Back to Journals » International Journal of General Medicine » Volume 15
Intraoperative Optical Coherence Tomography in Idiopathic Macular Epiretinal Membrane Surgery
Authors Mao ZQ, Wu HX, Fan HM, Li G, You ZP, Tan YY
Received 23 May 2022
Accepted for publication 25 July 2022
Published 8 August 2022 Volume 2022:15 Pages 6499—6505
DOI https://doi.org/10.2147/IJGM.S374630
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Zi-Qing Mao,1 Hong-Xi Wu,1 Hui-Min Fan,1 Gen Li,2 Zhi-Peng You,1 Yun-Yu Tan1
1Department of Ophthalmology, Affiliated Eye Hospital of Nanchang University, Nanchang, 330006, People’s Republic of China; 2Department of Ophthalmology, Zhangshu People’s Hospital, Zhangshu, 331299, People’s Republic of China
Correspondence: Zhi-Peng You, Department of Ophthalmology, Affiliated Eye Hospital of Nanchang University, Nanchang, 330006, People’s Republic of China, Email [email protected]
Objective: To evaluate the feasibility and practicability of intraoperative optical coherence tomography (IOCT) in the surgery of idiopathic macular epiretinal membrane (IMM) without internal limiting membrane staining in all patients.
Methods: Patients were selected from July 2018 to June 2020, and 32 patients (32 eyes) with IMM were operated with the use of IOCT. All patients underwent standard 23g vitrectomy. The internal limiting membrane was peeled off if there were obvious retinal folds. Intraoperative and postoperative complications, macular microstructural changes, and integrity of the detached membranes were recorded. The preoperative and postoperative best corrected visual acuity were compared.
Results: The macular epiretinal membrane was completely removed in 75% (24 eyes) patients without internal limiting membrane staining, and in 15.6% (5 eyes) patients with combined internal limiting membrane stripping. The “starting point” of macular epiretinal membrane stripping was found in 75% (24 eyes), and the time required to find the best starting point ranged from 28s to 140s (mean 66 ± 15s). At 3 months after operation, 96.8% of the patients had stable or improved BCVA (p < 0.05). The central macular thickness of the affected eyes decreased significantly at 1 and 3 months after operation (p < 0.05).
Conclusion: IOCT can significantly reduce the use of internal limiting membrane staining in idiopathic macular epiretinal membrane surgery, and it is safe, feasible and practical in idiopathic macular epiretinal membrane surgery without internal limiting membrane staining in all patients.
Keywords: intraoperative optical coherence tomography, epiretinal membrane, inner limiting membrane
Introduction
Macular epiretinal membrane (ERM) is a layer of transparent, non-vascular fibrotic tissue attached to the surface of the retina, and is formed by the migration of astrocytes, Müller cells and pigment epithelial cells. Further contraction and traction of ERM will change macular microanatomy, resulting in increased retinal thickness. However, long-term traction of the retina causes permanent functional damage. The smooth removal of the anterior membrane by surgery in time can prevent the destruction of macular structure and reduce the loss of visual function.1
Vitrectomy combined with internal limiting membrane (ILM) peeling has become a trend, and vitrectomy represented by 23 G has become the preferred surgical method in recent years.2 The residual ILM caused the recurrence of macular epiretinal membrane, and complete removal of ILM reduced the risk of ERM recurrence, and retinal folds were better resolved and visual function was better restored.1,3–5 However, the injury of optic nerve fiber layer, retinal edema, and retinal hemorrhage can occur simultaneously in the process of internal limiting membrane stripping.6
Optical coherence tomography (OCT) is a non-invasive tomography of tissue microscopic structures and has become an indispensable examination method in the field of ophthalmology. By measuring the thickness of macular fovea and the degree of macular epiretinal membrane adhesion, reliable information can be provided for preoperative evaluation and intraoperative risk prediction. OCT has been gradually applied in surgery, that is intraoperative OCT (IOCT), to observe the microscopic morphology of the macula and its relationship with the anterior membrane during the operation, and guide the complete removal of the anterior membrane.7
Therefore, this study aimed to evaluate the feasibility and practicability of IOCT in the surgery of idiopathic macular epiretinal membrane (IMM) without internal limiting membrane staining. We observed the morphological changes of macular epiretinal membrane and macula and the postoperative effect by IOCT assisted macular epiretinal membrane peeling.
