Back to Journals » Clinical Ophthalmology » Volume 14
Intravitreal Clindamycin as First-Line Therapy for Toxoplasmic Retinochoroiditis: A Case Series
Authors Verma L, Thulasidas M , Gupta A
Received 25 October 2020
Accepted for publication 18 November 2020
Published 7 December 2020 Volume 2020:14 Pages 4279—4285
DOI https://doi.org/10.2147/OPTH.S288725
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lalit Verma ,1 Mithun Thulasidas ,1 Avnindra Gupta 1
1 Centre for Sight, Delhi 110029, India
Correspondence: Lalit Verma; Mithun Thulasidas
Centre for Sight, B-5/24, Safdarjung Enclave, Delhi 110029, India
Email [email protected]; [email protected]
Purpose: To report a case series of four ocular toxoplasmosis patients who received intravitreal clindamycin as first-line treatment.
Materials and Methods: Retrospective interventional case series.
Results: Four (two females and two males) patients were diagnosed with active primary toxoplasmic retinochoroiditis based on their clinical presentation. All patients received intravitreal clindamycin 1mg/0.1mL as first-line therapy (two injections with 1-week interval). Oral corticosteroid 1mg/kg/day was also given in a tapering fashion over 4– 6 weeks. A remarkable response was seen in all cases with improved visual acuity, sharpening of the lesion borders, and resolution of inflammation within 4– 6 weeks. No recurrence or reactivation was noted until 2 years follow-up.
Conclusion: Intravitreal clindamycin, combined with oral corticosteroids, can be considered an effective and safe first-line therapy for active toxoplasmic retinochoroiditis. It provides the patient a more convenience, safer systemic side effect profile, increased availability, and fewer follow-up visits and hematologic investigations.
Keywords: toxoplasmosis, toxoplasmic retinochoroiditis, intravitreal clindamycin, uveitis, vitritis
Introduction
Ocular toxoplasmosis is the most common cause of posterior uveitis in immunocompetent patients.1 Focal retinitis adjacent to a pigmented chorioretinal scar, with overlying vitritis, is the frequent ocular presentation.2 Though the disease is commonly self-limited, the vision may be affected due to optic nerve or macular involvement and/or severe vitritis.2,3 Hence, early and adequate treatment is usually indicated for such lesions to avoid profound visual impairment. The conventional systemic medications include sulfadiazine, pyrimethamine with folinic acid, clindamycin, and trimethoprim-sulfamethoxazole double-strength (TMP- SMZ DS). Other antimicrobials, such as azithromycin and atovaquone, have also been used effectively.2–4 However, some patients are intolerant, allergic, or have infections resistant to systemic therapy.1,4 Intravitreal injections can deliver a high concentration of drug to the intraocular tissues, bypassing the ocular barriers, thereby acting directly on the target site.3 Intravitreal clindamycin alone or in combination with dexamethasone had been previously reported as an alternative treatment for ocular toxoplasmosis.2,3,5–13 However, the use of intravitreal clindamycin alone as the first-line therapy for toxoplasmic retinochoroiditis is not commonly practised. We report our experience with intravitreal clindamycin as first-line treatment in four patients of toxoplasmic retinochoroiditis.
Case Series
This retrospective consecutive interventional case series included patients who received intravitreal clindamycin, 1mg/0.1mL, as first-line therapy for active toxoplasmic retinochoroiditis at Centre for Sight eye hospital, New Delhi, India, between January 2018 and December 2018. Written informed consent was obtained from all the patients/legal guardians according to the tenets of the Declaration of Helsinki. Ethical approval was waived by the institutional ethics committee of Centre for Sight, given the retrospective nature of the case series that limits itself to collecting clinical data with no personal identifiers of the patient being captured from the records. The confidentiality of patients was maintained throughout all stages of the data analysis.
