Back to Journals » Clinical Ophthalmology » Volume 17
Intraretinal Macroaneurysms and Multimodal Imaging: A Retrospective Analysis
Authors Kayabaşı M , Köksaldı S , Mansour AM , Ayhan Z, Saatci AO
Received 23 August 2023
Accepted for publication 20 October 2023
Published 25 October 2023 Volume 2023:17 Pages 3195—3205
DOI https://doi.org/10.2147/OPTH.S436652
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Mustafa Kayabaşı,1 Seher Köksaldı,2 Ahmad M Mansour,3 Ziya Ayhan,1 Ali Osman Saatci1
1Department of Ophthalmology, Dokuz Eylul University, Izmir, Turkey; 2Department of Ophthalmology, Mus State Hospital, Mus, Turkey; 3Department of Ophthalmology, American University of Beirut, Beirut, Lebanon
Correspondence: Ali Osman Saatci, Department of Ophthalmology, Dokuz Eylul University, Mustafa Kemal Sahil Bulvarı, No: 73, A Blok, Daire 9, Narlıdere, İzmir, Turkey, Email [email protected]
Purpose: To analyze the multimodal imaging characteristics of intraretinal macroaneurysms.
Patients and Methods: Intraretinal aneurysms larger than 150 μm in diameter on fluorescein angiography were termed as intraretinal macroaneurysm and grouped as primary and secondary according to the absence or presence of any coexisting posterior segment diseases.
Results: A total of 20 intraretinal macroaneurysms were observed in 18 eyes of 18 patients. Mean age of the cohort was 65.44 ± 9.14 years (Range; 49– 82 years). Mean diameters of intraretinal macroaneurysms were 238.20 ± 61.12 μm (Range; 163.00– 292.50 μm) and 242.72 ± 49.58 μm (Range; 168.00– 328.00 μm) on fluorescein angiography and optical coherence tomography, respectively. Primary group had 10 eyes with 11 intraretinal macroaneurysms, whereas eight eyes had nine intraretinal macroaneurysms in the secondary group. Three of the eight eyes (37.5%) had diabetic retinopathy, four (50%), retinal vein occlusion, and one (12.5%), posterior uveitis in the secondary group. No statistically significant differences were found between the two groups in terms of age, sex, presence of intraretinal or subretinal fluid, the mean age, the mean central macular thickness, the mean distance of intraretinal macroaneurysms from the fovea, the mean diameter of intraretinal macroaneurysms measured on fluorescein angiography, and the mean diameter of intraretinal macroaneurysms measured on optical coherence tomography. Presence of intraretinal fluid was significantly more frequent than the presence of subretinal fluid in all eyes (p = 0.004).
Conclusion: Intraretinal macroaneurysms are diagnosed more and more with the utilization of multimodal imaging techniques. We propose a simple classification system in order to help achieving a standardized terminology and ensure consistent understanding. The classification can be simplified as primary or secondary intraretinal macroaneurysm according to the absence or presence of the associated posterior segment disorders.
Keywords: fluorescein angiography, intraretinal macroaneurysm, macular edema, optical coherence tomography, PEVAC, retinal capillary macroaneurysm
Introduction
Aneurysms of the retinal circulation usually involve the arterioles or capillaries. The aneurysms affecting the muscular arterioles are called retinal arterial macroaneurysms that typically involve the first few orders of the branching arterioles.1 Hypertension is considered to be the main cause of the aneurysms in arterioles and subretinal, intraretinal, and preretinal hemorrhage may accompany these lesions.2 Microaneurysms are saccular or fusiform dilatation of the retinal capillaries 50–100 μm in size.3 Though the presence of microaneurysms is the clinical hallmark of diabetic retinopathy (DR);4 larger macroaneurysms may also occur during DR.5,6 In addition to the DR, eyes with retinal vein occlusion (RVO) have been reported to have intraretinal macroaneurysms (IMAs), which are larger than microaneurysms but smaller than retinal arterial macroaneurysms.7,8 On color fundus images, IMAs seem hyalinized and have a white rim; on optical coherence tomography (OCT), they appear as vertically oval formations with a heterogeneous lumen and hyperreflective edge.9 Large IMAs are often accompanied by macular edema that responds poorly to intravitreal anti-vascular endothelial growth factor (VEGF) therapy.10,11
Large IMAs have been referred to by a variety of definitions and terminology, including perifoveal exudative vascular anomalous complex (PEVAC),12,13 PEVAC-like lesions,14,15 non-exudative perifoveal vascular anomalous complex (nePVAC),16 exudative perifoveal vascular anomalous complex (ePVAC),16 retinal capillary macroaneurysms,10 macroaneurysms,7 telangiectatic capillaries,17 and TelCaps.17,18
In this article, we describe the retrospective evaluation of the IMAs discovered through the analysis of fluorescein angiography (FA) and OCT, and we suggest a new terminology as primary and secondary IMAs by contrasting the lesion characteristics and additional clinical findings to streamline the current terminology.
