Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 12
Intravascular Large B Cell Lymphoma with CNS Involvement Successfully Treated with High-Dose Methotrexate and High-Dose Ara-C Based CNS-Directed Chemoimmunotherapy Alternating with Anthracycline Based Chemoimmunotherapy
Authors Wang J , Alhaj Moustafa M , Kuhlman JJ , Seegobin K, Jiang L , Gupta V , Tun HW
Received 26 February 2022
Accepted for publication 18 May 2022
Published 25 May 2022 Volume 2022:12 Pages 47—54
DOI https://doi.org/10.2147/BLCTT.S362736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wilson Gonsalves
Jing Wang,1 Muhamad Alhaj Moustafa,2 Justin J Kuhlman,1 Karan Seegobin,2 Liuyan Jiang,3 Vivek Gupta,4 Han W Tun2
1Department of Internal Medicine, Mayo Clinic Florida, Jacksonville, FL, 32224, USA; 2Division of Hematology and Medical Oncology, Mayo Clinic Florida, Jacksonville, FL, 32224, USA; 3Department of Pathology and Laboratory Medicine, Mayo Clinic Florida, Jacksonville, FL, 32224, USA; 4Department of Radiology, Mayo Clinic Florida, Jacksonville, FL, 32224, USA
Correspondence: Han W Tun, Mayo Clinic Florida Division of Hematology/Oncology, 4500 San Pablo Road S, Jacksonville, FL, 32224, USA, Tel +1 904-953-2000, Email [email protected]
Abstract: Intravascular large B cell lymphoma (IVL) is a rare subtype of diffuse large B cell lymphoma confined to small blood vessels with a predilection for CNS involvement. The prognosis of IVL with CNS involvement (CNS-IVL) is extremely poor. The optimal treatment for CNS-IVL is not well defined. Thus, we report three patients with CNS-IVL successfully treated with a CNS-centric approach consisting of high-dose methotrexate (HDMTX) and high-dose Ara-C (HiDAC) based CNS-directed chemoimmunotherapy (CIT) alternating with anthracycline-based CIT. Our rationale for intensifying the CNS-directed therapy is the presence of intracerebral bleeding in two of our patients which would result in extravasation of lymphoma cells into the cerebral parenchyma with the development of CNS lymphoma. All three patients have achieved excellent therapeutic outcomes. Two patients with intracerebral bleeding have been in complete remission (CR) for about 11 years and 4 years. One patient was successfully induced into CR about 10 months ago and currently is in CR. This unique therapeutic approach should be further explored for CNS-IVL.
Keywords: intravascular lymphoma, CNS involvement, CNS-directed chemoimmunotherapy, anthracycline-based chemoimmunotherapy, high-dose methotrexate, high-dose Ara-C
Introduction
The World Health Organization (WHO) classification of hematopoietic tumors defines intravascular large B cell lymphoma (IVL) as an extranodal diffuse large B cell lymphoma characterized by neoplastic lymphoid cells residing within the lumen of small vessels, particularly capillaries.1–3 The precise mechanisms responsible for this behavior of lodging and proliferation within vessels instead of forming a mass are largely unknown, but multiple studies have suggested that IVL cells express molecules involved in cell migration and molecules that make them capable of adhesion to the endothelium but lack those responsible for extravasation.4–13 The incidence of IVL is extremely rare, estimated at 0.095 per 1,000,000 per year in the United States.2,7 It can affect any organ in the body but most commonly involves the central nervous system (CNS), with CNS involvement seen in 48% of all IVL cases.14,15 Due to the rarity of CNS-IVL, data regarding its treatment regimen are currently lacking.
