Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Intraoperative dexmedetomidine attenuates postoperative systemic inflammatory response syndrome in patients who underwent percutaneous nephrolithotomy: a retrospective cohort study
Authors Tan F , Gan X , Deng Y, Li X, Guo N, Hei Z, Zhu Q , Chen ZG , Zhou S
Received 17 November 2017
Accepted for publication 22 December 2017
Published 14 February 2018 Volume 2018:14 Pages 287—293
DOI https://doi.org/10.2147/TCRM.S157320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Fang Tan,1,2,* Xiaoliang Gan,3,* Yingqing Deng,1 Xiaoyun Li,1 Na Guo,1 Ziqing Hei,1 Qianqian Zhu,1 Zhuang-Gui Chen,4 Shaoli Zhou1
1Department of Anesthesiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China; 2Department of Anesthesiology, The Seventh Affiliated Hospital of Sun Yat-sen University, Shenzhen, People’s Republic of China; 3Department of Anesthesiology, Zhongshan Ophthalmic Center of Sun Yat-sen University, Guangzhou, People’s Republic of China; 4Department of Pediatric Intensive Care Unit, Department of Pediatrics, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Purpose: Dexmedetomidine (DEX) has been reported to attenuate inflammation in rats. The present retrospective cohort study aimed to investigate whether intraoperative administration with DEX could reduce the incidence of postoperative systemic inflammatory response syndrome (SIRS) in patients following percutaneous nephrolithotomy (PCNL).
Patients and methods: A total of 251 patients were included in the analysis. Among these patients, 175 received intravenous DEX infusion during the intraoperative period and 76 did not. The primary outcome measures were the incidences of postoperative SIRS and fever. Secondary outcomes included patient-controlled analgesia (tramadol) requirements, length of postoperative hospitalization stay, serum creatinine (Scr) and serum blood urea nitrogen (BUN) concentration, and adverse events (bradycardia, hypotension, renal artery thrombosis).
Results: Administration of DEX not only significantly attenuated the incidence of SIRS and fever (P=0.029, P=0.042, respectively), but also reduced analgesia requirements (P=0.028). The length of postoperative hospitalization stay, Scr and BUN concentration, and adverse events did not differ significantly between the two groups. Further univariate and multivariate logistic regression analysis indicated that intraoperative DEX administration was a protective factor against SIRS after PCNL (OR 0.476 [95% CI: 0.257–0.835]; P=0.019).
Conclusion: Intraoperative administration of DEX might be associated with reductions in the incidences of SIRS and fever after PCNL.
Keywords: PCNL, SIRS, risk factor, dexmedetomidine
Introduction
Since its initial introduction in 1976,1 percutaneous nephrolithotomy (PCNL) has become the standard therapy for large renal calculi due to its lower surgical trauma and high stone-free rates. However, postoperative systemic inflammatory response syndrome (SIRS) and sepsis are common complications of the procedure, and can be associated with catastrophic consequences. The incidence of postoperative SIRS of PCNL is reported to range from 9.8 to 43%, which is significantly higher than other endourological surgeries.2,3 Therefore, investigating strategies to reduce the risk for postoperative SIRS of PCNL is warranted.
Dexmedetomidine (DEX) is a highly selective α2-adrenergic agonist that has demonstrated sedative, analgesic, and anxiolytic effects.4,5 Beyond these benefits, emerging data show that the medication also exhibits anti-inflammatory properties.6–9 Treatment with DEX has been shown to attenuate the release of cytokines in cells stimulated by endotoxin in a dose-dependent manner in in vitro studies.10,11 Specifically, empirical investigations have suggested that DEX has organ-protective effects against ischemia-reperfusion injury in the heart, brain, kidney, and lungs.9,12–15 In addition, intraoperative infusion of DEX can suppress inflammation and reduce cytokine levels in patients undergoing cardiac surgery.12 However, the anti-inflammatory effect of DEX has not been studied in patients undergoing PCNL.
To address this knowledge gap, we have retrospectively investigated the association between intraoperative infusion of DEX and the incidence of SIRS and prognosis in patients after PCNL.
Methods
Patients
A total of 415 consecutive adult patients who underwent PCNL at a single center between January 2011 and April 2014 were retrospectively reviewed. Patients who met the following criteria were enrolled in the present analysis: underwent first PCNL surgery; and physical status was evaluated as American Society of Anesthesiologists grade 1 or 2. Exclusion criteria included: age <18 years; combined with tumors, hematopathy, immunosuppressant treatments; diabetes mellitus; preoperative heart rate >90 beats/min; stone diameter <2 cm; heart or kidney disease(s); or preoperative fever. All patient data were extracted from a Hospital Information System database established by the Third Affiliated Hospital. This database is one of the largest all-payer inpatient care databases in the People’s Republic of China. In the current study, a total of 251 patients were included in the final analysis.
