Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Intrinsic Atopic Dermatitis and Extrinsic Atopic Dermatitis: Similarities and Differences
Authors Liu L , Song G, Song Z
Received 26 September 2022
Accepted for publication 8 November 2022
Published 7 December 2022 Volume 2022:15 Pages 2621—2628
DOI https://doi.org/10.2147/CCID.S391360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Lu Liu,1 Guoxuan Song,2 Zhiqiang Song1
1Department of Dermatology, Southwest Hospital, Third Military Medical University, Chongqing, 400038, People’s Republic of China; 2First School of Clinical Medicine, Changsha Medical College, Changsha, 410219, People’s Republic of China
Correspondence: Zhiqiang Song, Department of Dermatology, Southwest Hospital, Third Military Medical University, No. 29 Gaotanyan Str., Chongqing, 400038, People’s Republic of China, Tel +86 023 68754290, Email [email protected]
Abstract: In atopic dermatitis (AD), recent research advances have portrayed a complex disease profile based on different subtypes/phenotypes and underlying molecular mechanisms/endotypes. Extrinsic and intrinsic subdivision is one of the most common types, defined in terms of total serum immune globulin E (IgE) and/or specific IgE to food and environmental allergens. Extrinsic AD is the most common type (80%), with high IgE, impaired skin barrier, as well as high incidence of comorbid atopic diseases, whereas intrinsic AD accounted for 20% of AD, with normal IgE and intact barrier function. Clinical studies have shown that extrinsic AD was a classic Th2-based inflammatory dermatitis, while the intrinsic subtype presented increased Th1/Th17/Th22 but normal Th2 cytokines. Protein sensitization, a classic Th2 response, has been clearly characterized in extrinsic AD, while metal hapten induces intrinsic AD’s sensitization, which may be associated with suprabasin deficiency. In this review, we aimed to further expand our knowledge about similarities and differences between these two subtypes, which will expand our capability to further dissect pathophysiology of AD and enable us to develop personalized medicine approaches.
Keywords: intrinsic atopic dermatitis, extrinsic atopic dermatitis, epidermal barrier, immune dysfunction, immune globulin E
Introduction
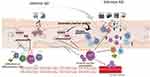
Atopic dermatitis (AD) is a chronic, relapsing, and inflammatory skin disorder. According to total serum immune globulin E (IgE) level or antigen specific-IgE, AD can be classified into extrinsic and intrinsic subtypes.1–5 Patients with extrinsic AD have high IgE level (>200 kU/L), atopic family history, and increased specific IgE level. But in intrinsic AD, the level of total serum IgE is below 200 kU/L, and there are no allergen-specific IgE antibodies and other atopic co-morbidities.6 Despite sharing similar clinical and histologic features, accumulating evidence has suggested that intrinsic AD is a distinct entity. In this review, we mainly clarified the differences of these two types of AD in epidemiology, skin barrier function, allergens, and inflammatory response, so as to provide new insights for further stratification of subtypes and more precise medicine approaches to prevent and treat AD (see Figure 1 and Table 1).
 |
Table 1 Summary of Similarities and Differences Between Extrinsic and Intrinsic Atopic Dermatitis |
Epidemiology
As a chronic inflammatory skin disease, AD is a global public health issue. AD affects 15–25% of the pediatric population and 2.0–17.6% of the adult population worldwide,7 imposing a considerable burden on individuals and society. With regard to extrinsic AD and intrinsic AD, study in German children reported a prevalence of 73% vs 27%, in Hungarian adults reported a prevalence of 88% vs 12%, in Dutch patients aged 13–37 reported a prevalence of 78.2% vs 21.8%, and in Korea patients reported a prevalence of 80% vs 20%, respectively,4 which indicated that environmental factors had only a minor influence on the prevalence of these two subtypes. A 5-year prospective birth cohort study in Germany found that one-third of pediatric AD were intrinsic types, and females had a higher prevalence.8 Subsequent study also demonstrated higher prevalence of extrinsic AD among adult patients. In the initial phase of that study, 18 (6.9%) patients met the criteria of intrinsic type. Afterwards, the incidence of intrinsic AD fell to 5.4% during the follow-up of 7.5 years, because four patients presented respiratory allergies or IgE-mediated sensitizations. This suggested that the distinction between the two types is not permanent, and intrinsic AD may become extrinsic type over time.9,10
Several studies have revealed female preponderance of intrinsic AD both in children and adults.11–14 Although the cause of this gender bias remains unclear, the effect of sexual hormones on immune responses and/or much more exposure to metal-containing jewelry in females could possibly induce occurrence of intrinsic AD. Moreover, according to a Korean study, intrinsic AD patients (100%) were more likely to deteriorate during pregnancy than extrinsic AD patients (47.1%), indicating that female sex hormones have a greater influences on intrinsic AD patients than on extrinsic AD patients.13,15 These interesting phenomena partially demonstrate the modulatory impact of sexual hormones on the progression of AD.