The optimal treatment for CNS-IVL is not established.7,15,16 Most patients with IVL with or without CNS involvement are treated with anthracycline-based chemoimmunotherapy (CIT), frequently rituximab, cyclophosphamide, hydroxydoxorubicin, vincristine and prednisone (RCHOP), and without any CNS-directed therapy.15,17,18 In the European patients with IVL with both CNS and non-CNS involvements, RCHOP has resulted in 88% complete remission rate (CRR), 91% overall response rate (ORR) and a 3-year overall survival (OS) in 81% of patients.17,18 In a larger retrospective study based on reported IVL cases with CNS involvement in the literature, the 3-year survival rate was 16.5% in the CIT group vs 12.5% in the chemotherapy group.15 Only 11 of 88 patients with CNS-IVL received CNS-penetrating agents.15 CNS-directed therapy consists of high-dose methotrexate (HDMTX) or intrathecal chemotherapy and did not add survival benefit compared with patients who did not receive CNS-directed therapy.15 Regardless, CNS-IVL cases are associated with poor prognosis with median OS of 240 days and 3-year survival rate of 4%.15 However, the experience with CNS-directed therapy in management of IVL patients is limited and further investigation and clinical experience are required.16
We present three cases of CNS-IVL treated with HDMTX and HiDAC-based CNS-directed CIT alternating with anthracycline-based CIT. All three patients have achieved excellent therapeutic outcomes.
Case 1
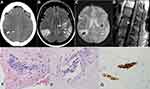
A 64-year-old female with a distant history of left-sided breast cancer had a sudden onset of dysarthria, right-sided numbness, and visual disturbances (Table 1). The initial computed tomography (CT) scan without contrast of the head and subsequent brain magnetic resonance imaging (MRI) and angiogram (MRA) of the head, neck, and arm showed no acute changes. Three weeks later, she was reevaluated with worsening altered mental status, persistent right-sided numbness, and a new burning back pain with radicular symptoms radiating down both lower extremities. CT scan of the head on the day of readmission showed intraparenchymal hemorrhage in the right parietal lobe measuring 7×13×20 mm with surrounding edema causing a mass effect and effacement of the adjacent parietal sulci (Figure 1A). A day later, MRI of the brain showed an additional left-sided hemorrhagic focus in the precentral gyrus that was not seen on the preceding CT scan (Figure 1B and C). A biopsy of the right parietal lobe showed CD20+ large cells inside the blood vessels consistent with IVL (Figure 1). No evidence of perivascular parenchymal infiltration was seen. The bone marrow aspirate and biopsy as well as the cerebrospinal fluid analysis were negative for IVL involvement. A spinal MRI T2-weighted image (T2WI) showed linear enhancement dorsal to the conus medullaris suggesting leptomeningeal involvement (Figure 1D). Lactate dehydrogenase (LDH) at the time of diagnosis was 725 U/L.
 |
Table 1 Patient Characteristics and Treatment Outcome |
The patient was treated with rituximab + HDMTX + HiDAC (RMA) (odd cycles) alternating with RCHOP (even cycles). RMA regimen consists of rituximab 375 mg/m2 IV (Day #1), HDMTX 3.5 g/m2 IV (Day #2), and HiDAC 2 g/m2 q12H IV (Days #3 and #4). RCHOP regimen consists of rituximab 375 mg/m2 IV (Day #1), cyclophosphamide 750 mg/m2 IV (Day #1), hydroxydoxorubicin 50 mg/m2 IV (Day #1), vincristine 1.4 mg/m2 IV with maximal dose of 2 mg (Day #1), and prednisone 100 mg/m2 PO (Days #1–5). RMA and RCHOP were given in an alternating schedule every three weeks for a total of eight cycles (four cycles each of RMA and RCHOP). No intrathecal chemotherapy or radiation therapy was given. Her first cycle of treatment with RMA was complicated by hypotension and seizure during rituximab infusion, which ultimately required intubation and intensive care unit (ICU) stay. She subsequently tolerated rituximab. She responded quickly with marked improvement in her mentation, motor, and cognitive functions. Complete remission (CR) was confirmed by imaging scans after 8 cycles of CIT. Currently, she has been in CR for 11 years at the time of writing. The only residual neurological symptom is numbness in the lower extremities.
Case 2
A 55-year-old female with sickle cell trait, hypertension, hyperlipidemia, rheumatoid arthritis and history of deep venous thrombosis, and cerebrovascular event presented to an outside facility with altered mental status, left arm paralysis, and left facial droop (Table 1). MRI of the brain showed multi-punctate ischemic infarctions within both cerebral hemispheres. CT abdomen and pelvis showed splenomegaly with 15 cm cephalocaudal span. A month later, she was diagnosed with left lower lobe pneumonia and Enterococcus faecalis urinary tract infection. In addition, she developed significant thrombocytopenia and anemia and was treated with IV methylprednisolone without significant improvement.