Materials and Methods
This retrospective study was performed under the tenets of the Declaration of Helsinki. The study was approved by the local ethics committee (Dokuz Eylul University, Approval ID: 2022/28-24). All patients first underwent ophthalmological examination and then appropriate diagnostic techniques were employed at the Dokuz Eylul University Department of Ophthalmology between January 2019 and September 2022 and the data were retrospectively reviewed. In this study population, we considered the presence of an IMA whenever we noticed a distinct hyperfluorescent lesion, circular in shape, with a minimum diameter of 150 μm that was observed as an isolated aneurysmal dilation exhibiting variable types of fluorescein filling on FA. Notably, our IMA definition was independent of the caliber/type of the vessels that it originated, as well as the presence or absence of any other posterior segment disease-related findings, such as capillary ischemia, subretinal or intraretinal fluid, hemorrhages, and exudates. The focus of this study does not encompass retinal arterial macroaneurysms, which is a totally distinct clinical phenomenon characterized by acquired dilatations that resemble sacs or spindles within the larger retinal arterioles typically occur within the first three orders of bifurcation.19
The presence of at least one IMA which had a vertical or horizontal diameter of ≥150 μm measured on a good-quality FA was the main eligibility criterion of this study. Corresponding OCT images were also assessed to measure the dimensions of the IMAs.
All patients received a full ophthalmic examination. Demographic characteristics, presence or absence of any posterior segment diseases, and the lens status were derived from the patients’ medical records. Color fundus pictures were obtained by VISCUAM 500 (Carl Zeiss Meditec, Jena, Germany). Fluorescein angiogram, indocyanine green angiography (ICGA), and OCT images were obtained with Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany). However, ICGA could be performed only in three patients due to reimbursement and supply issues in our country. Optical coherence tomography angiography (OCTA) images were obtained with DRI OCT Triton (Triton, TOPCON Inc, Tokyo, Japan).
Analysis of the Intraretinal Macroaneurysms
The anatomical horizontal and vertical diameters of the IMAs were measured manually on vertical and horizontal planes of FA and ICGA images. Even though IMAs did not consistently exhibit leakage during FA, we selected high-quality arteriovenous or early venous phase images to ensure precise diameter measurement. The closest distance of the IMA from the central fovea was measured manually on 55° FA images by using the built-in software of the device. The horizontal and vertical diameters of the IMAs were measured manually as parallel and perpendicular to the optical axis on the central OCT scan passing through the IMA. Mean central macular thickness (CMT) was measured as the closest vertical distance from retinal pigment epithelium (RPE) to the internal limiting membrane and the presence of intraretinal fluid (IRF) or subretinal fluid (SRF) was recorded as hyporeflective accumulations of fluid inside the retinal layers or between the neurosensory retina and RPE on a seven mm wide horizontal OCT scan centered at the fovea by using a built-in software of the device. The arithmetic mean of horizontal and vertical diameter measurements was recorded to compare the diameters measured on FA and OCT. 3 × 3 mm and 6 × 6 mm OCTA images utilizing angio retina scanning module were obtained to see the involvement of retinal capillary plexuses by the segmentation. Clinical and imaging data were reviewed by an experienced retina specialist (AOS). In addition, one of us (MK) manually measured the horizontal and vertical diameters of the IMAs on FA and OCT images.
