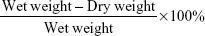Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Intravenous administration of adipose tissue-derived stem cells enhances nerve healing and promotes BDNF expression via the TrkB signaling in a rat stroke model
Authors Li X, Zheng W, Bai H, Wang J, Wei R, Wen H, Ning H
Received 23 January 2016
Accepted for publication 10 March 2016
Published 2 June 2016 Volume 2016:12 Pages 1287—1293
DOI https://doi.org/10.2147/NDT.S104917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Wai Kwong Tang
Xin Li,1 Wei Zheng,2 Hongying Bai,1 Jin Wang,3 Ruili Wei,1 Hongtao Wen,3 Hanbing Ning3
1Department of Neurology, 2Department of Nursing, The Second Affiliated Hospital of Zhengzhou University, 3Department of Digestive Diseases, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China
Abstract: Previous studies have shown the beneficial effects of adipose-derived stem cells (ADSCs) transplantation in stroke. However, the molecular mechanism by which transplanted ADSCs promote nerve healing is not yet elucidated. In order to make clear the molecular mechanism for the neuroprotective effects of ADSCs and investigate roles of the BDNF–TrkB signaling in neuroprotection of ADSCs, we, therefore, examined the neurological function, brain water content, and the protein expression in middle cerebral artery occlusion (MCAO) rats with or without ADSCs transplantation. ADSCs were transplanted intravenously into rats at 30 minutes after MCAO. K252a, an inhibitor of TrkB, was administered into rats by intraventricular and brain stereotaxic injection. Modified neurological severity score tests were performed to measure behavioral outcomes. The results showed that ADSCs significantly alleviated neurological deficits and reduced brain water content in MCAO rats. The protein expression levels of BDNF and TrkB significantly increased in the cortex of MCAO rats with ADSCs treatment. However, K252a administration reversed the ADSCs-induced elevation of BDNF, TrkB, and Bcl-2 and reduction of Bax protein in MCAO rats. ADSCs promote BDNF expression via the TrkB signaling and improve functional neurological recovery in stroke rats.
Keywords: stroke, adipose tissue-derived stem cells, brain-derived neurotrophic factor, TrkB
Introduction
Stroke is a devastating disease that leads to death and severe disability worldwide. According to the type of blood vessel injury, stroke is divided into ischemic and hemorrhagic subtypes. Almost 80% of strokes are ischemic, and the rest are hemorrhagic.1 The majority of patients suffer from different degrees of neurologic deficits such as behavior, sensory, memory, and cognitive disorders, most of which are caused by the blockages of blood vessels in different regions of the brain.2 Inflammation, oxidative stress, and apoptosis are all involved in the ischemic stroke-induced brain damage.3,4 No therapeutic strategies apart from thrombolytic drugs have been convincingly shown to be effective for the patients who suffered from ischemic stroke. However, due to the limitations of complications risk and narrow therapeutic time window, the method of thrombolysis is only appropriate for a few patients.5 In the recent years, accumulating researches have demonstrated that stem cell-based treatments may be a novel therapy for stroke.
Adipose-derived stem cells (ADSCs), a type of pluripotent stem cells, can be isolated from liposuction waste tissue by collagenase digestion and differential centrifugation. A growing body of evidence has shown the wound-healing functions of ADSCs transplantation in the central nervous system injuries to be associated with stroke, multiple sclerosis, Parkinson’s disease, and spinal cord injury.6,7 Several studies have shown that the intracerebroventricular or intravenous transplantation of ADSCs into the damaged brain area of mouse models contributed to tissue regeneration.8,9 ADSCs-based therapies for multiple tissues and organ injuries may offer a feasible solution for serious diseases. The transplantation of ADSCs for stroke patients has recently been regarded as a potentially workable therapeutic method because of the relative ease of being acquired, a greater possibility of autologous transplantation, and their protective biological effects against stroke.6 Besides neuroprotective functions, ADSCs have neurorestorative effects for contributing to recovery of damaged brain tissue. In animal models of stroke, ADSCs promoted recovery of stroke by increasing brain plasticity such as neurogenesis, remyelination, synaptogenesis, and angiogenesis.10–12
In the recent years, BDNF, one of the members of neurotrophin family, has been identified as an important neuroprotective factor in damaged brain. BDNF is produced by glutamate neurons and widely distributed in the brain, with the highest expression in the hippocampus. As the most active neurotrophins, BDNF plays an important role in neuroplasticity and inhibits inflammatory factor and neuronal apoptosis.13 BDNF has been proved to be involved in learning, memory formation, depression, and recovery from brain injury in animal models.14,15 Activation of TrkB is important for BDNF-mediated neuronal protection.16 TrkB knockout in mice enhanced hippocampal-dependent memory deficits and reduced hippocampal long-term potentiation.17 A previous study has shown that the BDNF signaling via TrkB is implicated in the neuroplasticity and functional recovery following cervical spinal cord injury.18 These discoveries suggest that pharmacological strategies for elevating the BDNF level may provide a novel therapeutic method for stroke-induced brain injury. In this study, we confirmed the protective effect of ADSCs on stroke-induced brain damage and investigated the mechanism of ADSCs on BDNF expression in middle cerebral artery occlusion (MCAO) rats by using a TrkB inhibitor.
