Back to Journals » Research and Reports in Urology » Volume 8
Intramuscular depot formulations of leuprolide acetate suppress testosterone levels below a 20 ng/dL threshold: a retrospective analysis of two Phase III studies
Authors Spitz A, Gittelman M, Karsh L, Dragnic S, Soliman A, Lele A, Gruca D, Norton M
Received 27 April 2016
Accepted for publication 14 June 2016
Published 23 August 2016 Volume 2016:8 Pages 159—164
DOI https://doi.org/10.2147/RRU.S111475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Aaron Spitz,1 Marc Gittelman,2 Lawrence I Karsh,3 Sanja Dragnic,4 Ahmed M Soliman,5 Aditya Lele,6 Damian Gruca,7 Michael Norton4
1Orange County Urology Associates, Laguna Beach, CA, 221st Century Oncology/UroMedix-Aventura Division, Aventura, FL, 3The Urology Center of Colorado, Denver, CO, 4US Medical Affairs, 5Health Economics and Outcomes Research, 6Data and Statistical Sciences, AbbVie Inc., North Chicago, IL, USA; 7Global Medical Affairs, AbbVie Deutschland, Ludwigshafen, Germany
Introduction: Androgen deprivation therapy (ADT) with gonadotropin-releasing hormone (GnRH) analogs is a standard treatment for advanced prostate cancer. GnRH analog therapy can reduce testosterone to “castrate” levels, historically defined as <50 ng/dL. With the advent of newer assays, a lower threshold of <20 ng/dL has recently been proposed. We report the results of a retrospective analysis of two Phase III trials of 4- and 6-month depot microsphere formulations of leuprolide acetate (LA), a GnRH agonist that has previously demonstrated efficacy in testosterone suppression to <50 ng/dL in patients on ADT. This analysis investigates the ability of these LA formulations to suppress to ≤20 ng/dL levels.
Methods: In two of five AbbVie/Abbott clinical trials of microsphere formulations of LA for ADT, analytic technology permitting testosterone detection as low as 3 ng/dL was used and thus was selected for this analysis. Both trials were open-label, fixed-dose studies in prostate cancer patients, naïve to ADT. Patients received either 30 mg (4-month formulation; n=49) or 45 mg (6-month formulation; n=151) depot injections of LA microspheres. Treatment duration was up to 32 weeks for the 4-month formulation and 48 weeks for the 6-month formulation. The proportion of patients achieving the 20 ng/dL threshold was determined every 4 weeks.
Results: Pooled analysis showed that 152 of 193 (79%) of patients achieved serum testosterone levels of ≤20 ng/dL at 4 weeks, and sustained the improvement at week 24 (169/189, 89%). Additionally, in the 6-month study, 127/135 (94.1%) patients were suppressed to ≤20 ng/dL at 48 weeks.
Conclusion: Both 4- and 6-month intramuscular depot formulations of LA achieved and maintained mean serum testosterone levels ≤20 ng/dL in the vast majority of patients as early as 4 weeks following treatment initiation. Additional research on the clinical relevance of this lower testosterone threshold is warranted.