Intraretinal macroaneurysms were divided into two groups: primary (no coexisting posterior segment disease) and secondary (associated with any posterior segment disease regardless of presence of systemic diseases). Two groups were compared in terms of age, sex, the mean CMT, presence of IRF, presence of SRF, the mean distance of IMAs from the fovea, the mean diameter of IMAs measured on FA, and the mean diameter of IMAs measured on OCT. The presence of IRF versus SRF and the mean diameter of the IMAs measured on FA versus OCT were also compared in all eyes.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics Version 28 (IBM, Armonk, New York, USA). Results of descriptive analyses are expressed as counts and percentages for categorical variables and as means ± standard deviations for quantitative variables.
Independent continuous variables were compared with the nonparametric Mann–Whitney U-test due to the small sample size of the groups. Categorical variables were compared individually with the chi-square test or Fisher’s exact test if necessary. The Wilcoxon signed-rank test was used to compare the mean diameter of IMAs measured on FA and OCT. The McNemar test was used to compare the frequency of the presence of IRF versus SRF. A p-value under 0.05 was considered statistically significant.
Results
Our cohort comprised a total of 20 IMAs in 18 eyes (seven right, 11 left eyes) of 18 patients. The mean age was 65.44 ± 9.14 years (Range; 49–82 years) and female-to-male ratio was 12/6. Three eyes (16.7%) had DR, four eyes (22.2%) had RVO, and one eye (5.6%) had posterior uveitis, while 10 eyes (55.6%) did not have any evidence of posterior segment disorder. Fifteen eyes (83.3%) were phakic and three (16.7%) were pseudophakic.
Fluorescein angiographic section analysis showed that the mean IMA diameter was 238.20 ± 61.12 μm (Range; 163.00–292.50 μm). The closest distance of IMAs from the fovea was estimated as 1497.65 ± 1789.83 μm (Range; 74–6380 μm).
In all eyes, transfoveal OCT sections were obtained. However, only 18 IMAs of 17 eyes OCT sections traversing the IMA were available due to the retrospective nature of the study. The mean diameter of IMAs was 242.72 ± 49.58 μm (Range; 168.00–328.00 μm) on OCT. The difference between the mean diameter of IMAs measured on FA versus OCT was not statistically significant (Wilcoxon signed-rank test, p = 0.068). The mean CMT in 18 eyes was 277.00 ± 87.37 μm (Range; 149–526 μm). Thirteen eyes (72.2%) had IRF, while only four eyes (22.2%) had SRF on the OCT section centered at the fovea. The presence of IRF was significantly more frequent than the presence of SRF (McNemar test, p = 0.004).
Four eyes (22.2%) had received anti-VEGF injections (Mean, 5.00 ± 2.16 injections) and one eye (5.5%) had received intravitreal dexamethasone implant (five injections) related to the coexistent DR or RVO before the diagnosis of IMA. One of the patients (Case 3) was misdiagnosed as unilateral diabetic macular edema and treated with eight intravitreal anti-VEGF injections before the diagnosis of IMA, though the clinical findings were because of the primary IMA. Retinal laser photocoagulation (panretinal or sectorial) had been performed in two eyes (11.1%) again for DR or RVO prior to IMA diagnosis. The demographic characteristics and clinical findings of all the patients and the imaging features of IMAs are summarized in Table 1.
 |
Table 1 Demographic Characteristics and Clinical Findings of the Patients with IMA |
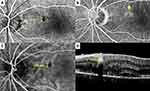
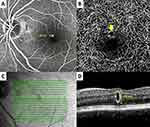
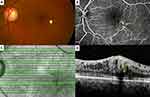
The primary IMA group comprised 11 IMAs in 10 eyes of 10 patients, while the secondary IMA group comprised nine IMAs in eight eyes of eight patients. There are no statistically significant differences between the groups in terms of sex, presence of IRF, and presence of SRF (Fisher’s exact test; p = 0.638, p = 0.608, and p = 1.000 respectively). The differences between the two groups in terms of the mean age, CMT, distance of IMAs from the fovea, diameter of IMAs measured on FA, and diameter of IMAs measured on OCT were also not statistically significant (Mann–Whitney U-test; p = 0.349, p = 0.790, p = 0.102, p = 0.676 and p = 0.964, respectively). The frequencies/mean values of these parameters for each group are shown in Table 2. Multimodal imaging characteristics of Case 4, Case 5, Case 7, and Case 13 are demonstrated in Figures 1–4.
 |
Table 2 The Clinical Characteristics of Two Groups |
Discussion
The present study demonstrated that there were no statistically significant differences between the primary IMA group and the secondary IMA group in terms of the presence of IRF, presence of SRF, mean CMT, mean distance of IMAs from the fovea, mean diameter of IMAs measured on FA, and mean diameter of IMAs measured on OCT (p = 0.608, p = 1.000, p = 0.790, p = 0.102, p = 0.676 and p = 0.964, respectively).