The toxoplasmic retinochoroiditis diagnosis was based on at least 1 of the following clinical findings: (a) optic nerve or macular involvement or threat thereof by an adjacent lesion, (b) a lesion adjacent to large retinal vessels, and (c) severe vitreous inflammation (≥ 2+ vitreous cells), in the absence of other identifiable causes, as well as positive serum titers of antibody (immunoglobulin [Ig] G or M) to Toxoplasma gondii. The degree of vitreous inflammation was determined on a scale from 0 to 4 as described by Kimura et al.14 A clinically significant response to the treatment was defined as Snellen visual acuity change of ≥ 2 lines or a final visual acuity of 6/6, sharpening of the lesion border with or without hyperpigmentation, and resolution of vitreous inflammation at the end of 6 weeks. A decrease of vitreous cells to the level of 0 or trace after treatment was considered to be vitreous inflammation resolution.
Table 1 summarizes the patient data.
 |
Table 1 Summary of Patient Data |
Case 1
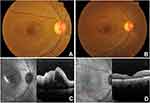
A 40-year-old woman was diagnosed with ocular toxoplasmosis based on her presentation with a corrected distance visual acuity (CDVA) of counting fingers (CF) at 1 metre, 2+ vitreous cells, and an active juxtamacular retinitis (Figure 1A) in the right eye (RE). There was no history of consumption of raw meat or contact with animals. The examination of the left eye (LE) was within normal limits with a CDVA of 6/6. Intraocular pressure (IOP) was normal in both eyes. Spectral-domain optical coherence tomography (SD-OCT) of RE revealed macular edema with intra- and sub-retinal fluid, and vitreous hyperreflectivity (Figure 1C). She was treated with intravitreal clindamycin 1mg/0.1mL (two injections with 1-week interval) administered under sterile conditions. Oral corticosteroid 1mg/kg/day was also initiated with gradual tapering over 6 weeks. One month later, CDVA improved to 6/9 with a quiescent lesion free of inflammation and macular edema reduced. At 2 months, CDVA was 6/6 with an inactive lesion and complete resolution of macular edema (Figure 1B and D). No recurrence of the disease was noted until 2 years follow-up.
Case 2
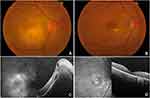
A 57-year-old woman presented with a CDVA of CF at ½ metre, 3+ vitreous cells, and focal juxtamacular retinochoroiditis (Figure 2A) in the RE. She did not give any specific exposure history like consumption of undercooked meat, ingestion of contaminated water, or contact with animals. IOP was normal in both eyes. RE SD-OCT showed elevation of the neurosensory retina and retinal pigment epithelium (RPE) with the presence of intra- and sub-retinal fluid (Figure 2C). LE examination was unremarkable. IgG antibody titres to Toxoplasma gondii were elevated, and a diagnosis of ocular toxoplasmosis was made. She received intravitreal clindamycin 1mg/0.1mL twice with an interval of one week and oral corticosteroid 1mg/kg/day in a descending regime over 6 weeks. CDVA improved to 6/24 at 1 month with the sharpening of the borders of retinochoroidal lesion. At 2 months, CDVA was 6/12, vitreous inflammation resolved, and the lesion remained quiescent with some evidence of outer retinal and RPE atrophy (Figure 2B and D). No reactivation of the lesion was noted up to 2 years follow-up.
Case 3
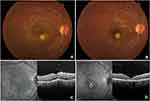
A 12-year-old boy was diagnosed with active toxoplasmosis based on his presentation with a CDVA of 6/18, 2+ vitreous cells, and focal juxtamacular retinitis (Figure 3A) in the RE. There was no history of consumption of undercooked meat or contact with animals. The examination of LE was unremarkable with normal IOP in both eyes. SD-OCT of RE revealed macular edema with increased retinal thickness, sub-retinal fluid, and vitreous hyperreflectivity (Figure 3C). He was treated with two injections of intravitreal clindamycin 1mg/0.1mL and oral corticosteroid 1mg/kg/day in a tapering regime over 4 weeks. One month later, CDVA improved to 6/6 with the sharpening of the lesion borders, resolution of vitritis and macular edema (Figure 3B and D). He remained free of inflammation with a CDVA of 6/6 at 3 months. No recurrence was observed for 2 years until the last follow-up.