Keywords: androgen deprivation therapy, gonadotropin-releasing hormone analog, prostate cancer, castrate levels
Introduction
Excluding nonmelanoma skin cancer, prostate cancer is the most prevalent malignancy and is the second leading cause of cancer death among males in the US today.1,2 After local therapy, most commonly surgery or radiation, androgen deprivation therapy (ADT) is used for biochemical or prostate-specific antigen relapse and is standard treatment for progressive disease. ADT is also the standard of care for metastatic prostate cancer.3–5 ADT may be achieved via medical treatment with gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, or surgical castration (orchiectomy).3–5 ADT using GnRH agonists is the most common and well-established treatment for prostate cancer and avoids the stigma associated with orchiectomy.4–6 The first administration of a GnRH agonist produces an initial transient increase in testosterone and its metabolite 5α-dihydrotestosterone (“testosterone surge” or “flare”). Continuous regular dose administration, however, results in suppression of luteinizing hormone and follicle-stimulating hormone. The net effect is a decrease in testosterone concentrations to castrate levels.5 This decrease in serum testosterone levels inhibits androgen receptor signaling pathways within the tumor cells, resulting in tumor cell death or apoptosis, thereby limiting the cancer’s growth.7
GnRH agonists have been shown to suppress testosterone levels to <50 ng/dL (1.7 nmol/L), which has been the standard endpoint for treating prostate cancer with ADT for many years.4–6,8 Recent studies have shown that testosterone levels closer to a 20 ng/dL (0.7 nmol/L) threshold may improve survival in patients with prostate cancer when compared to those closer to 50 ng/dL.9–13 The mean value of serum testosterone after surgical castration, as determined by current analytical methods, is 15 ng/dL (0.5 nmol/L),14 and the most recent 2015 European Association of Urology guidelines now suggest <20 ng/dL is a more appropriate target castrate level rather than the original <50 ng/dL threshold.5
Leuprolide acetate (LA) is the most widely used GnRH agonist15 and is available in depot formulations allowing for administration every 1, 3, 4, or 6 months, depending on the formulation and/or dose.10,15 These depot formulations are of two types: intramuscularly-administered LA-containing microspheres16 and a subcutaneously-administered biodegradable LA-containing polymer solid.17 Both microsphere-18–21 and solid polymer-based LA22–24 have been approved by the US Food and Drug Administration for treating prostate cancer based on successful suppression of testosterone levels in randomized clinical trials to the <50 ng/dL castrate threshold. At the end of a 12-month clinical study in prostate cancer patients, 88% of patients were shown to have serum testosterone levels <20 ng/dL when treated with a 6-month subcutaneous solid depot LA formulation.23
Given the current interest in testosterone suppression to levels ≤20 ng/dL rather than the 50 ng/dL threshold during ADT,10 we carried out a retrospective analysis of two Phase III trials to examine the ability of 4- and 6-month LA intramuscular, microsphere depot formulations (Lupron®, AbbVie, North Chicago, IL, USA) to achieve testosterone castrate levels ≤20 ng/dL.
Methods
To date, AbbVie/Abbott has conducted five clinical trials that examined the impact of microsphere formulations of LA therapy on testosterone levels among prostate cancer patients. Among the five trials, two trials used analytic technology (radioimmunoassay or liquid chromatography/mass spectrometry) that allowed testosterone detection to <20 ng/dL levels and thus were selected as data sources for the current paper (M93-01325 and L-PC07-169 [NCT00626431]21).
Study M93-013 (4-month depot formulation) was an unblinded, multicenter study conducted at 17 centers that enrolled 49 patients with metastatic prostate cancer for a total treatment period of 32 weeks.25
Study L-PC07-169 (6-month depot formulation) was an open-label, noncomparative, multicenter study in 151 males with prostate cancer that included a screening period, a 12-month (48-week) treatment period, and a 30-day follow-up period. This study evaluated the efficacy and safety of two formulations 6-month depot LA (A and B). Only formulation A, however, met prespecified criteria for efficacy, and only the results from formulation A were reported originally21 and are reported here.
Patients
For the 4-month depot LA formulation study, patients with histologically confirmed prostatic adenocarcinoma and two or more clinically evaluable lesions with prestudy serum testosterone levels of 150 ng/dL (5.2 nmol/L) or higher and a performance status grade ≤2 as defined by the Eastern Cooperative Oncology Group scale were enrolled.26 None of the patients had previously received a GnRH analog, other hormonal therapy, or chemotherapy within 4 weeks preceding the initial depot injection. Ongoing radiation therapy precluded entry into the study.25
For the 6-month depot LA formulation study, patients were required to have histologically confirmed prostate cancer, TNM staging cT1b-4NanyMany, or rising prostate-specific antigen following radical prostatectomy (≥0.2 ng/dL increase from previous test on two consecutive assessments) or prostate irradiation (≥2.0 ng/dL increase above the nadir). Patients required a prestudy serum testosterone level of >150 ng/dL, an Eastern Cooperative Oncology Group performance score of ≤2, and at least 32 weeks to have passed since prior hormone therapy.21
These studies were approved by institutional review boards for each site and all patients provided written informed consent before screening or any study-related procedures.