By definition, microaneurysms are 50–100 μm in size, usually involve the capillaries, and are associated with various ischemic conditions such as DR and RVO. In comprehensive postmortem examinations, microaneurysms were observed in up to 24% of the aged retinas.20 Various mechanisms have been implicated in the development of microaneurysms such as pericyte death, endothelial proliferation, impaired angiogenesis, alterations in intraluminal rheology, and damage to the blood vessel basement membrane.21
Lopez-Luppo et al21 stated that it might be difficult to detect the small microaneurysms using ancillary clinical imaging modalities as most of the microaneurysms lie in the deep vascular plexus. Unlike macroaneurysms, microaneurysms reveal a bright contrast in the early stage on FA, while no change in size, contrast, or brightness is observed in the later stages.22
They also histopathologically disclosed that small microaneurysms had cross-linked fibers of type III collagen, not present in the normal retinal capillaries. In larger microaneurysms, there was an increased expression of matrix metalloproteinase-9 that could facilitate the breakdown of basement membrane proteins. They suggested that matrix metalloproteinases might weaken the structural integrity of the basement membrane and decrease the vessel wall integrity, thereby potentially lessen the resistance to aneurysmal formation. Therefore, such expansionary factors might result in increased leakage from the aneurysm that might be unresponsive to anti-VEGF therapy. In the present study, Case 3 had diabetes mellitus but did not show any signs of DR. Despite receiving eight intravitreal anti-VEGF injections for presumed diabetic macular edema prior to recognition of IMA; IRF was still unchanged. Concurrently, the limited efficacy of intravitreal anti-VEGF treatment has also been emphasized in the literature for these lesions.10,11,21
Macroaneurysms can be originated from the arteries, capillaries, veins, or collateral vessels.23,24 Capillaries have lower flow rates and thus develop dilatations of smaller caliber.25 Varying from author to author, retinal capillary macroaneurysms have been defined in size between 100 and 300 μm.7,10,17 Localized increase in capillary hydrostatic pressure, selective pericyte loss, and smooth muscle cell death were thought to be the possible causes of retinal capillary macroaneurysm formation. Spaide et al10 reported five patients with solitary, large, persistent intraretinal aneurysms derived from the capillaries in the macular area. They defined an intraretinal aneurysm larger than 200 μm as a “retinal capillary macroaneurysm” by measuring the lesion with OCT and also used OCTA to identify these aneurysmal dilations. Anti-VEGF treatment provided a partial response in one case and no apparent response was noted in the remaining four patients. We feel that it is most likely that these lesions have a capillary origin than the other speculated vessel types based on the assumption outlined in the study by Spaide and Barquet,10 although no conclusive evidence regarding the specific caliber of vessels from where IMAs originate has been established.
Subretinal, intraretinal, and preretinal hemorrhage, exudation, and fluid have been observed in association with IMAs.25 Paques et al9 observed that capillary macroaneurysms were usually located at the center of the circinate deposits. However, these lesions were commonly associated with intraretinal cystic fluid accumulation with relatively well-preserved outer retinal layers.25,26 In the present study, the presence of IRF was significantly more frequent than the presence of SRF in eyes with IMA. Intraretinal fluid and SRF were observed in eight (80%) and two (20%) eyes in the primary IMA group; while five (62.5%) and two (25%) eyes had IRF and SRF in the secondary IMA group, respectively (p = 0.608 and p = 1.000 for IRF and SRF, respectively).
Hussain et al24 reported an otherwise healthy 45-year-old male who had an isolated retinal venous macroaneurysm, a circular lesion with a hyperreflective border in the middle retinal layers with intraretinal edema and hyperreflective exudates on OCT. The venous phase of FA showed pinpoint leakage of the dye at the branching of the second‐order vein.