Epidermal Barrier Dysfunction
A number of studies have demonstrated the extreme importance of barrier integrity in AD.16–18 Transepidermal water loss (TEWL), skin surface hydration (capacitance), temperature, erythema index, and elasticity are commonly used to evaluate skin barrier function. And TEWL and temperature were reported to be positively associated with AD’s severity.19 Notably, research had repeatedly shown that extrinsic AD exhibits increased TEWL but decreased capacitance.18 However, study on the correlation between erythema index and temperature and AD’s subtypes has received little attention.
Additionally, skin electric current perception threshold (CPT) can be used to quantify pruritic sensory threshold of sensory nerves to external stimulation in AD. Yin et al found that the CPT in non-lesional skin of extrinsic AD was higher than in healthy control, but not found in intrinsic AD.20 T. Mori also reported this phenomenon.21 Some dermatologists propose that epidermal nerve fibers are in an activated state in extrinsic AD, with decreased sensitivity to external stimuli, while the homeostasis between epidermal barrier and sensory nerve fiber in intrinsic AD is not breached.21,22 Altogether, it can be considered that in patients with intrinsic AD, the barrier function and sensory reactivity to external pruritic stimuli remain normal.
Filaggrin (FLG) is an epidermal structural protein that mediates cornification and hydration (osmolytes are formed during the breakdown of FLG).23 FLG-null (FLG−/−) mouse showed spontaneous atopiform dermatitis, with substantially abundant type 2 innate lymphocytes infiltrating into the skin lesions.24 According to a Japanese report, FLG mutations are detected in 10.5% of intrinsic AD cases, in 44.4% of extrinsic AD cases, and in 3.7% of healthy controls.25 Furthermore, FLG mutations are closely linked to palmar hyperlinearity in extrinsic AD patients, which was a common feature of extrinsic AD and ichthyosis vulgaris. This is consistent with the report that palmar hyperlinearity is inversely correlated with intrinsic subtype.26 Certainly, FLG mutation is not the single factor for the disrupted barrier as several reports demonstrate that the levels of loricrin, involucrin, and keratin in some AD patients without FLG mutations were significantly down-regulated compared to those in healthy controls.
The acidic pH in skin maintains the skin barrier function. First, the acidic mantle decreases the skin colonization of pathogenic bacteria by exerting a strong antimicrobial effect.27,28 In addition, there is evidence that the pH of the skin surface plays a role in desquamation, homoeostasis of the permeability barrier, and integrity/cohesion of the stratum corneum. AD patients with FLG deficiency have increased skin pH, which activates degrading proteases and inhibits lipid synthesis enzymes.28 Taken together, elevated skin pH can hinder barrier recovery and aggravate barrier breakdown. In a pilot study by a German group, AD patients with FLG loss-of-function mutations had higher epidermal pH than those without mutations.8 Thus, the surface pH may also be important to differentiate extrinsic and intrinsic AD due to a higher FLG mutation rate in extrinsic AD.