Case 4
A 22-year-old male presented with a CDVA of 6/9, 2+ vitreous cells, and an active juxtapapillary retinitis (Figure 4A) in the RE. He had no history of consuming undercooked meat, ingesting contaminated water, or contact with animals. The examination of LE was normal, with a CDVA of 6/6. A positive titre of IgG antibody to Toxoplasma gondii confirmed the diagnosis of ocular toxoplasmosis. He was administered intravitreal clindamycin 1mg/0.1mL twice and given oral corticosteroid 1mg/kg/day in a tapering fashion over 4 weeks. CDVA improved to 6/6 at 1 month with a quiescent toxoplasmic lesion free of inflammation (Figure 4B). The disease remained inactive at 3 months, maintaining a CDVA of 6/6. No reactivation was seen till 2 years follow-up.
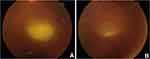 |
Figure 4 (A) Fundus photograph showing active juxtapapillary retinitis before treatment, (B) quiescent lesion after treatment with intravitreal clindamycin. |
Discussion
Many of the traditional systemic anti-toxoplasmosis medications used may have side effects that compromise patient care. Pyrimethamine has been shown to produce leukopenia and thrombocytopenia in 26% of patients.15 Sulpha-containing medications such as sulfadiazine and TMP-SMZ DS may cause skin rashes in >10% of patients.1,2 Oral clindamycin may generate vomits and/or diarrhoea due to the risk of pseudomembranous colitis.1,2,5 Two alternative treatment options that avoid these systemic side effects include local treatment with alternate day subconjunctival clindamycin injections for 1 month or the intravitreal injection of clindamycin.2,3,5,6,15
Our patients received intravitreal clindamycin as first-line therapy due to its direct action on the target site at high concentration and lack of systemic side effects. It is believed that 1mg of intravitreal clindamycin allows concentrations of 1.6μg/mL for 40 hours, a level exceeding the 50% inhibitory concentration for toxoplasma.5 Also, no retinal toxicity has been reported yet with intravitreal clindamycin.2,3,5–9 All our patients improved without recurrence following two injections of intravitreal clindamycin despite continuing on a tapering dose of systemic prednisolone without antibiotic cover. This could be due to the high concentration of clindamycin available to the retina and choroid when the treatment was given via intravitreal route and the possible superior potential of clindamycin to treat the encysted form of toxoplasma.
Previous studies have compared the use of intravitreal clindamycin and dexamethasone against classic oral treatment, demonstrating similar effectiveness with both treatment modalities.7–9 An intravitreal dose of 1mg/0.1mL clindamycin at weekly intervals and up to four injections had been administered by most of the authors.2,3,5–7,9 In our series, all patients received two intravitreal injections with a 1-week interval and responded well. There is no consensus on the best mode of corticosteroid administration in ocular toxoplasmosis cases. We believe that oral administration provides better results as intravitreal administration exhibits short mean life. Some authors believe that co-administration of intravitreal steroids may interfere with the effectiveness of intravitreal clindamycin.2,7 Also, reactivation of the disease has been reported with intravitreal and periocular steroids.16–18 All patients in this series received oral steroids in a tapering regime for 4–6 weeks and no recurrence was noted until 2 years follow-up.
The risk of recurrence of ocular toxoplasmosis during the first few years after an active episode is found to be significantly greater in patients with a primary lesion, which then decreases with increasing disease-free intervals.19–21 Therefore, patients should be followed up more often in the first year after suffering from an active lesion of ocular toxoplasmosis. The age of the patient also seems to influence the risk of recurrence, which is controversially discussed in the literature. However, recent studies suggest that elderly individuals are more likely to have a higher risk of recurrence.19,20 Our case series included patients of all age groups ranging from 12 to 57 years with a primary active lesion and were followed up regularly up to 2 years. None of our patients had a recurrence at 2 years of follow-up, similar to the report by Lasava et al.8 However, studies by Soheilian et al, Baharivand et al, and Kishore et al showed episodes of recurrence with intravitreal clindamycin and dexamethasone.7,9,10 It is important to note that the risk of recurrence is additionally affected by other host-specific and parasite-specific factors such as immune status, cytokine profile, the genotype of the patient, and strain of the parasite.20,22
Possible complications of intravitreal injections include inadvertent retinal damage and retinal detachment, infectious endophthalmitis, and damage to the posterior capsule of the lens. However, these risks can be avoided by following the guidelines for the intravitreal injection of drugs and sterilization procedures.23 Only one case report described a hypersensitivity reaction following intravitreal clindamycin in a patient with ocular toxoplasmosis.24 In our patients, injections were well tolerated without any associated complications during or after the procedure.