In 2011, Querques et al12 defined the unilateral, isolated perifoveal aneurysms that might be associated with retinal hemorrhages, intraretinal fluid, and hard exudates and named it as “PEVAC” without specifying a cut-off diameter for those lesions. They visualized the so-called PEVAC cases with the help of OCT, FA, ICGA, and color fundus imaging in two cases without any known systemic or ocular diseases. The term “PEVAC-like” was suggested by several authors for the lesions resembling PEVAC in eyes with vascular retinopathies such as DR, RVO, prepapillary arterial loops or age-related macular degeneration,27 lamellar macular hole,28 and pathologic myopia.14–16,29 The lesions defined as PEVAC in the literature exhibited similarities in terms of characteristics and definition with the primary IMA group in our study.
Sacconi et al16 reported the follow-up features of four eyes exhibiting microvascular abnormalities characterized by the presence of an isolated, large intraretinal aneurysm detected at the perifoveal region. Notably, these eyes did not exhibit any exudation, and the authors named the lesions as nePVAC. However, three eyes developed signs of exudation during the course.
The perifoveal vascular anomalous complex was believed to be due to a focal or progressive endothelial cell degeneration/injury and reduction in pericyte cell protection related to basement membrane breakdown. The response to anti-VEGF treatment was poor.11,12,16,30,31 It appeared as a round hyperreflective lesion with a reflective wall, surrounding a dark lumen containing reflective material in a variable nature. Intraretinal cystic spaces generally accompanied these lesions on OCT. A fluorescein angiogram showed the hyperfluorescent lesion, characterized as an isolated aneurysmal dilation with a variable filling of the aneurysm associated with some leakage on the late frames. On ICGA, the same hyperfluorescent lesion was also seen, but no late leakage was present.32 Type 3 neovascularization might develop in patients with PEVAC.13
Farias et al17 suggested the term ‘telangiectatic capillaries’ to denote the intraretinal aneurysms larger than 150 μm with the help of ICGA in eyes with DR. They highlighted the association of hard exudates with telangiectatic capillaries and concluded that these lesions could be considered as a subtype of microaneurysms but rather as a specific pathological entity. They emphasized the prolonged, focal ICGA staining of telangiectatic capillaries and suggested that hydrophobic intraluminal materials present in these lesions might induce incomplete and delayed filling on ICGA.17
Intraretinal macroaneurysms can be visualized with OCT, FA, and ICGA. Bourhis et al7 used the term “macroaneurysm” for the aneurysms greater than 100 μm that were detected by FA and/or ICGA in six patients. They reported that some macroaneurysms could be depicted better with the ICGA than with FA. Two of their cases had DR, while the other four had RVO and all of their cases had hard exudates in association with the macroaneurysms. They argued that macroaneurysms originated from the microaneurysms. They added that ICG dye had amphiphilic properties and stained the intraluminal fibrinogen more often than the fluorescein dye in FA and this might be the fact while explaining the superiority of ICGA over FA.7 In their series, ICGA showed a prolonged and complete filling with minimal extravascular leakage but FA showed fading of the lesion hyperfluorescence. There was a 20–30 μm difference between the ICGA and OCT measurements and OCT yielded a larger macroaneurysm size than the ICGA in most of the cases.7 This difference might be due to the inclusion of the IMA wall on the OCT measurements, whereas indocyanine green dye bound only to intraluminal material.7 In our cohort, there was a small discrepancy between the diameters measured on FA versus OCT, but the difference was not statistically significant. In Case 4 and Case 5, where ICGA could be performed, the diameter of IMAs appeared larger on ICGA compared to FA and OCT (Figures 1 and 2, respectively).
Depth-resolved analysis of the retinal blood flow and the visualization of the retinal capillary plexuses have become possible with the introduction of OCTA.33 Sacconi et al13 reported aneurysmal dilations and remarkable rarefaction of the retinal capillaries in the perilesional area especially at the superficial and deep capillary plexus slabs with the OCTA examination in 15 patients diagnosed as PEVAC. Similar findings on both superficial and deep capillary plexus slabs (especially the deep capillary plexus slab) were noted in our cohort. Although OCTA could demonstrate most of the IMAs, some of them could not be visualized due to a low flow rate within the aneurysm.34 Unfortunately, IMAs could only be demonstrated in 14 of 15 eyes in our study where OCTA examination was performed and in Case 4 where we could not visualize the IMA with OCTA despite meticulous segmentation attempts.