Materials and Methods
Patients
Patients diagnosed with idiopathic macular epiretinal membrane in the Affiliated Ophthalmological Hospital of Nanchang University in Nanchang City, Jiangxi Province from July 2018 to June 2020 were enrolled. All patients received vitrectomy combined with macular epiretinal membrane peeling. Inclusion criteria: (1) All patients had high retinal reflection signal in the inner layer of the macular area after OCT. (2) The visual acuity was less than 0.5, or patient complaints that the visual distortion and discomfort seriously affected the life. (3) The fundus examination showed tinfoil reflection or gray-white translucent membrane accompanied by macular edema. Exclusion criteria: (1) Patients with macular hole, macular degeneration, maculoschisis, macular atrophy and other eye diseases that can lead to changes in the morphology and structure of the macula; (2) Patients with a history of eye trauma, eye surgery and intraocular drug injection; (3) Secondary ERM, such as inflammation, postoperative hemorrhage, retinal vein occlusion and diabetic retinopathy; (4) Severe cardiac, pulmonary, hepatic and renal insufficiency and other patients who could not tolerate surgical treatment. This study was reviewed and approved by the Ethics Committee of Affiliated Eye Hospital of Nanchang University and complied with the Declaration of Helsinki. All patients were informed and signed the consent form.
Operations
All patients were examined with best corrected visual acuity (BCVA), non-contact tonometer, slit-lamp microscope with anterior lens and indirect ophthalmoscope, and OCT before operation. The BCVA was checked using the logarithm of the minimum angle of resolution (logMAR) fraction. The patients were followed up for 1 to 3 months after operation. The best corrected visual acuity, fundus oculi, OCT and postoperative complications were reexamined at 1 week, 1 month and 3 months after operation.
All patients underwent surgical treatment. The standard pars plana 23 G vitrectomy system was used for three-channel vitrectomy under local anesthesia and performed by the same experienced surgeon. First, the iOCT device (RESCAN 700 Carl Zeiss Medical Technologies, Germany) was used to scan the vitreo-retinal interface at the posterior pole to observe posterior vitreous detachment. The vitreous body should be removed as far as possible, and if there is no posterior vitreous detachment before operation, the posterior vitreous detachment is feasible. IOCT field of view was 6 mm × 6 mm.
During the operation, the scanning area was selected by freely moving the foot pedal, so that the changes of the retinal anatomical structure can be observed in real time during the peeling process, the position relationship between the internal limiting membrane forceps and the anterior membrane can be accurately positioned, and the traction force of the anterior membrane on the retina and the microstructure changes between the nerve fiber layers can be evaluated during the peeling process.8 The beginning time of membrane stripping was marked as the beginning time, and the end time was marked as the end time. The time of membrane stripping was recorded. All the image data of the operation process could be stored.
Phacoemulsification and intraocular lens implantation were performed simultaneously when cataract affected visual acuity or membrane stripping was affected during operation. All eyes were filled with balanced saline solution.
Statistical Analysis
Data were presented as mean and standard deviation, and analyzed using IBM SPSS version 22.0 software (IBM SPSS, Inc., Chicago, Illinois, USA). BCVA was expressed as the logarithm of the minimum resolution angle. Baseline and postoperative BCVA were compared by analysis of variance (ANOVA) test. The choice level of statistical significance was two-sided p < 0.05.
Results
Characteristics of Clinical Data
A total of 35 patients (35 eyes) were initially enrolled in this study, of which 2 eyes were excluded because IOCT images could not be clearly displayed during the operation due to equipment reasons, and 1 patient was excluded because of loss of follow-up one week after the operation. Finally, a total of 32 patients (12 males and 20 females) aged 38–75 years were included. Among them, 10 patients (10 eyes) were in grade 2 of ERM and 22 patients (22 eyes) were in grade 3 of ERM. Twenty-six eyes were complicated with cataract that affected visual acuity, and 23 G vitrectomy combined with membrane peeling and phacoemulsification combined with intraocular lens implantation were performed. Cataract in 6 eyes was mild and did not affect visual acuity and iOCT imaging, so cataract extraction was not performed. Air tamponade was performed in 4 eyes because of the tight adhesion between the anterior membrane and the retina during the operation, the relative difficulty of membrane peeling and the severe macular edema after membrane peeling.