Sobrin et al initially reported the efficacy of intravitreal clindamycin in the treatment of toxoplasmic retinochoroiditis. Their study included six patients treated with intravitreal clindamycin (1.0 mg/0.1 mL) because of intolerance to systemic therapy or disease progression despite oral antimicrobial treatment. Four patients underwent concomitant pars plana vitrectomy at the time of injection. They concluded that intravitreal clindamycin was associated with control of toxoplasmosis and resolution of vitreous inflammation.2
Wong et al described a patient with ocular toxoplasmosis with an atypical presentation managed successfully with intravitreal clindamycin because of intolerance to oral therapy. After two injections of intravitreal clindamycin and oral prednisolone without concomitant systemic antibiotics, this patient continued to improve without recurrence.5 Tabuenca Del Barrio et al reported using intravitreal clindamycin and oral steroids in a case of bilateral ocular toxoplasmosis in a sulfamide allergic patient with unilateral activation. They concluded that weekly intravitreal clindamycin treatment is a suitable therapeutic alternative in severe ocular toxoplasmosis and/or in patients with a contraindication to classical treatment.6 Hosseini et al explained a case of toxoplasmic chorioretinitis resistant to standard oral treatment that responded dramatically to intravitreal clindamycin.3
In contrast to the published literature, our patients had no contraindication to standard oral therapy and received intravitreal clindamycin as first-line therapy. The lesion became inactive, the visual acuity improved, and the vitritis resolved within 4–6 weeks in all four patients. Three out of four patients had a final CDVA of 6/6. One patient’s CDVA was limited to 6/12 due to the development of RPE and outer retinal atrophy. Moreover, no recurrence or reactivation of the lesion was noted till 2 years follow-up.
This case series demonstrates that intravitreal clindamycin, together with oral corticosteroids, suppose an effective and safe first-line strategy for patients with toxoplasmic retinochoroiditis due to better patient compliance, improved systemic safety profile, enhanced availability at the target site, and lesser follow-up visits and hematologic investigations. It could be a better choice of treatment for pregnant and pediatric patients. However, further prospective clinical studies are needed to validate the efficacy of intravitreal clindamycin as a first-line treatment.
Data Sharing Statement
The data are available within the manuscript.
Ethics Approval and Informed Consent
Written informed consent was obtained from all the patients/legal guardians for the publication of the case details and any accompanying images. Ethical approval was waived by the institutional ethics committee of Centre for Sight, given the retrospective nature of the case series that limits itself to collecting clinical data with no personal identifiers of the patient being captured from the records.
Consent for Publication
Written informed consent was obtained from the patients (Cases 1, 2, 4) and legal guardian (Case 3) for the publication of the case details and any accompanying images. This report does not contain any personal identifying information.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
No funding or grant support.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005;20(3):129–141. doi:10.1080/08820530500231961
2. Sobrin L, Kump LI, Foster CS. Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina. 2007;27(7):952–957. doi:10.1097/IAE.0b013e31804b3f0d
3. Hosseini SM, Abrishami M, Mehdi Zadeh M. Intravitreal clindamycin in the treatment of unresponsive zone one toxoplasmic chorioretinitis: a case report. Iran Red Crescent Med J. 2014;16(11):e15428. doi:10.5812/ircmj.15428
4. Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MSA, et al. A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol. 2002;134(1):34–40. doi:10.1016/s0002-9394(02)01537-4
5. Wong R, Omo R, Marino M, et al. Toxoplasma gondii: an atypical presentation of toxoplasma as optic disc swelling and hemispherical retinal vein occlusion treated with intravitreal clindamycin. Int Ophthalmol. 2009;29(3):195–198. doi:10.1007/s10792-008-9192-8
6. Tabuenca Del Barrio L, Heras MH, Mozo CM, et al. Clindamicina intravítrea como alternativa terapéutica en la toxoplasmosis ocular severa. Arch de la Sociedad Española de Oftalmología. 2019;94(12):602–604. doi:10.1016/j.oftal.2019.09.005
7. Kishore K, Conway MD, Peyman GA. Intravitreal clindamycin and dexamethasone for toxoplasmic retinochoroiditis. Ophthalmic Surg Lasers. 2001;32(3):183–192.