Our study has a larger number of patients compared to the previous manuscripts, and there were no statistically significant differences observed in the presence of IRF/SRF, average diameter on OCT and FA, distance to the fovea, and CMT between the primary and secondary IMA groups. These findings imply that the lesions may possess a similar underlying nature. Additionally, multifocal lesions were observed in both the primary and secondary IMA groups. Although Smid et al14 claimed that there was a tendency for multifocality for the lesions they named “PEVAC-like”, we believe that multifocality cannot be a distinctive feature to classify these lesions.
To our best knowledge, there is no single-center study with a significant number of patients with IMAs thus far. These lesions have been primarily defined in the case reports or case series, using various terms such as PEVAC, PEVAC-like, nePVAC, ePVAC, capillary macroaneurysm, telangiectatic capillaries, or TelCaps as summarized above.7,10,12–18 However, all of the terms likely point out the same lesion or different stages of the lesion using different cut-off diameter sizes ranging from 100 to 200 μm. Despite existing various terminologies, we believe that the terms “intraretinal” and “macroaneurysm” are more appropriate due to the location of these lesions on OCT sections, as well as their aneurysmal appearance exceeding 150 microns. Furthermore, we would like to reiterate that the lesions discussed in this study are different from the retinal arterial macroaneurysms, which constitute a separate clinical entity originating from the large-caliber muscular arteries.
There are certain limitations within the scope of our study that need to be underlined. First, IMA size measurements were performed by a single grader. Second, the sample size was relatively small despite the fact that it was the largest study coming from a single center. Third, ICGA images could not be obtained in all patients due to abovementioned reimbursement issues. Fourth, a head-to-head comparison could not be performed as there was no well-established classification system for these lesions.
We feel that the multiplicity of these terms creates confusion and ambiguity among the retina specialists. Thus, we propose a simple classification system that encompasses visually similar lesions described by several authors and aim to help achieving a standardized terminology and ensure consistent understanding. The terminology can be simplified as primary or secondary IMA according to the absence or presence of associated posterior segment disorders.
Conclusion
Intraretinal macroaneurysms are relatively newly described and recognized retinal vascular changes that can be seen in otherwise healthy individuals or patients with other systemic and/or ocular diseases. These lesions might be more frequent than previously thought and the careful evaluation of the multimodal images may help to recognize and distinguish these lesions. Their presence may alter the approach of clinicians when selecting the most suitable therapeutic approach. Further, preferably prospective studies with larger number of patients will help the clinicians to determine the ideal imaging method and treatment modalities in patients with intraretinal macroaneurysms.
Abbreviations
CMT, Central macular thickness; DR, Diabetic retinopathy; ePVAC, Exudative perifoveal vascular anomalous complex; FA, Fluorescein angiography; ICGA, Indocyanine green angiography; IMA, Intraretinal macroaneurysms; IRF, Intraretinal fluid; nePVAC, Non-exudative perifoveal vascular anomalous complex; OCT, Optical coherence tomography; OCTA, Optical coherence tomography angiography; PEVAC, Perifoveal exudative vascular anomalous complex; RPE, Retinal pigment epithelium; RVO, Retinal vein occlusion; SRF, Subretinal fluid; VEGF, Vascular endothelial growth factor.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This retrospective study was performed under the tenets of the Declaration of Helsinki on the patients examined at the Dokuz Eylul University Department of Ophthalmology between January 2019 and September 2022. The study was approved by the local ethics committee (Dokuz Eylul University, Approval ID: 2022/28-24).
Consent for Publication
The permission document which guarantees the use of personal information solely in an anonymous manner has been obtained from the local ethics committee together with the ethics approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no conflict of interest.