Ethical standard
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University and was carried out in compliance with the Helsinki Declaration. The requirement for informed consent was waived because of the retrospective nature of the study and using data from which the patients’ identification information had been removed.
DEX, anesthesia and analgesia administration
The definition of the DEX administered group was the patient who received a bolus dose 1 μg/kg of DEX after anesthesia induction in no less than 15 min and continuously infused (typically administered it at range from 0.1 to 0.6 μg/kg/h) until 30 min before the end of surgery. The nontreated group (non-DEX) was defined as those who did not receive DEX throughout the perioperative period.
For all PCNL patients, after standard monitoring, induction of general anesthesia consisted of midazolam, fentanyl/sufentanil, propofol and cisatracurium. Maintenance of anesthesia was facilitated with sevoflurane (1%–3%) and oxygen. Ventilation was controlled with 8–10 mL/kg tidal volume with end tidal CO2 of 35–45 mmHg. Vasoactive drugs including dopamine, dobutamine, nitroglycerine, and phenylephrine were used to maintain blood pressure in normal range according to the hemodynamic responses when necessary, and atropine was used if heart rates were <50 beats/min.
Patients were intravenously infused with flurbiprofen axetil (1 mg/kg) as an analgesic before the end of surgery. Use of flurbiprofen axetil before the end of surgery was routine in our department unless there was a contraindication. If patients had the contraindication of nephrogenic syndrome of inappropriate antidiuresis, 0.05 mg/kg morphine was given alternatively. Tramadol (100 mg) intramuscular injection was administered without pain score assessed when patient complained of a pain after recovery from anesthesia.
Outcome measures
The primary outcome measures included the presence of postoperative SIRS and fever. SIRS definition criteria included a body temperature >38°C or <36°C; a heart rate >90/min; a respiratory rate >20 breaths/min; and a white blood cell count >12,000/mm3 or <4,000 mm3. The presence of ≥2 criteria was accepted as SIRS. Postoperative fever was defined as a body temperature >38.5°C. These primary outcome measures were recorded in the 3-day period after surgery.
In addition, a variety of secondary outcome measures were recorded, including patient-controlled analgesia (tramadol) requirements, postoperative hospital length of stay, serum creatinine (Scr) and serum blood urea nitrogen (BUN) concentration, as well as adverse events including bradycardia, hypotension, and renal artery thrombosis. The postoperative hospital length of stay was defined using the first day after operation and discharge status. Bradycardia was defined as a heart rate <50 beats/min, and hypotension was defined as mean arterial pressure <30% from baseline for 60 s.
Statistical methods
Continuous and categorical variables, respectively, are presented as mean ± standard deviation and percentages. The Student’s t-test was used to compare normally distributed variables between the two groups, and the Mann–Whitney U-test was used for non-normally distributed data. Categorical data were compared using the chi-squared or Fisher’s exact tests. A multivariable logistic regression (LR) analysis (forward LR method) was used to determine risk factors for SIRS after PCNL; P<0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 251 patients who met the study inclusion criteria were included in the present retrospective analysis. Among these patients, 175 received DEX administration and 76 did not (Figure 1). Demographics and surgical aspects did not differ significantly between groups with and without DEX (Table 1).
  | Figure 1 Flow diagram. |
Postoperative SIRS was common in this cohort, with an incidence between 0.3% and 21.1% in the first 5 days after PCNL; >90% of these SIRS events occurred in the first 3 days (Figure 2). Similarly, the same trend was observed in postoperative fever events.
  | Figure 2 Days between surgery and SIRS and fever. |
Primary and secondary outcomes
In the first 3 days after PCNL, 41 patients in the DEX group developed SIRS, which was a significantly lower proportion than in the non-DEX group (23.4% vs 36.8%, respectively; P=0.029) (Table 2). Moreover, the incidence of fever in patients treated with DEX was significantly lower compared with the non-DEX group (16.0% vs 22.4%, respectively; P=0.042) (Table 2).