8. Lasave AF, Diaz-Llopis M, Muccioli C, et al. Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology. 2010;117(9):1831–1838. doi:10.1016/j.ophtha.2010.01.028
9. Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011;118(1):134–141. doi:10.1016/j.ophtha.2010.04.020
10. Baharivand N, Mahdavifard A, Fouladi RF. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. Int Ophthalmol. 2013;33(1):39–46. doi:10.1007/s10792-012-9634-1
11. Zamora YF, Arantes T, Reis FA, et al. Local treatment of toxoplasmic retinochoroiditis with intravitreal clindamycin and dexamethasone. Arq Bras Oftalmol. 2015;78(4):216–219. doi:10.5935/0004-2749.20150056
12. Bor’i A, Mahrous A, Al-Aswad MA, et al. Intravitreal Clindamycin and Dexamethasone Combined with Systemic Oral Antitoxoplasma Therapy versus Intravitreal Therapy Alone in the Management of Toxoplasma Retinochoroiditis: A Retrospective Study. J Ophthalmol. 2018;2018:4160837. doi:10.1155/2018/4160837
13. Park JH, Lee SY, Lee EK. Morphological characteristics of ocular toxoplasmosis and its regression pattern on swept-source optical coherence tomography angiography: a case report. BMC Ophthalmol. 2019;19(1):199. doi:10.1186/s12886-019-1209-8
14. Kimura SJ, Thygeson P, Hogan M. Signs and symptoms of uveitis. II. Classification of the posterior manifestations of uveitis. Am J Ophthalmol. 1959;47:171–176. doi:10.1016/s0002-9394(14)78240-6
15. Rothova A, Meenken C, Buitenhuis HJ, et al. Therapy for ocular toxoplasmosis. Am J Ophthalmol. 1993;115(4):517–523. doi:10.1016/s0002-9394(14)74456-3
16. Nicholson DH, Wolchok EB. Ocular toxoplasmosis in an adult receiving long-term corticosteroid therapy. Arch Ophthalmol. 1976;94(2):248–254. doi:10.1001/archopht.1976.03910030120009
17. Nozik RA. Results of treatment of ocular toxoplasmosis with injectable corticosteroids. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:811–818.
18. O’Connor GR, Frenkel JK. Editorial: dangers of steroid treatment in toxoplasmosis. Periocular injections and systemic therapy. Arch Ophthalmol. 1976;94:213. doi:10.1001/archopht.1976.03910030093001
19. Reich M, Ruppenstein M, Becker MD, Mackensen F. Time patterns of recurrences and factors predisposing for a higher risk of recurrence of ocular toxoplasmosis. Retina. 2015;35(4):809–819. doi:10.1097/IAE.0000000000000361
20. Aleixo ALQDC, Vasconcelos C, de Oliveira R, et al. Toxoplasmic retinochoroiditis: the influence of age, number of retinochoroidal lesions and genetic polymorphism for IFN-γ +874 T/A as risk factors for recurrence in a survival analysis. PLoS One. 2019;14(2):e0211627. doi:10.1371/journal.pone.0211627
21. Holland GN, Crespi CM, Ten Dam-van Loon N, et al. Analysis of recurrence patterns associated with toxoplasmic retinochoroiditis. Am J Ophthalmol. 2008;145(6):1007–1013. doi:10.1016/j.ajo.2008.01.023
22. Saffra NA, Seidman CJ, Weiss LM. Ocular Toxoplasmosis: controversies in Primary and Secondary Prevention. J Neuroinfect Dis. 2013;4:235689.
23. Grzybowski A, Told R, Sacu S, et al. Update on Intravitreal Injections: Euretina Expert Consensus Recommendations. Ophthalmologica. 2018;239:181–193. doi:10.1159/000486145
24. Kim P, Younan N, Coroneo MT. Hypersensitivity reaction to intravitreal clindamycin therapy. Clin Experiment Ophthalmol. 2002;30(2):147–148. doi:10.1046/j.1442-6404.2002.00502.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.