References
1. Fichte C, Streeten BW, Friedman AH. A histopathologic study of retinal arterial aneurysms. Am J Ophthalmol. 1978;85(4):509–518. doi:10.1016/S0002-9394(14)75249-3
2. Lewis RA, Norton EW, Gass JD. Acquired arterial macroaneurysms of the retina. Br J Ophthalmol. 1976;60(1):21–30. doi:10.1136/bjo.60.1.21
3. Dubow M, Pinhas A, Shah N, et al. Classification of human retinal microaneurysms using adaptive optics scanning light ophthalmoscope fluorescein angiography. Invest Ophthalmol Vis Sci. 2014;55(3):1299–1309. doi:10.1167/iovs.13-13122
4. Barot M, Gokulgandhi MR, Patel S, Mitra AK. Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Med Chem. 2013;5(3):301–314. doi:10.4155/fmc.12.206
5. Stitt AW, Gardiner TA, Archer DB. Histological and ultrastructural investigation of retinal microaneurysm development in diabetic patients. Br J Ophthalmol. 1995;79(4):362–367. doi:10.1136/bjo.79.4.362
6. Zhang Z, Xu L, Wu Z, Zhang J. Case Report: perifoveal Exudative Vascular Anomalous Complex in a Chinese Patient with Diabetes Mellitus. Optom Vis Sci. 2019;96(7):531–535. doi:10.1097/OPX.0000000000001401
7. Bourhis A, Girmens JF, Boni S, et al. Imaging of macroaneurysms occurring during retinal vein occlusion and diabetic retinopathy by indocyanine green angiography and high resolution optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248(2):161–166. doi:10.1007/s00417-009-1175-6
8. Parodi MB, Da Pozzo S, Saviano S, Ravalico G. Branch retinal vein occlusion and macroaneurysms. Int Ophthalmol. 1997;21(3):161–164. doi:10.1023/A:1026420707368
9. Paques M, Philippakis E, Bonnet C, et al. Indocyanine-green-guided targeted laser photocoagulation of capillary macroaneurysms in macular oedema: a pilot study. Br J Ophthalmol. 2017;101(2):170–174. doi:10.1136/bjophthalmol-2015-308142
10. Spaide RF, Barquet LA. Retinal Capillary Macroaneurysms. Retina. 2019;39(10):1889–1895. doi:10.1097/IAE.0000000000002406
11. Kim JH, Kim JW, Kim CG, Lee DW. Characteristics of Perifoveal Exudative Vascular Anomalous Complex in Korean Patients. Semin Ophthalmol. 2019;34(5):353–358. doi:10.1080/08820538.2019.1626450
12. Querques G, Kuhn D, Massamba N, Leveziel N, Querques L, Souied EH. Perifoveal exudative vascular anomalous complex. J Fr Ophtalmol. 2011;34(8):559 e1–4. doi:10.1016/j.jfo.2011.03.002
13. Sacconi R, Freund KB, Yannuzzi LA, et al. The Expanded Spectrum of Perifoveal Exudative Vascular Anomalous Complex. Am J Ophthalmol. 2017;184:137–146. doi:10.1016/j.ajo.2017.10.009
14. Smid LM, Verhoekx JSN, Martinez Ciriano JP, Vermeer KA, Yzer S. Multimodal imaging comparison of perifoveal exudative vascular anomalous complex and resembling lesions. Acta Ophthalmol. 2021;99(5):553–558. doi:10.1111/aos.14650
15. Ayachit AG, Sacconi R, Ayachit GS, Joshi S, Querques G. Perifoveal Exudative Vascular Anomalous Complex-Like Lesion as a Complication of Prepapillary Arterial Loops. Ophthalmic Surg Lasers Imaging Retina. 2018;49(12):974–978. doi:10.3928/23258160-20181203-11
16. Sacconi R, Borrelli E, Sadda S, et al. Nonexudative Perifoveal Vascular Anomalous Complex: the Subclinical Stage of Perifoveal Exudative Vascular Anomalous Complex? Am J Ophthalmol. 2020;218:59–67. doi:10.1016/j.ajo.2020.04.025
17. Castro Farias D, Matsui Serrano R, Bianchi Gancharov J, et al. Indocyanine green angiography for identifying telangiectatic capillaries in diabetic macular oedema. Br J Ophthalmol. 2020;104(4):509–513. doi:10.1136/bjophthalmol-2019-314355
18. Sagar P, Shanmugam M, Ayachit G, Joshi S, Ayachit A. Commentary: a thesaurus for aneurysms - Anomalous exudative complexes, capillary macroaneurysms, TelCaps, macro- microaneurysms. Indian Journal of Ophthalmol. 2022;2(2):453. doi:10.4103/ijo.IJO_2900_21