Although patients with intrinsic AD possess a normal epidermal barrier, itch induced by inflammatory cytokines inevitably leads to scratching, thus creating a secondary barrier defect. It is possible that intrinsic AD evolves into an inflammatory pattern similar to that of extrinsic AD once a secondary barrier defect has occurred. Hence, simultaneous control of itch is particularly critical in the primary management of intrinsic AD in addition to anti-inflammatory therapy (Figure 2).
Inflammatory Response Pattern
AD is widely recognized as a Th2-based disease. Patients with extrinsic AD have elevated levels of Th2 cytokines (IL-4, IL-5, and IL-13), while those with intrinsic AD have high Th1 but low CCL17/thymus and activation-regulated chemokine (TARC) levels.10,29 Accordingly, eosinophil numbers and the eosinophilic cationic protein (ECP) serum levels are higher in extrinsic AD. In an in vitro experiment, extrinsic AD patients produced less IFN-γ and GM-CSF than intrinsic AD patients when peripheral blood mononuclear cells were stimulated with an anti-CD3 antibody.30 When co-cultured with normal B cells, T cells isolated from extrinsic AD skin biopsies were able to induce high levels of IL-13-mediated IgE production, but not in intrinsic AD, suggesting that there are some differences in cytokine micro-environment between the two subtypes’ serum.31,32
Allergens interact with local antigen-presenting cells (APCs) through defective epidermis, further triggering activation of T cells. Activated effector T cells then are induced to express skin homing receptor (CLA+CD45RO+), migrating to the epidermis and secreting Th2-dominated cytokines to initiate flare of dermatitis.33 Interestingly, individuals with atopic diseases express high levels of Fas and Fas-ligand in the Th1 compartment, resulting in activation-induced cell death (AICD). Those Th1 cells present characteristic apoptosis in vivo by degrading procaspase and activating caspase-8, skewing the immune response in favor of surviving Th2 cells. However, in non-atopic patients (intrinsic AD, intrinsic-type asthma, psoriasis, and contact dermatitis), circulating Th1 cells did not undergo apoptosis.5 These findings may explain the mechanism about high Th2 cytokines in extrinsic AD. In contrast, effector T cells in the epidermis of AD patients do not demonstrate any apoptosis despite expressing Fas and Fas-ligand, because they are protected against apoptosis by IL-2, IL-15, and extracellular matrix proteins.31
Evolving clinical data have found that there is a similar or higher level of Th2 and Th1 activity in intrinsic lesional skin, but a more significant increase in Th17 and Th22 responses compared to extrinsic AD lesions.34 Accordingly, a higher level of IL-17/IL-22-controlled antimicrobials, S100A9 and S100A12, were found in intrinsic lesions. In addition, clinical trials showed that, in extrinsic AD, Th2 cytokines correlated positively with disease severity and negatively with barrier products (loricrin, periplakin, and FLG), while in intrinsic AD Th1 and CCL20 levels correlated with disease severity. Studies afterwards revealed a lipid metabolism and inflammatory pathway (Il-36RN, IL-36α/γ, IL-22, and CCL22) overlap between psoriasis and intrinsic AD,35–37 indicating IL-23/Th17 and IL-22 as pathological cytokines of the two diseases.