IOCT of Posterior Vitreous Detachment and the Starting Point of Membrane Peeling
All 32 eyes underwent standard 3-channel pars plana 23 G vitrectomy without vitreous staining. The macula and optic disc were scanned by iOCT during the operation. Posterior vitreous detachment was successfully performed in 24 eyes, and 8 eyes still had posterior vitreous cortex adhesion to the optic disc or macula (Figure 1). The macular area and the retina in the vascular arch of the macular area were scanned with the cross method during the operation. In some areas, the retina adhered tightly to the anterior membrane. The iOCT images showed that the retinal surface was flat, and in some areas the adhesion between the anterior membrane and the retina was loose. The high reflection lines in front of the retina were observed, and there was a low reflection dark area between the anterior membrane and the omentum. The size of the gap in the dark area was different, and the area with the lar gap was taken as the starting point of film strip (Figure 2). The “starting point” was found in 24 eyes (75%), and the retinal forceps successfully clamped and stripped the anterior membrane, while the thickest area of the anterior membrane was selected as the starting point in 8 eyes without obvious starting point. The time for iOCT scanning to find the best starting point was 28S-140S (mean time 66 ± 15s).
 |
Figure 1 During the vitrectomy, ioct scan was performed to observe posterior vitreous cortex and posterior detachment. The arrow indicated posterior vitreous cortex. |
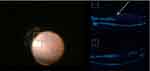 |
Figure 2 Before stripping, ioct scanning was performed to find the “starting point”. The arrow indicated the loose part of the anterior membrane, which represented the best part of stripping. |
Removal of Anterior Membrane and Internal Limiting Membrane
In 24 eyes, the anterior membrane was completely removed without internal limiting membrane staining, and the integrity of the anterior membrane was judged by brilliant blue staining. In 8 eyes, part of the residual anterior membrane was found by brilliant blue staining after membrane stripping (Figure 3), of which 3 eyes were 2 pupillary distance (PD) away from the central fovea of the macula without special treatment, and 5 eyes were closely adhered to the retina during membrane stripping, and the anterior membrane was dense and extensive. The macular retina was significantly wrinkled and edematous, and was stripped intact after staining. In 10 eyes, the retinal folds were obvious after simple stripping of the anterior membrane, and there was no significant improvement, so the internal limiting membrane was stripped to the vascular arch under brilliant blue staining.
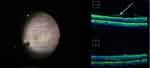 |
Figure 3 Edge remained during front film peeling. The ILM was stained in the color photo. The arrow indicated that the front film was broken in the middle and the edge remains. |
Changes of Visual Acuity and Macular Microstructure After Operation
The postoperative visual acuity was (0.61 ± 0.31) logMAR at 1 month and (0.52 ± 0.23) logMAR at 3 months, which were significantly higher than those before operation (p < 0.05). At 3 months postoperatively, BCVa improved in 28 patients and decreased in 1 patient (Figure 4).
The central macular thickness (CMT) of the affected eye was (311.50 ± 62.7843 µm) at 1 month after operation and (278.68 ± 57.63 µm) at 3 months after operation, significantly lower than that before operation (p < 0.05).
Intraoperative and Postoperative Complications and Recurrence Rate
There were several capillary bleeding spots in 10 eyes (31.25%) during the operation, including 8 eyes after stripping the internal limiting membrane and 2 eyes after stripping the anterior membrane. The bleeding stopped spontaneously without special treatment. No vitreous hemorrhage, retinal break or detachment occurred during the operation.
Vitreous hemorrhage occurred in one eye 2 days after the operation, and the hemorrhage in the vitreous cavity was removed. The reason was that there was about 1/2 PD hole in the peripheral retina, which led to vitreous hemorrhage. There was no hemorrhage or retinal detachment in the macular area. There were no complications such as retinal detachment, vitreous hemorrhage, macular hole and macular epiretinal membrane recurrence within 3 months after operation.