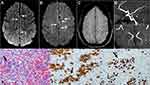
The patient was transferred to our institution for further management. Her hospital course was characterized by multiple episodes of major as well as minor strokes. On admission, hemoglobin was 7.8 g/dl with mean corpuscular volume (MCV) 82.3 fL, white count 4.2×109/L with normal differential, platelet count was 85×109/L while she was on 4 mg dexamethasone daily. Peripheral blood smear was negative for schistocytes, fibrinogen was within normal limits, and Coomb’s test was negative. LDH was elevated at 1073 U/L. MRI of the brain (Figure 2) showed multiple foci of T2 FLAIR hyperintensity within bilateral cerebral hemispheres and subcortical microscopic hemorrhagic foci. Cytology of the cerebrospinal fluid (CSF) was negative for malignant cells. Splenectomy was performed for therapeutic and diagnostic purposes to relieve patient’s abdominal pain and elucidate the underlying pathology. Pathologic findings in the spleen were consistent with diffuse large B-cell lymphoma (DLBCL) with infiltrating neoplastic lymphocytes filling the sinusoid and small vessels within the red pulp (Figure 2). The neoplastic lymphocytes were positive for CD79a, CD20, BCL2, and MUM1 (focal and weak) with a high proliferative rate by Ki-67 (90%) (Figure 2); and were negative for CD10, BCL6, MYC, TdT, and cyclin D1 by immunohistochemistry studies. Fluorescence in situ hybridization (FISH) study was positive for BCL6 gene rearrangement but negative for MYC or BCL2 gene rearrangement. Based on the finding of IVL in the spleen, she was thought to have CNS involvement by IVL resulting in multiple strokes. The patient received weekly rituximab for 4 weeks while recovering from the surgery, followed by RMA alternating with RCHOP for a total of 8 cycles as described for Case 1. CR was confirmed by imaging scans after 4 cycles of CIT. Patient was clinically improved with normalization of her counts and no further strokes. She has been in CR for about 4 years at the time of writing.
Case 3
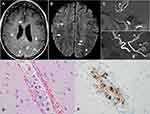
A 50-year-old female with a history of systemic lupus nephritis who had a progressive neurological deterioration over one year presented to an outside hospital with cognitive decline, personality changes, dysarthria, ataxia, visual hallucinations, and two episodes of tonic/clonic seizure (Table 1). She was treated with steroids and immunosuppressants for suspected lupus cerebritis and vasculitis. However, she continued to have progressive neurologic symptoms and was transferred to our institution for further evaluation and management. During hospitalization, she was noted to have multiple episodes of neurological manifestations consistent with strokes. MRI of the brain showed multiple foci of leptomeningeal enhancement involving the supratentorial brain and the cerebellum (Figure 3). MRA of the brain showed multifocal mild narrowing of both anterior and posterior circulation arteries, even though there was no significant vessel wall enhancement to suggest vasculitis (Figure 3). Cytology of the CSF was negative for malignant cells. Repeat MRI of the brain a day later showed persistent abnormal leptomeningeal enhancement and nonspecific T2 hyperintense areas. Whole body positron emission tomography–computed tomography (PET-CT) scan showed multi-station hypermetabolic lymphadenopathy. Stereotactic biopsy of left temporal lobe lesion showed findings consistent with intravascular large B-cell lymphoma. Intravascular malignant cells were identified with associated micro-infarctions. Lymphoma cells were positive for CD19 and negative for programmed death-ligand 1 (PDL-1) (Figure 3). No evidence of perivascular parenchymal infiltration was seen. As lymphadenopathy is uncommon in IVL, the patient may have nodal DLBCL with intravascular involvement in the brain. LDH at the time of diagnosis was 353 U/L. The patient was initiated on treatment with rituximab, HDMTX, HiDAC, and thiotepa (MATRIX) to alternate with RCHOP. MATRIX regimen consists of rituximab 375 mg/m2 IV (Day #1), HDMTX 3.5 g/m2 IV (Day #2), HiDAC 2 g/m2 q12H IV (Days #3 and #4) and thiotepa 30 mg/m2 IV (Day #5). No intrathecal chemotherapy or radiation therapy was given. Due to severe cytopenias, her treatment was modified. Her whole treatment consisted of three cycles of CNS-centric regimen (one cycle each of MATRIX, RMA, and rituximab + HDMTX) alternating with three cycles of RCHOP. After six cycles of CIT, she had an additional treatment with 8 weeks of rituximab. She achieved CR after three cycles of CIT based on imaging scans. She has been in CR for 10 months at the time of writing. She is currently doing well with no significant neurological deficits.