Study on the molecular mechanism of AD’s pathogenesis is the basis of targeted therapy. Although dupilumab (anti-IL-4R α) has significantly improved AD patients’ symptoms, including lesions, pruritus, and life quality, not all patients have a good efficacy.40 Considering that AD is a systematic atopic disease, some patients usually have other concomitant diseases, including alopecia areata, chronic rhinosinusitis with nasal polyposis, and asthma.41 Janus kinase inhibitor may have a better efficacy for refractory AD with concomitant diseases via blocking upstream signals.42 At present, specific therapy targeted on intrinsic and extrinsic AD is very limited. However, study on this aspect enables us to further dissect AD’s inflammatory mechanism. There have been extensive studies on drugs targeted on other cytokines, including IL-22, IL-31, and TSLP. In a recent phase II clinical study, anti-IL-22 (fezakinumab7) was effective and safe at ameliorating AD clinical manifestations and transcriptome.38 Anti-p40 (IL-12/IL-23 common subunit) by ustekinumab presented a limited efficacy on AD severity, but with the improvement of common immune markers mRNA (IL-22/IL-17-induced S100As), which may be correlated with dose of medicine and sub-group analysis.39
IgE Level
Immunoglobulin class switching in AD lesions is affected by the cytokine milieu. In many studies, the increase in IL-13 and IL-4 produced by skin-homing T cells induces the activation of B cells and the production of IgE. According to a recent study, both AD types produce similar levels of mRNA of Th2 cytokines (IL-4, IL-13, and IL-31).37 Therefore, the level of IgE is not exclusively explained by Th2 activation. A Japanese study in vitro found that upregulation of interferon-γ could suppress IgE production, thus contributing to the normal IgE level in intrinsic AD.43 In intrinsic lesional skin, FOXP3, a regulatory T-cell marker, was also upregulated, which could relieve immune inflammation via inducing IgG4 production and inhibiting IgE production. Moreover, cytokines in the peripheral blood and lymph nodes may reduce IgE production by interfering with maturity of B cells. The absence of allergen-specific IgE in intrinsic AD may be explained by unidentified allergens (such as self-allergen), or there may be production of other immunoglobulin classes with unknown function.44
Previous studies have shown that severity of extrinsic AD is significantly correlated with the serum total IgE levels. IgE activates effector cells via FcεRI and/or FcεRII to initiate allergic inflammation. It has been observed in animals that the expression of FcεRI on APCs (such as Langerhans cells, epidermal dendritic cells), basophils, and mast cells was correlated with the total IgE level.2 An increased FcεRI on epidermal DC (EDC) from the epidermis of AD patients in both types has been demonstrated, but Oppel et al found a lower level of FcεRI on EDC in intrinsic AD compared to extrinsic AD.45 FcεRI/FcεRII expression was 1.5 in extrinsic AD, while a ratio of 0.5 was observed in intrinsic AD.37 It is still not clear whether lower expression of FcεRI on EDC is the reason or result of lower epidermal and serum IgE in IAD patients compared with that in extrinsic AD. Although SCORAD scores are positively associated with IgE levels in extrinsic AD, effects of anti-IgE treatment are ambiguous. Thus, serum total IgE may be a key effector of allergic sensitization, involved in atopy march and aggravation of AD. In view of this, targeted anti-IgE intervention could be a preventative measure for high-risk groups.
Allergens
It has been reported that intrinsic AD patients manifested higher positive rate to nickel, cobalt, and/or chrome in patch tests than the extrinsic group, and they have higher metal concentrations in blood and sweat.46,47 Therefore, some dermatologists propose that although protein allergens that induce typical Th2 responses are not sensitized by intrinsic AD patients, patients with intrinsic AD may be sensitized to other antigens such as metals.44
Another Japanese report found that intrinsic AD patients showed higher sweat concentration of nickel, inversely related to serum IgE.48 These data are in accordance with the normal IgE level and high metal allergy incidence in intrinsic AD, further demonstrating that metal hapten is an allergen of intrinsic AD. A recent observation suggests that bacterial superantigens bind to Toll-like receptor-2 (TLR-2) on monocytes and upregulate FcεRI, initiating flare of dermatitis in extrinsic AD, whereas nickel/cobalt activates APCs via TLR-4 in intrinsic AD.45 Furthermore, eliminating metals from the diet and contact could reduce skin eruptions in some AD patients; meanwhile their serum metal levels were decreased compared to healthy controls. Considering the ubiquity of metals in the environment and inconsistent correlation of symptoms and intake of metals, avoidance of metals is not recommended.49
A recent report showed that high incidence of metal allergy in intrinsic AD may correlate with the absence of suprabasin (Sbsn). Sbsn is expressed in epithelia’s suprabasal layers, involved in epidermal differentiation, metal’s absorption, and allergy in the upper digestive tract. And the SBSN−/− mice exhibit intact skin barrier function, but abnormal upper digestive tract epithelium that presents increased Ni absorption and high allergy to Ni, which is similar in intrinsic AD.50 The implication of Sbsn’s deficiency in human intrinsic AD warrants further investigation.