Regarding the analgesic effect of DEX, patient-controlled analgesia (tramadol) requirements in the DEX group were lower than those in the non-DEX group (20% vs 32.9%, respectively; P=0.028) (Table 2). The length of postoperative hospitalization stay, and Scr and BUN concentration were not statistically different between the two groups. Similarly, the incidence of adverse events, including bradycardia, hypotension and renal artery thrombosis, did not differ significantly between the two groups (all P>0.05) (Table 2).
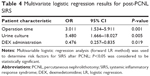
On univariate and multivariate LR analysis, independent risk factors for the incidence of SIRS were related to operation time and preoperative positive-urine culture (OR 3.011 [95% CI: 1.534–5.911], P=0.001; and OR 5.480 [95% CI: 1.666–18.027], P=0.005, respectively) (Tables 3 and 4). DEX administration was shown to be a protective factor for SIRS after PCNL (OR 0.476 [95% CI: 0.257–0.835]; P=0.019) (Tables 3 and 4).
Discussion
In the present analysis of 251 consecutive patients undergoing PCNL, we found that the intraoperative use of DEX was associated with reduced rates of SIRS after surgery compared with those who did not receive DEX. Moreover, significant reductions in the incidence of postoperative fever and lower requirements for patient-controlled analgesia (tramadol) were observed in patients who received DEX. In addition, multivariable LR analysis further demonstrated that DEX treatment was a protective factor against SIRS. PCNL was identified to be associated with high risk for infection and other complications, with reported rates of up to 83% for total complications.1 These postoperative complications included fever (10.5%–32.1%), SIRS (9.8%–43%), and sepsis (0.3%–4.7%).1–3,16–18 These events consequently require additional treatment and longer hospital stay; occasionally, severe septic shock leading to death may occur. In the present study, >50% SIRS and fever occurred in the first 3 days after surgery. Consistent with previous studies,19,20 the overall incidence of SIRS and fever in the first 3 days was 27.5% and 17.9%, respectively.
Previous studies have suggested that several perioperative factors, including positive-urine culture, stone diameter, staghorn calculus, operation time, and blood transfusion, are associated with SIRS after PCNL.21–24 The present study demonstrated that stone diameter, staghorn calculus, urine culture, operation time, and DEX treatment were factors associated with SIRS after PCNL. Preoperative positive-urine culture and operative time were identified as independent risk factors for SIRS. We have speculated that increased fluid and toxins translocated into the systemic circulation with prolonged durations of surgery could subsequently result in high incidences of postoperative SIRS. However, Caddedu et al reported that there were no clear correlations between duration of surgery and the incidence of postoperative fever.25 Different criteria may contribute to these differences. Studies have identified the significance of perioperative urine culture for infection after PCNL.26,27 Results of present study suggest that perioperative urine culture is an independent risk factor for postoperative SIRS. Consequently, clinicians should consider the use of preoperative antibiotics for patients who present with positive-urine culture(s). Interestingly, results of the current study also suggest that DEX administration is a protective factor against the development of SIRS after PCNL. It would be reasonable to speculate that intraoperative DEX treatment may be an effective strategy for reducing the risk for postoperative SIRS after PCNL.
DEX is highly selective α2-adrenergic agonist, and is widely used for anxiolysis, sedation, and analgesia.4,5 Recently, studies have demonstrated the protective benefits of DEX, which are exerted through its anti-inflammatory properties.6–9 Administration of DEX could alleviate systemic inflammation in animals through stabilization of the sympathetic nervous system.28,29 DEX significantly reduced ischemia/reperfusion damage in diabetic rats, and the mechanism may be related to down-regulated expression of pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-6.30 Our previous study has demonstrated that DEX had protected against acute kidney injury by down-regulating inflammatory reactions in endotoxemia.31 In clinical practice, DEX has demonstrated its anti-inflammatory effects in cardiac surgery, where it has significantly reduced sepsis in patients with cardiac disease by suppressing the production of pro-inflammatory cytokines.12,13 Moreover, it has also produced neuroprotective effects by attenuating inflammation and oxidative stress.32 In the present retrospective analysis, >50% of the patients who underwent PCNL received intraoperative administration of DEX, which was associated with a lower incidence of postoperative SIRS and fever after PCNL compared with those who did not receive DEX. Furthermore, as commonly reported about the inflammation and neuropathic pain after PCNL, the results of our study supported the analgesic benefit of DEX. Patient-controlled analgesia (tramadol) requirements were significantly lower in the DEX group than in the non-DEX group. Beyond its anti-inflammatory properties, DEX also produces its analgesic effect by central and spinal cord α2 receptor modulation.33 Of note, these benefits did not result in a better prognosis because the length of postoperative hospital stay was similar regardless of whether patients received DEX. Consistent with our results, a previous study also suggested that DEX could decrease the incidence of sepsis, but could not reduce the length of hospital stay.13 This would be reasonable because many patient-specific factors are associated with postoperative hospital length of stay.