Discussion
We report three cases of IVL with CNS involvement successfully treated with a unique treatment protocol consisting of HDMTX and HiDAC-based CNS-directed CIT alternating with standard RCHOP CIT. The first case had multifocal CNS involvement with lesions in the brain as well as the conus medullaris without evidence of involvement outside the CNS. As such, the diagnosis was primary CNS-IVL. The other two cases had both CNS and non-CNS involvement and can be labeled as secondary CNS-IVL. In agreement with stroke-like symptoms being the most frequent manifestation of CNS-IVL,15 all three patients presented with multifocal stroke not confined to any major cerebral arterial distribution with the first and second cases having hemorrhagic strokes.19–22 The clinical implication is that IVL lymphoma cells could escape into the brain parenchyma in these bleeding sites resulting in establishment of CNS parenchymal lymphoma. This provides rationale to using CNS-directed CIT in treatment of CNS-IVL.
Our therapeutic approach is unique in that we alternate HDMTX and HiDAC-based CNS-directed CIT with non-CNS-directed RCHOP to target lymphoma cells inside the CNS blood vessels as well as those already in the CNS parenchyma. We adapted this approach from therapies for primary central nervous system lymphoma (PCNSL). HDMTX and HiDAC-based CIT has shown significant therapeutic activity in PCNSL.23 Therapeutic agents in the CHOP chemotherapy have poor CNS penetration and have not shown survival benefit in PCNSL,24 and therefore RCHOP alone will not have any significant impact on the lymphoma cells in the CNS parenchyma. Addition of rituximab to CHOP chemotherapy has resulted in significant survival improvement in survival of IVL patients, with 3-year overall survival of 81% (n = 34) compared with 35% (n = 22) in the pre-rituximab era.25,26 This is likely due to improved therapeutic activity against lymphoma cells inside the blood vessels. Most CNS-directed therapy for IVL in the literature consists of HDMTX or intrathecal chemotherapy. Our approach is unique in that CNS-directed therapy is intensified by including additional CNS-penetrating agents. Based on our literature search, we have found a recently published retrospective study27 that reported similar CNS-directed regimen for IVL with hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating rituximab with high-dose MTX (1.0 g/m2 on day 1) /cytarabine (3 g/m2 twice a day on days 3–4) in one out of the 16 reported patients who received CNS-directed therapies. Only four out of the 16 patients had CNS-IVL.27 In addition, rituximab + HDMTX-based CIT has been used for treatment of IVL with CNS involvement.28–31 For IVL without CNS involvement, RCHOP with HDMTX for CNS prophylaxis has been used.32,33
For all three patients, we initiated treatment with CNS-directed CIT to achieve impact on lymphoma cells in the vascular compartment as well as those in CNS parenchyma. The first two cases received four cycles of RMA for CNS-directed therapy and four cycles of RCHOP in alternating fashion. The third case was initiated on MATRIX regimen due to her rapidly deteriorating neurological condition (Table 1). Although her neurological condition improved significantly after MATRIX, she developed severe cytopenia requiring modifications of treatment. MATRIX regimen is essentially an addition of thiotepa to RMA. In a phase 2 randomized trial on newly diagnosed PCNSL (International extranodal lymphoma study group 32 trial), MATRIX was shown to have significantly higher complete response than RMA, 49% vs 30%. However, no significant improvement was seen for progression-free survival or overall survival.23 There has been no extensive experience on the use of MATRIX for CNS-IVL. Based on higher incidence of toxicity with MATRIX, we would prefer using RMA in the future. Moreover, intrathecal (IT) chemotherapy and whole brain radiation (WBR) have been used in treatment of CNS lymphoma. However, their role in management of CNS-IVL is not clear. Due to intravascular lymphoma cells being outside the blood–brain barrier, it is not likely for IT chemotherapy to have any impact. As for the possible extravascular involvement by CNS-IVL, IT chemotherapy will not be ideal as CNS involvement by IVL is predominantly intracerebral. As for WBR, the median age at diagnosis of IVL is in the sixth and seventh decades.25,26,34 Because of the high risk of neurotoxicity in elderly patients, WBR is not a good option.