Summary
AD is an extremely complex skin disease, with high heterogeneity. Current guidelines about the evaluation and treatment of atopic dermatitis almost are underpinned by clinical severity. In this review, we investigated the similarities and differences between extrinsic and intrinsic atopic dermatitis from epidemiology, skin barrier function, allergens, and inflammatory response. Extrinsic AD is a classic Th2-based disease, with defective skin barrier and high serum and lesional IgE, and extrinsic AD patients commonly have atopic history and are easily allergic to protein allergens. Intrinsic AD patients only make up about 20% of the total AD patients, and the condition is more common in females and children. With normal serum and lesional IgE and preserved skin barrier, patients with intrinsic AD have a more complex inflammatory mechanism, implicating Th2, Th1, Th17, and Th22. However, intrinsic AD may transform into extrinsic AD with the development and progression of disease. Although the distinction between intrinsic and extrinsic AD subtypes has expanded our understanding about the pathogenesis of AD, there is still much work to be done to understand the patho-mechanisms and heterogeneity of AD, which potentially contributes to future endotypic- and genetically based management of AD.
In recent years, some scholars put forward the concept of “residual disease genomic profile (RDGP)”, which indicates that although the dermatitis has been clinically resolved, differentially expressed genes coding inflammation-associated cytokines did not reach 75% improvement. Comparing the RDGP between extrinsic AD and intrinsic AD subjected to the same therapy is another area of interest. In aggregate, AD is mainly a Th2-mediated systemic rather than lesion-limited disease. Classifying AD into different endotypes, not merely intrinsic and extrinsic forms, will add important new aspects to strive for a target, the tolerated and effective prevention and treatment for AD.
Funding
This work was supported by the National Natural Science Foundation of China (No.82073442, 82273529).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Tokura Y, Hayano S. Subtypes of atopic dermatitis: from phenotype to endotype. Allergol Int. 2022;71(1):14–24. doi:10.1016/j.alit.2021.07.003
2. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi:10.1016/S0140-6736(20)31286-1
3. Karimkhani C, Silverberg JI, Dellavalle RP. Defining intrinsic vs. extrinsic atopic dermatitis. Dermatol Online J. 2015;21(6):1.
4. Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58(1):1–7. doi:10.1016/j.jdermsci.2010.02.008
5. Akdis CA, Akdis M. Immunological differences between intrinsic and extrinsic types of atopic dermatitis. Clin Exp Allergy. 2003;33(12):1618–1621. doi:10.1111/j.1365-2222.2003.01803.x
6. Atopic D W G, Immunology G, Chinese S O D. Guidelines for diagnosis and treatment of atopic dermatitis in China (2020). Int J Dermatol Venereol. 2021;04(01):1–9.
7. Napolitano M, Fabbrocini G, Martora F, et al. Children atopic dermatitis: diagnosis, mimics, overlaps, and therapeutic implication. Dermatol Ther;2022:e15901. doi:10.1111/dth.15901
8. Zutavern A, Hirsch T, Leupold W, et al. Atopic dermatitis, extrinsic atopic dermatitis and the hygiene hypothesis: results from a cross-sectional study. Clin Exp Allergy. 2005;35(10):1301–1308. doi:10.1111/j.1365-2222.2005.02350.x
9. Del PD, Zhu Y, Mitra N, et al. The risk of atopic comorbidities and atopic march progression among black and white children with mild-to-moderate atopic dermatitis: a cross-sectional study. J Am Acad Dermatol. 2022;87(5):1145–1147. doi:10.1016/j.jaad.2022.02.023
10. Nomura T, Kabashima K. Advances in atopic dermatitis in 2019–2020: endotypes from skin barrier, ethnicity, properties of antigen, cytokine profiles, microbiome, and engagement of immune cells. J Allergy Clin Immunol. 2021;148(6):1451–1462. doi:10.1016/j.jaci.2021.10.022
11. Gratton R, Del VC, Zupin L, et al. Unraveling the role of sex hormones on keratinocyte functions in human inflammatory skin diseases. Int J Mol Sci. 2022;23(6):1. doi:10.3390/ijms23063132
12. De Martinis M, Sirufo MM, Suppa M, et al. Sex and gender aspects for patient stratification in allergy prevention and treatment. Int J Mol Sci. 2020;21(4):1535. doi:10.3390/ijms21041535
13. Kanda N, Hoashi T, Saeki H. The roles of sex hormones in the course of atopic dermatitis. Int J Mol Sci. 2019;20(19):4660. doi:10.3390/ijms20194660
14. Kasperska-Zajac A, Brzoza Z, Rogala B. Serum concentration of dehydroepiandrosterone sulfate and testosterone in women with severe atopic eczema/dermatitis syndrome. J Investig Allergol Clin Immunol. 2007;17(3):160–163.