Discussion
The main surgical treatment of idiopathic macular epiretinal membrane is vitrectomy combined with macular epiretinal membrane peeling, but whether it is necessary to peel off the internal limiting membrane is still controversial. It has been reported that macular epiretinal membrane combined with internal limiting membrane peeling and simple macular epiretinal membrane peeling have advantages in the restoration of macular foveal structure and the reduction of foveal thickness, and in the macular microvision after surgery. Patients with internal limiting membrane stripping have higher absolute scotoma, which may be due to the attachment of the fibrous scaffold footplate of Muller cells to the internal limiting membrane, while Muller cells support the photoreceptor cells in the macular area and maintain the close connection of nerve fibers.9 Therefore, the removal of the internal limiting membrane may lead to the loss of support and the damage of nerve fibers in the macula.10
It was reported that BCVA was better in the ERM and ILM stripping groups within 12 months after surgery, but BCVA was better in the ERM stripping group at 18 months after surgery.5 A meta-analysis showed that BCVA was better in the ERM stripping group within 12 months after surgery and in the ERM and ILM stripping groups at 18 months after surgery.11 After the anterior membrane stripping, the macular retinal edema is serious and the macular retinal folds are obvious. It is recommended to perform internal limiting membrane stripping, which is conducive to the recovery of macular microstructure after surgery.12 In this study, we attempted to perform epimacular membrane peeling without internal limiting membrane staining in all patients, and then perform internal limiting membrane brilliant blue G staining after peeling to verify whether there is any residue of ERM after the removal of the anterior membrane. In this study, 24 eyes were confirmed without any residual anterior membrane after staining. In the 8 eyes with partial residual epimembranous membrane, 3 eyes had the position of epimembranous membrane more than 2 PD away from the central fovea of the macula, and the residual area was small, and no further stripping was performed. In 5 eyes, the residual area was located in the macular area, and all of them were stage 3 epimembranous membrane, and the internal limiting membrane was stripped at the same time under staining.
The key to the success of stripping is to find the best “starting point” for stripping. The “starting point” is considered to be the relatively loose area between the anterior membrane and the retina. With the help of iOCT, we successfully found the “starting point” in 24 eyes, and the strip was successfully completed. During the operation, no obvious starting point was found in 8 eyes. We chose the dense area as the starting point and kept it away from the center of the macula as far as possible to reduce possible mechanical damage to the retina during membrane peeling. The time for scanning to find the starting point was 28–140 s, with an average of 66 ± 15s, which was similar to previous reported.13 During the stripping process, iOCT visualized the residual area of the anterior membrane.14 In this study, iOCT guided visualization of retinal morphological changes during detachment showed macular or subretinal hypo-reflective cysts in 8 eyes (25%), resulting in damage to the intersection zone. It is considered to be an important cause affecting the vision of patients.7 Among them, 3 eyes had small cysts before operation, and 5 eyes had no obvious macular low reflection cysts. The capsule slowly recovered after stripping, and the macular anterior membrane was dense, and it adhered closely to the retina during stripping, so it could not be completely stripped one time and it needs to be stripped many times and in small quantities.
One week after the operation, there was no low reflection area under the macula, but the shape of macular edema recovered slowly, and the visual acuity improved to varying degrees 6 months after the operation, which may be determined by the improvement of refractive status of some patients after cataract extraction and the recovery of macular function after macular epiretinal membrane stripping. Intraoperative phacoemulsification and intraocular lens implantation did not increase the complications of macular epiretinal membrane injury.15 There were no complications such as retinal detachment and vitreous hemorrhage in all the eyes, and some of the eyes with combined ILM stripping had macular punctate capillary bleeding, which did not require special treatment. Vitreous hemorrhage occurred in one eye two days after the operation. Six months after surgery, no macular epiretinal membrane recurrence was found.
This study has some limitations. First, this is a single center study. Second, cataract surgery is a confounding factor in 26 out of 32 eyes. Therefore, BCVA improvement only due to ERM peeling may be difficult to assess. Third, the follow-up is relatively short. We need to increase follow-up duration to assess long-term remodeling in idiopathic ERM.