Materials and methods
Isolation and culture of adipose tissue-derived stem cells
The subcutaneous adipose tissue was separated from the inguinal region of rats following the procedure of MCAO surgery. Adipose tissue was washed with phosphate-buffered saline (PBS) three times under sterile conditions to remove red blood cells and cellular debris. Then, the extracellular matrix was enzymatically digested with equal volume of 0.5 mg/mL collagenase (Sigma-Aldrich Co., St Louis, MO, USA) and 0.25% trypsin (Sigma-Aldrich Co.) for 1 hour at 37°C. The cell suspension was centrifuged at 1,200× g for 10 minutes. The ADSCs were cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% antibiotic/antimycotic solution (Thermo Fisher Scientific) and maintained at a temperature of 37°C and 5% humidified carbon dioxide. After 24 hours of incubation, the cells were washed thoroughly with PBS to remove nonadherent cells. On the third pass, ADSCs were trypsinized and counted before being administered to the experimental animals.
Flow cytometry
ADSCs of rats were digested by 0.25% trypsinase for 15 minutes at 37°C. After being washed twice with PBS, the cells were incubated with monoclonal antibodies, including anti-CD29 antibody (BioLegend, San Diego, CA, USA), anti-CD90 antibody (BioLegend), anti-CD105 antibody (R&D Systems, Inc., Minneapolis, MN, USA), anti-CD45 antibody (BioLegend), anti-CD106 antibody (BioLegend), or anti-CD34 antibody (BioLegend). The secondary antibodies, antirabbit or goat fluorescein isothiocyanate-conjugated antibodies (BD, Franklin Lakes, NJ, USA), were used according to the manufacturer’s instructions. Negative controls were conducted by omitting the primary antibodies. The scatter parameters of ADSCs were analyzed using FACScan flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA) and CellQuest analysis software (BD).
Animals and MCAO surgical procedure
The adult male Sprague Dawley rats were purchased from the Shanghai Experimental Animal Centre. The animals were kept under standard conditions at a temperature of 25°C and a 12-hour light/dark cycle and had free access to food and water. The rats were randomly assigned into four groups: the sham-operated group, the MCAO group, the MCAO + vehicle group, and the MCAO + ADSCs group. The rats were anesthetized with 5% isoflurane in O2 and treated with 2% isoflurane throughout surgery. Throughout the surgery, the rats were maintained at 37°C±0.5°C. The right carotid artery was clamped with a small vascular clip. The middle cerebral artery was occluded until the tip occluded the origin of the middle cerebral artery. After closure of the operative sites, the animals were allowed to awake from the anesthesia.19
The animal experiment was reviewed and approved by the Animal Care and Use Committee of the Second Affiliated Hospital of Zhengzhou University and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals.
Transplants of ADSCs and drug treatment
The ADSCs were washed with PBS and centrifuged twice at 1,000× g for 5 minutes. Viability was measured using the trypan blue dye exclusion method, and cell concentration was adjusted to 1×104 cells/μL. In the experimental group, a total of 5×105 ADSCs in 500 μL PBS were infused into each rat by tail intravenous injection after 24 hours of surgical operation. In parallel, an equal amount of PBS was injected into the rats in the control group. Injection time for all was 4 minutes. Nothing was done with the sham-operated group.