Beyond its reported protective effects, attention should be devoted to the fact that DEX infusion may result in bradycardia and hypotension due to decreased sympathetic tone and increased vagal activity. Furthermore, Scheinin et al reported that young volunteers exhibited bradycardia and sinus arrest with infusion of DEX.34 However, in the present study, we found that the incidence of hypotension and bradycardia were similar in our patients, regardless of whether they received DEX. Intraoperative stress may compensate for this discrepancy. It has been reported that unaltered hemodynamics has been recorded in patients even with high doses of DEX.35,36
There were several limitations to the current study, the first of which was its retrospective cohort design. Although multivariate regression analysis was used without any apparent adjustment to reduce evident biases, potential confounding biases are inherent because this was a nonrandomized study. Second, the number of patients treated with DEX is more than twice those untreated. The total number of participants was small. Thus, study with large number is needed in future. Thirdly, many perioperative factors were associated with SIRS after PCNL; therefore, the exact role of DEX in postoperative SIRS and fever remains to be determined, preferably in further prospective studies. Finally, this was a single-institution study and, as such, was limited by the involvement of different surgeons, surgical techniques, and treatment protocols, which may have impacted the results.
Conclusion
Collectively, findings from the current study suggest that intraoperative administration of DEX might be associated with reductions in the incidence of SIRS and fever after PCNL, as well as lower requirements for patient-controlled analgesia (tramadol). However, these results are only hypothesis-generating, and a large, well-conducted randomized controlled trial is required to confirm the exact role DEX plays in postoperative SIRS and fever.
Acknowledgments
This study was supported by Sun Yat-sen University Clinical Research 5010 Program (Grant no 2015006) and Technology Planning Project of Guangdong Province (Grant no 2013B021800197).
Author contributions
Shaoli Zhou, Zhuang-Gui Chen, and Qianqian Zhu, helped design the study, conduct of the study and prepared the manuscript. Fang Tan helped data collection, data analysis, and manuscript preparation. Xiaoliang Gan helped manuscript preparation. Xiaoyun Li, Yingqing Deng, Na Guo, and Ziqing Hei helped data collection and data analysis. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
de la Rosette J, Assimos D, Desai M, et al. The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: indications, complications, and outcomes in 5803 patients. J Endourol. 2011;25(1):11–17. | ||
Korets R, Graversen JA, Kates M, Mues AC, Gupta M. Post-percutaneous nephrolithotomy systemic inflammatory response: a prospective analysis of preoperative urine, renal pelvic urine and stone cultures. J Urol. 2011;186(5):1899–1903. | ||
Moses RA, Agarwal D, Raffin EP, et al. Postpercutaneous nephrolithotomy systemic inflammatory response syndrome is not associated with unplanned readmission. Urology. 2017;100:33–37. | ||
Khan ZP, Ferguson CN, Jones RM. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54(2):146–165. | ||
Ergenoglu P, Akin S, Bali C, Eker HE, Cok OY, Aribogan A. Effect of low dose dexmedetomidine premedication on propofol consumption in geriatric end stage renal disease patients. Braz J Anesthesiol. 2015;65(5):326–332. | ||
Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin. 2009;25(3):551–570. | ||
Hofer S, Steppan J, Wagner T, et al. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13(1):R11. | ||
Tüfek A, Kaya S, Tokgöz O, et al. The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clin Invest Med. 2013;36(2):E95–E102. | ||
Yang CL, Chen CH, Tsai PS, Wang TY, Huang CJ. Protective effects of dexmedetomidine-ketamine combination against ventilator-induced lung injury in endotoxemia rats. J Surg Res. 2011;167(2):e273–e281. | ||
Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22(3):221–228. | ||
Peng M, Wang YL, Wang CY, et al. Dexmedetomidine attenuates lipopolysaccharide-induced proinflammatory response in primary microglia. Surg Res. 2013;179(1):e219–e225. | ||