In conclusion, our CNS-centric approach to management of CNS-IVL is associated with excellent therapeutic outcome. All three patients have achieved excellent survival outcomes from the treatment with CR for about 11 years, 4 years, and 10 months. The combination of HDMTX and HiDAC-based CNS-directed CIT with anthracycline-based systemic CIT produces long-term survival for patients with this rare and aggressive disease. We propose that intracerebral bleeding provides a mechanistic basis for extravasation of lymphoma cells into the CNS parenchyma in CNS-IVL. Therefore, we suggest that CNS-directed therapy should be included in management of CNS-IVL. Further research is necessary to determine the optimal treatment for CNS-IVL.
Ethics and Consent
Written informed consents have been provided by all patients to have the case details and any accompanying images published. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jaffe ES. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Iarc. Vol. 3; 2001.
2. Ponzoni M, Ferreri AJM, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168–3173. doi:10.1200/JCO.2006.08.2313
3. Ferreri AJM, Campo E, Ambrosetti A, et al. Anthracycline-based chemotherapy as primary treatment for intravascular lymphoma. Ann Oncol. 2004;15(8):1215–1221. doi:10.1093/annonc/mdh274
4. Ponzoni M, Arrigoni G, Gould VE, et al. Lack of CD 29 (β1 integrin) and CD 54 (ICAM-1) adhesion molecules in intravascular lymphomatosis. Hum Pathol. 2000;31(2):220–226. doi:10.1016/S0046-8177(00)80223-3
5. Starr JS, Jiang L, Li Z, et al. CD47 and osteopontin expression in diffuse large B-cell lymphoma with nodal and intravascular involvement. Clin Lymphoma Myeloma Leuk. 2013;13(5):597–601. doi:10.1016/j.clml.2013.05.001
6. Jaiswal S, Jamieson CHM, Pang WW, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi:10.1016/j.cell.2009.05.046
7. Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132(15):1561–1567. doi:10.1182/blood-2017-04-737445
8. Ferry J, Harris NL, Picker LJ, et al. Intravascular lymphomatosis (malignant angioendotheliomatosis). A B-cell neoplasm expressing surface homing receptors. Mod pathol. 1988;1(6):444–452.
9. Kato M, Ohshima K, Mizuno M, et al. Analysis of CXCL9 and CXCR3 expression in a case of intravascular large B-cell lymphoma. J Am Acad Dermatol. 2009;61(5):888–891. doi:10.1016/j.jaad.2009.02.007
10. Alon R, Shulman Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res. 2011;317(5):632–641. doi:10.1016/j.yexcr.2010.12.007
11. Kasuya A, Fujiyama T, Shirahama S, et al. Decreased expression of homeostatic chemokine receptors in intravascular large B-cell lymphoma. Eur J Dermatol. 2012;22(2):272–273. doi:10.1684/ejd.2012.1639
12. Kinoshita M, Izumoto S, Hashimoto N, et al. Immunohistochemical analysis of adhesion molecules and matrix metalloproteinases in malignant CNS lymphomas: a study comparing primary CNS malignant and CNS intravascular lymphomas. Brain Tumor Pathol. 2008;25(2):73–78. doi:10.1007/s10014-008-0232-x
13. Shimada K, Shimada S, Sugimoto K, et al. Development and analysis of patient-derived xenograft mouse models in intravascular large B-cell lymphoma. Leukemia. 2016;30(7):1568–1579. doi:10.1038/leu.2016.67
14. Murase T, Nakamura S, Kawauchi K, et al. An Asian variant of intravascular large B‐cell lymphoma: clinical, pathological and cytogenetic approaches to diffuse large B‐cell lymphoma associated with haemophagocytic syndrome. Br J Haematol. 2000;111(3):826–834.