The rats were administered intraventricularly with K252a (10 μL, 0.5 μg/μL in 1% dimethyl sulfoxide; Cell Signaling Technology, Inc., Danvers, MA, USA) or the same volume of dimethyl sulfoxide after MCAO. One hour after MCAO, the heads of rats were fixed on a stereotaxic instrument (David Kopf Instruments, Tujunga, CA, USA) and then injected using a microsyringe (Hamilton, Reno, NV, USA) at a rate of 0.5 μL/min. Based on the stereotaxic map of the rat brain, a 1 mm hole was drilled (0.8 mm posterior and 1.5 mm lateral to the bregma and 3.3 mm from the dura mater).
Neurologic severity scores
The modified neurological severity score (mNSS), a composite of motor, sensory, reflex, and balance tests, was used to evaluate neurologic deficits of rats d1, d7, d14, d21, and d28 after MCAO, as previously described.19–21 The items of motor tests of the mNSS mainly reflect the function of the motor representation area in the contralateral cortex, damage to which causes contralateral limb paralysis, resulting in a high score on the motor tests. Sensory tests mainly reflect a combination of visual, tactile, and deep sensation. Balance tests mainly reflect hindlimb placing performance. If the hindlimb of rats is not placed on the beam or the hindlimb is placed on the vertical surface of the beam in order to support the weight of rats and maintain balance, the rats obtain high scores on balance tests. The neurologic function were graded 0–5 scores (0, no neurologic deficit; 5, maximal deficit). Rats with a score of 1 were not able to perform the test or did not have the tested reflex. A higher score indicated a more severe injury.
Analysis of brain water content
Brains were separated at 72 hours after MCAO and divided into two hemispheres, and cerebella were abandoned. The brain samples were weighed to acquire the wet weights. The samples were dried in an electric oven at 100°C for 24 hours for dry weights. Water contents are represented as percentages of wet weights in the following formula:22
|
|
Western blot analysis
The brain tissues were lysed using radioimmunoprecipitation lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, People’s Republic of China). Proteins mixed with loading buffer were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a hybrid polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). Then, nonspecific binding was blocked by incubating with 5% (w/v) nonfat milk in Tris-buffered saline with Tween-20 (Sigma-Aldrich Co.) at room temperature for 1 hour. The membranes were incubated with primary antibodies, including anti-BDNF antibody (Cell Signaling Technology), anti-TrkB antibody (Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-Bcl-2 antibody (Santa Cruz Biotechnology Inc.), anti-Bax antibody (Santa Cruz Biotechnology Inc.), and anti-β-actin antibody (Sigma-Aldrich Co.). After overnight incubation with the primary antibody at 4°C, blots were incubated with horseradish peroxidase-conjugated secondary antibodies (CWBIO, Beijing, People’s Republic of China) for 1 hour at room temperature. Following washing three times (15 minutes each) with TBST (Tris 20 mM, NaCl 150 mM, and Tween 20 0.1%, pH 7.6) at room temperature, the membranes were subjected to enhanced chemiluminescence (Pierce, Rockford, IL, USA) and exposed to film. Each Western blot band was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The Student’s t-test and one-way analysis of variance were used for statistical analyses. A P-value of <0.05 was considered statistically significant. Data were expressed as mean ± standard error of the mean.
Results
Characterization of adipose tissue-derived stem cells
Aimed to confirm phenotype and purity of ADSCs, cell surface markers were determined by flow cytometry. The flow cytometric analysis showed that CD29, CD90, and CD105 were highly expressed in almost all ADSCs. On the contrary, CD34, CD45, and CD106 were nearly not expressed in the ADSCs (Figure 1). These results were consistent with the previous reports for porcine ADSCs.23
Intravenous administration of ADSCs improves functional recovery of rats
In order to assess the effects of administration of ADSCs on functional recovery of rats, mNSS tests were performed to measure behavioral outcomes at different time points after MCAO. As shown in Figure 2, the scores of rats in the ADSCs group were significantly lower than those in the vehicle group at day 7 after ADSCs transplantation. These data showed that transplantation of ADSCs by tail vein injection alleviated neurological deficits in the rat model of stroke.
ADSCs treatment reduced brain water content after MCAO
The water content is an important marker of brain injury, and it reaches the peak 3 days after brain damage. To test whether ADSCs transplantation decreases brain edema, brain water content was determined. The percentage of brain water content in the vehicle group significantly increased compared with that of the sham control group. However, the brain water content in the ADSCs group was lower than that in the vehicle group (Figure 3).
