contain two distinct Myb–related proteins, TRF1 and TRF2. Nat Genet. 1997;17(2):231. doi:10.1038/ng1097-231
5. Chibi M, Meyer M, Skepu A, Rees DJG, Moolman-Smook JC, Pugh DJ. RBBP6 interacts with multifunctional protein YB-1 through its RING finger domain, leading to ubiquitination and proteosomal degradation of YB-1. J Mol Biol. 2008;384(4):908–916. doi:10.1016/j.jmb.2008.09.060
6. Fitzmaurice C, Dicker D, Pain A, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi:10.1001/jamaoncol.2015.0735
7. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
8. Jordan S, Michelow P, Simoens C, Bogers J. Challenges and progress of policies on Cervical cancer in South Africa. Health Care current review. 2013;5(188):2.
9. Iyoke CA, Ugwu GO. Burden of gynaecological cancers in developing countries. World J Obstet Gynecol. 2013;2(1):1–7. doi:10.5317/wjog.v2.i1.1
10. Jordaan S, Michelow P, Richter K, Simoens C, Bogers J. A review of cervical cancer in South Africa: previous, current and future. Health Care Curr Rev. 2016;4(180):2.
11. Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012;125(2):292–296. doi:10.1016/j.ygyno.2012.01.034
12. Kirkpatrick K, Clark G, Ghilchick M, Newbold R, Mokbel K. hTERT mRNA expression correlates with telomerase activity in human breast cancer. Eur J Surg Oncol. 2003;29(4):321–326. doi:10.1053/ejso.2002.1374
13. Koivusalo R, Mialon A, Pitkänen H, Westermarck J, Hietanen S. Activation of p53 in cervical cancer cells by human papillomavirus E6 RNA interference is transient, but can be sustained by inhibiting endogenous nuclear export–dependent p53 antagonists. Cancer Res. 2006;66(24):11817–11824.
14. Sandin S, Rhodes D. Telomerase structure. Curr Opin Struct Biol. 2014;25:104–110. doi:10.1016/j.sbi.2014.02.003
15. Rose PG, Java JJ, Whitney CW, Stehman FB, Lanciano R, Thomas GM. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol Oncol. 2014;135(2):208–212. doi:10.1016/j.ygyno.2014.08.018
16. Jiang J, Zhao L-J, Zhao C, et al. Hypomethylated CpG around the transcription start site enables TERT expression and HPV16 E6 regulates TERT methylation in cervical cancer cells. Gynecol Oncol. 2012;124(3):534–541. doi:10.1016/j.ygyno.2011.11.023
17. Di Giammartino DC, Li W, Ogami K, et al. RBBP6 isoforms regulate the human polyadenylation machinery and modulate expression of mRNAs with AU-rich 3′ UTRs. Genes Dev. 2014;28(20):2248–2260. doi:10.1101/gad.245787.114
18. Motadi LR, Bhoola KD, Dlamini Z. Expression and function of retinoblastoma binding protein 6 (RBBP6) in human lung cancer. Immunobiology. 2011;216(10):1065–1073. doi:10.1016/j.imbio.2011.05.004
19. Moela P, Choene MM, Motadi LR. Silencing RBBP6 (Retinoblastoma Binding Protein 6) sensitises breast cancer cells MCF7 to staurosporine and camptothecin-induced cell death. Immunobiology. 2014;219(8):593–601. doi:10.1016/j.imbio.2014.03.002
20. Motadi LR, Lekganyane MM, Moela P. RBBP6 expressional effects on cell proliferation and apoptosis in breast cancer cell lines with distinct p53 statuses. Cancer Manag Res. 2018;10:3357. doi:10.2147/CMAR.S169577
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.