15. Liu Z, Zhang Y, Zhu Y, et al. Prognosis of Intravascular Large B Cell Lymphoma (IVLBCL): analysis of 182 patients from global case series. Cancer Manag Res. 2020;12:10531. doi:10.2147/CMAR.S267825
16. Shimada K, Kiyoi H. Current progress and future perspectives of research on intravascular large B-cell lymphoma. Cancer Sci. 2021;112(10):3953–3961. doi:10.1111/cas.15091
17. Ferreri AJ, Dognini GP, Bairey O, et al. The addition of rituximab to anthracycline‐based chemotherapy significantly improves outcome in ‘Western’patients with intravascular large B‐cell lymphoma. Br J Haematol. 2008;143(2):253–257. doi:10.1111/j.1365-2141.2008.07338.x
18. Ferreri AJ, Dognini GP, Govi S, et al. Can rituximab change the usually dismal prognosis of patients with intravascular large B-cell lymphoma? J Clin Oncol. 2008;26(31):5134–5137. doi:10.1200/JCO.2008.19.1841
19. Suzuki Y, Tanaka H, Suyama K, et al. Secondary central nerve system lymphoma with intratumoral hemorrhage suggested as intravascular lymphoma by autopsy: a case report. J Clin Med Res. 2017;9(11):953–957. doi:10.14740/jocmr3177w
20. Gan LP, Ooi W, Lee H, et al. A case of large B-cell intravascular lymphoma in the brain. Surg Neurol Int. 2013;4:99. doi:10.4103/2152-7806.115709
21. Marino D, Sicurelli F, Cerase A, et al. Fulminant intravascular lymphomatosis mimicking acute haemorrhagic leukoencephalopathy. J Neurol Sci. 2012;320(12):141–144. doi:10.1016/j.jns.2012.05.043
22. Koujanian S, Al-Rawaf S, Zang E, Provias J. Intravascular large B-cell lymphoma of the central nervous system, a masquerader on radiography and clinical presentation: a case report. Hum Pathol. 2020;19:200297.
23. Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–e227. doi:10.1016/S2352-3026(16)00036-3
24. Mead GM, Bleehen NM, Gregor A, et al. A medical research council randomized trial in patients with primary cerebral non‐Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer. 2000;89(6):1359–1370. doi:10.1002/1097-0142(20000915)89:6<1359::AID-CNCR21>3.0.CO;2-9
25. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’ 1. Br J Haematol. 2004;127(2):173–183. doi:10.1111/j.1365-2141.2004.05177.x
26. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189–3195. doi:10.1200/JCO.2007.15.4278
27. Takahashi H, Nishimaki H, Nakanishi Y, et al. Clinical impact of central nervous system‐directed therapies on intravascular large B‐cell lymphoma: a single institution’s experience. eJHaem. 2022. doi:10.1002/jha2.380
28. Shimada K, Murase T, Matsue K, et al. Central nervous system involvement in intravascular large B‐cell lymphoma: a retrospective analysis of 109 patients. Cancer Sci. 2010;101(6):1480–1486. doi:10.1111/j.1349-7006.2010.01555.x
29. Chen R, Singh G, McNally JS, et al. Intravascular lymphoma with progressive CNS hemorrhage and multiple dissections. Case Rep Neurol Med. 2020;2020:6134830. doi:10.1155/2020/6134830
30. Chwalisz BK, Douglas VP, Douglas KAA, et al. Episodic visual distortions and stroke-like symptoms in a 56-year-old man with intravascular lymphoma. J Neuro-Ophthalmol. 2020;40(2):265–270. doi:10.1097/WNO.0000000000000900
31. Cruto C, Taipa R, Monteiro C, et al. Multiple cerebral infarcts and intravascular central nervous system lymphoma: a rare but potentially treatable association. J Neurol Sci. 2013;325(1–2):183–185. doi:10.1016/j.jns.2012.10.012
32. Shimada K, Yamaguchi M, Atsuta Y, et al. Favorable Outcomes of newly diagnosed intravascular large B-cell lymphoma patients treated with R-CHOP combined with high-dose methotrexate plus intrathecal chemotherapy: results from a multicenter phase 2 trial (PRIMEUR-IVL). Blood. 2019;134:350. doi:10.1182/blood-2019-126798
33. Shimada K, Yamaguchi M, Atsuta Y, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone combined with high-dose methotrexate plus intrathecal chemotherapy for newly diagnosed intravascular large B-cell lymphoma (PRIMEUR-IVL): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(4):593–602. doi:10.1016/S1470-2045(20)30059-0
34. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478–485. doi:10.1182/blood-2006-01-021253
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.