15. Kische H, Hannemann A, Voss C, et al. Lack of significant association between sex hormone concentrations and atopic dermatitis in adolescents and adults in two population-based studies. J Invest Dermatol. 2022;142(2):486–489. doi:10.1016/j.jid.2021.07.133
16. Yang G, Seok JK, Kang HC, et al. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int J Mol Sci. 2020;21(8):5.
17. Napolitano M, Fabbrocini G, Martora F, et al. Role of aryl hydrocarbon receptor activation in inflammatory chronic skin diseases. Cells. 2021;10(12):3559. doi:10.3390/cells10123559
18. Tsakok T, Woolf R, Smith CH, et al. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol. 2019;180(3):464–474. doi:10.1111/bjd.16934
19. Montero-Vilchez T, Segura-Fernandez-Nogueras MV, Perez-Rodriguez I, et al. Skin barrier function in psoriasis and atopic dermatitis: transepidermal water loss and temperature as useful tools to assess disease severity. J Clin Med. 2021;10(2):359. doi:10.3390/jcm10020359
20. An Y, Yin L, Wang S, et al. 砷致健康危害生物标志物研究进展 [An overview on biomarkers of arsenic-induced health hazardsan]. Wei Sheng Yan Jiu. 2008;37(2):237–241. Chinese.
21. Mori T, Ishida K, Mukumoto S, et al. Comparison of skin barrier function and sensory nerve electric current perception threshold between IgE-high extrinsic and IgE-normal intrinsic types of atopic dermatitis. Br J Dermatol. 2010;162(1):83–90. doi:10.1111/j.1365-2133.2009.09440.x
22. Fujii M. Current understanding of pathophysiological mechanisms of atopic dermatitis: interactions among skin barrier dysfunction, immune abnormalities and pruritus. Biol Pharm Bull. 2020;43(1):12–19. doi:10.1248/bpb.b19-00088
23. Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol. 2020;124(1):36–43. doi:10.1016/j.anai.2019.10.008
24. Saunders SP, Moran T, Floudas A, et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J Allergy Clin Immunol. 2016;137(2):482–491. doi:10.1016/j.jaci.2015.06.045
25. Nomura T, Wu J, Kabashima K, et al. Endophenotypic variations of atopic dermatitis by age, race, and ethnicity. J Allergy Clin Immunol Pract. 2020;8(6):1840–1852.
26. Bradshaw LE, Haines RH, Thomas KS, et al. Clinical examination for hyperlinear palms to determine filaggrin genotype: a diagnostic test accuracy study. Clin Exp Allergy. 2021;51(11):1421–1428.
27. Choi SJ, Song MG, Sung WT, et al. Comparison of transepidermal water loss, capacitance and pH values in the skin between intrinsic and extrinsic atopic dermatitis patients. J Korean Med Sci. 2003;18(1):93–96.
28. Zainal H, Jamil A, Md NN, et al. Skin pH mapping and its relationship with transepidermal water loss, hydration and disease severity in adult patients with atopic dermatitis. Skin Res Technol. 2020;26(1):91–98.