19. Robertson DM. Macroaneurysms of the retinal arteries. Trans Am Acad Ophthalmol Otolaryngol. 1973;77(1):OP55–67.
20. Ashton N. Retinal micro-aneurysms in the non-diabetic subject. Br J Ophthalmol. 1951;35(4):189–212. doi:10.1136/bjo.35.4.189
21. Lopez-Luppo M, Nacher V, Ramos D, et al. Blood Vessel Basement Membrane Alterations in Human Retinal Microaneurysms During Aging. Invest Ophthalmol Vis Sci. 2017;58(2):1116–1131. doi:10.1167/iovs.16-19998
22. Karti O, Ipek SC, Saatci AO. Multimodal Imaging Characteristics of a Large Retinal Capillary Macroaneurysm in an Eye With Severe Diabetic Macular Edema: a Case Presentation and Literature Review. Med Hypothesis Discov Innov Ophthalmol. 2020;9(1):33–37.
23. Cousins SW, Flynn HW, Clarkson JG. Macroaneurysms associated with retinal branch vein occlusion. Am J Ophthalmol. 1990;109(5):567–570. doi:10.1016/S0002-9394(14)70687-7
24. Hussain SZM, Siddiqui MAR. Multimodal imaging of an isolated retinal venous macroaneurysm. Oman J Ophthalmol. 2020;13(2):102–103. doi:10.4103/ojo.OJO_231_2019
25. Rehmani AS, Banaee T, Makkouk F. Subretinal leakage of a retinal capillary macroaneurysm - a case report. BMC Ophthalmol. 2021;21(1):221. doi:10.1186/s12886-021-01984-6
26. Fernandez-Vigo JI, Burgos-Blasco B, Dolz-Marco R, Jimenez-Santos M, Lopez-Guajardo L, Donate-Lopez J. Atypical perifoveal exudative vascular anomalous complex (PEVAC) with multifocal and bilateral presentation. Am J Ophthalmol Case Rep. 2020;18:100717. doi:10.1016/j.ajoc.2020.100717
27. Verhoekx JSN, Smid LM, Vermeer KA, Martinez Ciriano JP, Yzer S. Anatomical Changes on Sequential Multimodal Imaging in Perifoveal Exudative Vascular Anomalous Complex. Retina. 2021;41(1):162–169. doi:10.1097/IAE.0000000000002809
28. Siedlecki J, Vounotrypidis E, Vogt D, Wolf A, Priglinger SG, Schumann RG. Lamellar hole-associated epiretinal proliferation presenting with perifoveal exudative vascular anomalous complex. Am J Ophthalmol Case Rep. 2019;14:112–116. doi:10.1016/j.ajoc.2019.03.008
29. Mrejen S, Le HM, Nghiem-Buffet S, Tabary S, Quentel G, Cohen SY. Insights Into Perifoveal Exudative Vascular Anomalous Complex. Retina. 2020;40(1):80–86. doi:10.1097/IAE.0000000000002435
30. Arruabarrena C, Liaño G, Hernáez-Leonato JM, Martínez-Sánchez M, Cañas-Martín J, Teus MA. Selective Focal Laser Therapy as the Primary Treatment for Exudative Perifoveal Vascular Anomalous Complex (ePVAC): case Series and Literature Review. Ophthalmic Surg Lasers Imaging Retina. 2023;54(1):43–49. doi:10.3928/23258160-20221219-01
31. Nataraj A, Sheth J, Soman M, Nair U. Refractory perifoveal exudative vascular anomalous complex like lesion responding to intravitreal dexamethasone implant: a therapeutic challenge. Indian Journal of Ophthalmol. 2022;2(2):449–452. doi:10.4103/ijo.IJO_1000_21
32. Venkatesh R, Yadav NK, Bavaharan B, Prabhu V, Sinha S. Multimodal imaging in perifoveal exudative vascular anomalous complex with co-existent diabetic retinopathy. Clin Exp Optom. 2019;102(5):528–532. doi:10.1111/cxo.12868
33. Gao SS, Jia Y, Zhang M, et al. Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57(9):t27–36. doi:10.1167/iovs.15-19043
34. de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015;1(1):5. doi:10.1186/s40942-015-0005-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.