Ueki M, Kawasaki T, Habe K, et al. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69(7):693–700. | ||
Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127(15):1576–1584. | ||
Sugita S, Okabe T, Sakamoto A. Continuous infusion of dexmedetomidine improves renal ischemia-reperfusion injury in rat kidney. J Nippon Med Sch. 2013;80(2):131–139. | ||
Sahin T, Begec Z, Toprak HI, et al. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res. 2013;183(1):385–390. | ||
Michel MS, Trojan L, Rassweiler JJ. Complications in percutaneous nephrolithotomy. Eur Urol. 2007;51(4):899–906. | ||
Vorrakitpokatorn P, Permtongchuchai K, Raksamani EO, Phettongkam A. Perioperative complications and risk factors of percutaneous nephrolithotomy. J Med Assoc Thai. 2006;89(6):826–833. | ||
Armitage JN, Irving SO, Burgess NA; British Association of Urological Surgeons Section of Endourology. Percutaneous nephrolithotomy in the United Kingdom: results of a prospective data registry. Eur Urol. 2012;61(6):1188–1193. | ||
Margel D, Ehrlich Y, Brown N, Lask D, Livne PM, Lifshitz DA. Clinical implication of routine stone culture in pereutaneous nephrolithotomy-a prospective study. Urology. 2006;67(1):26–29. | ||
Hosseini MM, Basiri A, Moghaddam SM. Percutaneous nephrolithotomy of patients with staghom stone and incidental purulent fluid suggestive of infection. J Endourol. 2007;21(12):1429–1432. | ||
Dogan HS, Guliyev F, Cetinkaya YS, Sofikerim M, Ozden E, Sahin A. Importance of microbiological evaluation in management of infectious complications following percutaneous nephrolithotomy. Int Urol Nephrol. 2007;39(3):737–742. | ||
Gutierrez J, Smith A, Geavlete P, et al. Urinary tract infections and post-operative fever in percutaneous nephrolithotomy. World J Urol. 2013;31(5):1135–1140. | ||
Chen L, Xu QQ, Li JX, Xiong LL, Wang XF, Huang XB. Systemic inflammatory response syndrome after percutaneous nephrolithotomy: an assessment of risk factors. Int J Urol. 2008;15(12):1025–1028. | ||
Gonen M, Turan H, Ozturk B, Ozkardes H. Factors affecting fever following percutaneous nephrolithotomy: a prospective clinical study. J Endourol. 2008;22(9):2135–2138. | ||
Cadeddu JA, Chen R, Bishoff J, et al. Clinical significance of fever after percutaneous nephrolithotomy. Urology. 1998;52(1):48–50. | ||
Sharifi Aghdas F, Akhavizadegan H, Aryanpoor A, et al. Fever after percutaneous nephrolithotomy: contributing factors. Surg Infect(Larchmt). 2006;7(4):367–371. | ||
Wolf JS, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179(4):1379–1390. | ||
Qiao H, Sanders RD, Ma D, Wu X, Maze M. Sedation improves early outcomes in severely septic Sprague Dawley rats. Crit Care. 2009;13(4):R136. | ||
Knaus AE, Muthig V, Schickinger S, et al. Alpha2-adrenoceptor subtypes–unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem Int. 2007;51(5):277–281. | ||
Zeng X, Wang H, Xing X, Wang Q, Li W. Dexmedetomidine protects against transient global cerebral ischemia/reperfusion induced oxidative stress and inflammation in diabetic rats. PLoS One. 2016;11(3):e0151620. | ||
Tan F, Chen Y, Yuan D, Gong C, Li X, Zhou S. Dexmedetomidine protects against acute kidney injury through downregulating inflammatiory reactions in endotoxemia rats. Biomed Rep. 2015;3(3):365–370. | ||
Rodríguez-González R, Sobrino T, Veiga S, et al. Neuroprotective effects of dexmedetomidine conditioning strategies: Evidences from an in vitro model of cerebral ischemia. Life Sci. 2016;144:162–169. | ||
Grewal A. Dexmedetomidine: new avenues. J Anaesthesiol Clin Pharmacol. 2011;27(3):297–302. | ||
Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998;89(3):574–584. | ||
Rajan S, Hutcherson MT, Sessler DI, et al. The effects of dexmedetomidine and remifentanil on hemodynamic stability and analgesic requirement after craniotomy: a randomized controlled trial. J Neurosurg Anesthesiol. 2016;28(4):282–290. | ||
Park HY, Kim JY, Cho SH, Lee D, Kwak HJ. The effect of low-dose dexmedetomidine on hemodynamics and anesthetic requirement during bis-spectral index-guided total intravenous anesthesia. J Clin Monit Comput. 2016;30(4):429–435. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.