29. Suarez-Farinas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132(2):361–370.
30. Yang L, Fu J, Zhou Y. Research progress in atopic march. Front Immunol. 2020;11:1907.
31. Rho NK, Kim WS, Lee DY, et al. Immunophenotyping of inflammatory cells in lesional skin of the extrinsic and intrinsic types of atopic dermatitis. Br J Dermatol. 2004;151(1):119–125.
32. Custovic A, Custovic D, Kljaic BB, et al. Atopic phenotypes and their implication in the atopic march. Expert Rev Clin Immunol. 2020;16(9):873–881.
33. de la O-Escamilla NO, Sidbury R. Atopic Dermatitis: update on Pathogenesis and Therapy. Pediatr Ann. 2020;49(3):e140–e146.
34. Kabashima-Kubo R, Nakamura M, Sakabe J, et al. A group of atopic dermatitis without IgE elevation or barrier impairment shows a high Th1 frequency: possible immunological state of the intrinsic type. J Dermatol Sci. 2012;67(1):37–43.
35. Schabitz A, Eyerich K, Garzorz-Stark N. So close, and yet so far away: the dichotomy of the specific immune response and inflammation in psoriasis and atopic dermatitis. J Intern Med. 2021;290(1):27–39.
36. Kemeny L, Varga E, Novak Z. Advances in phototherapy for psoriasis and atopic dermatitis. Expert Rev Clin Immunol. 2019;15(11):1205–1214.
37. Martel BC, Litman T, Hald A, et al. Distinct molecular signatures of mild extrinsic and intrinsic atopic dermatitis. Exp Dermatol. 2016;25(6):453–459.
38. Brunner PM, Pavel AB, Khattri S, et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol. 2019;143(1):142–154.
39. Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2017;26(1):28–35.
40. Montero-Vilchez T, Rodriguez-Pozo JA, Diaz-Calvillo P, et al. Dupilumab improves skin barrier function in adults with atopic dermatitis: a prospective observational study. J Clin Med. 2022;11(12):1.
41. Napolitano M, Maffei M, Patruno C, et al. Dupilumab effectiveness for the treatment of patients with concomitant atopic dermatitis and chronic rhinosinusitis with nasal polyposis. Dermatol Ther. 2021;34(6):e15120.
42. Mariateresa Cantelli FMMN. Dermatologic therapy - 2022 - Cantelli - Upadacitinib improved alopecia areata in a patient with atopic dermatitis A case. Dermatol Ther. 2022;35:e15346.
43. Toncic RJ, Jakasa I, Hadzavdic SL, et al. Altered levels of sphingosine, sphinganine and their ceramides in atopic dermatitis are related to skin barrier function, disease severity and local cytokine milieu. Int J Mol Sci. 2020;21(6):1.
44. Yin H, Wang S, Gu C. Identification of molecular signatures in mild intrinsic atopic dermatitis by bioinformatics analysis. Ann Dermatol. 2020;32(2):130–140.
45. Song Z, Deng X, Chen W, et al. Toll-like receptor 2 agonist Pam3CSK4 up-regulates FcepsilonRI receptor expression on monocytes from patients with severe extrinsic atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29(11):2169–2176.
46. Hiragun T, Hiragun M, Ishii K, et al. Sweat allergy: extrinsic or intrinsic? J Dermatol Sci. 2017;87(1):3–9.
47. Cabanillas B, Brehler AC, Novak N. Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol. 2017;17(4):309–315.
48. Yamaguchi H, Kabashima-Kubo R, Bito T, et al. High frequencies of positive nickel/cobalt patch tests and high sweat nickel concentration in patients with intrinsic atopic dermatitis. J Dermatol Sci. 2013;72(3):240–245.
49. Rustad AM, Nickles MA, Bilimoria SN, et al. The role of diet modification in atopic dermatitis: navigating the complexity. Am J Clin Dermatol. 2022;23(1):27–36.
50. Nakazawa S, Shimauchi T, Funakoshi A, et al. Suprabasin-null mice retain skin barrier function and show high contact hypersensitivity to nickel upon oral nickel loading. Sci Rep. 2020;10(1):14559.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.