In conclusion, iOCT-assisted staining-free surgery for macular epiretinal membranes can successfully find the starting point of epiretinal membrane stripping and visualize the changes in macular microstructure, but some idiopathic macular epiretinal membranes still need ILM stripping. Our results confirm the safety and reliability of iOCT in idiopathic macular epretinal membrane surgery, and provide a new and effective method for the removal of idiopathic macular epiretinal membrane.
Data Sharing Statement
Data available on request from the corresponding author.
Acknowledgment
This study was supported by Scientific Project of Department of Education of Jiangxi, China (No. 190152).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schechet SA, DeVience E, Thompson JT. The effect of internal limiting membrane peeling on the idiopathic epiretinal membrane surgery, with a review of the literature. Retina. 2017;37(5):873–880.
2. Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Double staining with brilliant blue G and double peeling for epiretinal membranes. Ophthalmology. 2009;116(7):1370–1376. doi:10.1016/j.ophtha.2009.01.024
3. Azuma K, Ueta T, Eguchi S, Aihara M. Effects of internal limiting membrane peeling combined with removal of idiopathic epiretinal membrane: a systematic review of literature and meta-analysis. Retina. 2017;37(10):1813–1819. doi:10.1097/IAE.0000000000001537
4. Diaz-Valverde A, Wu L. To peel or not to peel the internal limiting membrane in idiopathic epiretinal membranes. Retina. 2018;38(Suppl 1):S5–S11. doi:10.1097/IAE.0000000000001906
5. Liu H, Zuo S, Ding C, Dai X, Zhu X. Comparison of the effectiveness of pars plana vitrectomy with and without internal limiting membrane peeling for idiopathic retinal membrane removal: a meta-analysis. J Ophthalmol. 2015;2015:974568.
6. Almony A, Nudleman E, Shah GK, et al. Techniques, rationale, and outcomes of internal limiting membrane peeling. Retina. 2012;32(5):877–891. doi:10.1097/IAE.0b013e318227ab39
7. Leisser C, Hackl C, Hirnschall N, et al. Visualizing macular structures during membrane peeling surgery with an intraoperative spectral-domain optical coherence tomography device. Ophthalmic Surg Lasers Imaging Retina. 2016;47(4):328–332.
8. Cai C, Sun H, Hu L, Fan Z. Visualization of integrin molecules by fluorescence imaging and techniques. Biocell. 2021;45(2):229–257. doi:10.32604/biocell.2021.014338
9. Deltour JB, Grimbert P, Masse H, Lebreton O, Weber M. Detrimental effects of active internal limiting membrane peeling during epiretinal membrane surgery: microperimetric analysis. Retina. 2017;37(3):544–552. doi:10.1097/IAE.0000000000001179
10. Clark A, Balducci N, Pichi F, et al. Swelling of the arcuate nerve fiber layer after internal limiting membrane peeling. Retina. 2012;32(8):1608–1613. doi:10.1097/IAE.0b013e3182437e86
11. Chang WC, Lin C, Lee CH, Sung TL, Tung TH, Liu JH. Vitrectomy with or without internal limiting membrane peeling for idiopathic epiretinal membrane: a meta-analysis. PLoS One. 2017;12(6):e0179105. doi:10.1371/journal.pone.0179105
12. Falkner-Radler CI, Glittenberg C, Gabriel M, Binder S. Intrasurgical microscope-integrated spectral domain optical coherence tomography-assisted membrane peeling. Retina. 2015;35(10):2100–2106. doi:10.1097/IAE.0000000000000596
13. Tao J, Wu H, Chen Y, et al. Use of iOCT in vitreoretinal surgery for dense vitreous hemorrhage in a Chinese population. Curr Eye Res. 2019;44(2):219–224. doi:10.1080/02713683.2018.1533982
14. Pfau M, Michels S, Binder S, Becker MD. Clinical experience with the first commercially available intraoperative optical coherence tomography system. Ophthalmic Surg Lasers Imaging Retina. 2015;46(10):1001–1008. doi:10.3928/23258160-20151027-03
15. Savastano A, Savastano MC, Barca F, Petrarchini F, Mariotti C, Rizzo S. Combining cataract surgery with 25-gauge high-speed pars plana vitrectomy: results from a retrospective study. Ophthalmology. 2014;121(1):299–304. doi:10.1016/j.ophtha.2013.06.022
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