ADSCs transplantation increases BDNF level by activating TrkB signaling in the cortex of MCAO rats
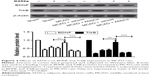
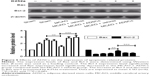
We examined the expression of BDNF and TrkB in the cortex of rats at day 7 after MCAO by Western blot analysis. The protein expression levels of BDNF and TrkB in the MCAO group significantly decreased compared with those in the sham-operated group. ADSCs transplantation augmented BDNF and TrkB expression in the brain of MCAO rats. However, the protein expression of BDNF decreased in the presence of K252a (Figure 4). The results showed that TrkB activation was essential for BDNF expression in MCAO rats with ADSCs transplantation.
ADSCs transplantation regulates apoptosis-related protein expression in the cortex of rats after MCAO
As shown in Figure 5, the expression levels of Bcl-2 in MCAO and vehicle + MCAO groups were significantly decreased compared with those of the sham-operated group. Moreover, Bax in MCAO and vehicle + MCAO groups increased compared with that of the sham-operated group. ADSCs transplantation significantly elevated the expression level of Bcl-2 and attenuated the expression level of Bax. However, the ADSCs-induced increase in Bcl-2 and decrease in Bax protein were reversed in MCAO rats treated with K252a. These data demonstrated that the antiapoptotic function of transplanted ADSCs may be dependent on BDNF-TrkB signaling in rat cortical neurons.
Discussion
In the recent years, multiple ways have been explored to find an effective therapeutic approach for stroke. The inflammation-induced neuron loss leads to irreversible damages and infarction areas in the brain of patients with stroke, and there is currently no cure for this disorder.8 The key point to solve this problem is to explore an effective treatment strategy to regenerate neuronal cells. Therefore, stem cells transplantation may be an effective method for curing stroke.
A large number of evidence indicated that ADSCs transplantation remarkably improved the neurological defects and functions via various molecular mechanisms, and no serious complications have been observed so far. A study demonstrated that ADSCs transplantation via tail intravenous injection or brain stereotaxic injection promoted restoration and regeneration of nerve injury in animal models.24 A large number of evidence indicated that ADSCs were capable of secreting multiple neurotrophic factors and vascular endothelial growth factors that contributed to early peripheral nerve regeneration. ADSCs were capable of producing a mass of vascular endothelial growth factor, which contributed to implant biocompatibility. Transplantation of ADSCs attenuated functional deficits in MCAO rats.25 Human ADSCs administration with MCAO mice could apparently attenuate stroke symptoms and ameliorate neurological functions by enhancing immunosuppression and evaluating the viability of endogenous neurons.26 Inflammatory and apoptosis factors have been found to be involved in brain aging and neurodegeneration.27 Adipose-derived mesenchymal stem cells therapy contribute to recovery of neurological function in rats after acute ischemic stroke by promoting vasculogenesis and neurogenesis as well as its inhibitory properties on inflammation and apoptosis.28 In this study, we demonstrated that transplanted ADSCs improved neurological function in rats after MCAO.
The growth factors, especially BDNF and glial cell-derived neurotrophic factor, have antiapoptotic and neurotrophic effects on neurons.29 Decreasing the BDNF mRNA level by intracerebroventricular injection of antisense oligonucleotide prevented recovery of behavioral functions in rats after stroke.30 A previous study suggested that adipose stromal cells prevented hypoxia-induced brain damage by elevating expression of BDNF.31 Here, we further investigated the mechanism of BDNF upregulation in MCAO rats with ADSCs transplantation. We demonstrated that inhibition of TrkB receptor signaling with K252a prevented the increase in BDNF induced by ADSCs treatment. The antiapoptotic protein Bcl-2 regulates permeabilization of the mitochondrial outer membrane and further controls apoptosis.32 We found that Bcl-2 expression was elevated in the brain of MCAO rats with ADSCs transplantation. Although the mechanisms underlying the therapeutic potential of ADSCs transplantation in promoting neurological functional recovery of rats with MCAO have been clarified in this study, the challenges that exist in the clinical application of ADSCs for stroke may be more difficult. The short-term outcome of ADSCs transplantation for neurologic deficits after stroke was impressive, while the long-term outcome was needed to be followed up to evaluate the therapeutic effect accurately.
Conclusion
These findings suggested that ADSCs contributed to nerve healing and enhanced BDNF expression by activating TrkB signaling in MCAO rats. ADSCs may be developed as a novel cell-based therapy for the recovery of stroke. Further investigations should be conducted to clarify the mechanisms of antiapoptosis of ADSCs.
Acknowledgment
This study was supported by the Key Project of Natural Science Foundation of Henan Province Department of Education (13A320445).
Disclosure
The authors report no conflicts of interest in this study.
References
Dubow J, Fink ME. Impact of hypertension on stroke. Curr Atheroscler Rep. 2011;13(4):298–305. | ||
Shyu WC, Chen CP, Lin SZ, Lee YJ, Li H. Efficient tracking of non-iron-labeled mesenchymal stem cells with serial MRI in chronic stroke rats. Stroke. 2007;38(2):367–374. | ||
Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62(5):712–718. | ||
Gu LJ, Xiong XX, Ito T, et al. Moderate hypothermia inhibits brain inflammation and attenuates stroke-induced immunodepression in rats. CNS Neurosci Ther. 2014;20(1):67–75. | ||
Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. | ||
Yeh DC, Chan TM, Harn HJ, et al. Adipose tissue-derived stem cells in neural regenerative medicine. Cell Transplant. 2015;24(3):487–492. | ||
Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129(6):1277–1290. | ||
Chan TM, Harn HJ, Lin HP, et al. The use of ADSCs as a treatment for chronic stroke. Cell Transplant. 2014;23(4–5):541–547. | ||
Gutiérrez-Fernández M, Rodríguez-Frutos B, Otero-Ortega L, Ramos-Cejudo J, Fuentes B, Díez-Tejedor E. Adipose tissue-derived stem cells in stroke treatment: from bench to bedside. Discov Med. 2013;16(86):37–43. | ||
di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63(9):1544–1552. | ||
Tajiri N, Acosta SA, Shahaduzzaman M, et al. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J Neurosci. 2014;34(1):313–326. | ||
Liu XL, Zhang W, Tang SJ. Intracranial transplantation of human adipose-derived stem cells promotes the expression of neurotrophic factors and nerve repair in rats of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol. 2014;7(1):174. | ||
Bekinschtein P, Cammarota M, Katche C, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105(7):2711–2716. | ||
Schäbitz WR, Steigleder T, Cooper-Kuhn CM, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38(7):2165–2172. | ||
Schäbitz WR, Berger C, Kollmar R, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35(4):992–997. | ||
Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. | ||
Minichiello L, Korte M, Wolfer D, et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24(2):401–414. | ||
Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol. 2013;247:101–109. | ||
Shehadah A, Chen J, Kramer B, et al. Efficacy of single and multiple injections of human umbilical tissue-derived cells following experimental stroke in rats (S45. 007). Plos One. 2013;8(1):173–185. | ||
Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. | ||
Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61(3):596–602; discussion 602–603. | ||
Lee ST, Chu K, Jung KH, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(3):616–629. | ||
Meliga E, Strem BM, Duckers HJ, Serruys PW. Adipose-derived cells. Cell Transplant. 2007;16(9):963–970. | ||
Liu G, Cheng Y, Guo S, et al. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med. 2011;28(4):565–572. | ||
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183(2):355–366. | ||
Zhou F, Gao S, Wang L, et al. Human adipose-derived stem cells partially rescue the stroke syndromes by promoting spatial learning and memory in mouse middle cerebral artery occlusion model. Stem Cell Res Ther. 2015;6(1):92–92. | ||
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–873. | ||
Leu S, Lin YC, Yuen CM, et al. Research adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med. 2010;8:63. | ||
Glavaski-Joksimovic A, Virag T, Mangatu TA, McGrogan M, Wang XS, Bohn MC. Glial cell line-derived neurotrophic factor-secreting genetically modified human bone marrow-derived mesenchymal stem cells promote recovery in a rat model of Parkinson’s disease. J Neurosci Res. 2010;88(12):2669–2681. | ||
Müller HD, Hanumanthiah KM, Diederich K, Schwab S, Schäbitz WR, Sommer C. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke. 2008;39(3):1012–1021. | ||
Wei X, Du Z, Zhao L, et al. IFATS collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27(2):478–488. | ||
Shamas-Din A, Kale J, Leber B, Andrews DW. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5(4):a008714. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.