Dosing and testosterone assessments
In the 4-month depot LA formulation study, patients received 30 mg intramuscular depot injections of LA microspheres every 16 weeks (112 days) until there was lack of clinical benefit (at the discretion of the investigator). Testosterone levels were assessed by standard radioimmunoassay. The lower limit of quantitation for the assay was 3.0 ng/dL. Sharifi et al25 previously reported the outcomes of the first 32 weeks of treatment using the 50 ng/dL cutoff.
In the 6-month formulation study, patients received two 45 mg intramuscular depot injections of LA microspheres 6 months (24 weeks) apart for a 48-week treatment period. Testosterone levels were assessed by liquid chromatography/mass spectrometry. The lower limit of quantitation for the assay was 2.5–3.0 ng/dL. Outcomes based on a threshold of 50 ng/dL and safety are reported elsewhere.21
In the original studies, the suppression rate was calculated using the testosterone castration level of ≤50 ng/dL and this is still the castrate level considered by the regulatory authorities.5 For this analysis, we used the more stringent cutoff level of 20 ng/dL. The number of patients who had testosterone levels ≤20 ng/dL at each assessment timepoint was determined and the proportion of the total calculated as a percentage. The patients from both studies were pooled at each common timepoint, so that the resulting numbers and percentages represent the total from both studies combined. Analysis of testosterone data was based on observed data. Data were summarized using SAS® Version 9.1 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Four patients were excluded from the 4-month depot LA formulation’s efficacy analysis because no pretreatment testosterone levels were available (two patients) or there was uncertainty of disease stage (two patients).25 A total of 29 out of 45 patients (64%) had had no previous treatment for prostate cancer. The remaining subjects had undergone transurethral prostatic resection, radical prostatectomy, radiation therapy, or a combination of these therapies.
Demographic details and baseline characteristics of the 45 and 148 patients evaluated for efficacy in the 4- and 6-month depot LA formulations, respectively, are shown in Table 1. These demographic and baseline details are taken from the original publications.21,25
  | Table 1 Patient demographics and baseline characteristics Abbreviations: NA, not available; SD, standard deviation. Notes: Data from a previous study.25 Adapted with permission from Prostate Cancer Prostatic Dis. Spitz et al. Efficacy and safety of leuprolide acetate 6-month depot for suppression of testosterone in patients with prostate cancer. 2012;15(1):93–99.21 |
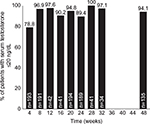
Both 4- and 6-month intramuscular depot injections suppressed serum testosterone below the 20 ng/dL threshold in the majority of patients. After 4 weeks of treatment, 152 out of 193 patients (78.8%) achieved serum testosterone levels of ≤20 ng/dL; at the 24-week timepoint, 169/189 (89.4%) of patients were similarly suppressed. At 48 weeks (6-month formulation only), 127/135 (94.1%) were suppressed ≤20 ng/dL (Figure 1).
Mean testosterone levels for the 45 evaluable patients on the 4-month LA depot formulation decreased from a baseline mean of 423.7 ng/dL (14.7 nmol/L) to 10.7 ng/dL (0.4 nmol/L) by week 6 and then stayed within the range of 9.7–21.0 ng/dL (0.3–0.7 nmol/L) through week 32.25 In the 6-month LA depot formulation, baseline mean testosterone was 434.6 ng/dL (15.1 nmol/L) and testosterone levels reached 15.9 ng/dL (0.6 nmol/L) by week 4 and remained ≤11.0 ng/dL (0.4 nmol/L) at the end of each treatment cycle until the end of the study.21
The safety profile of intramuscular LA depot formulations is well established from previous studies18–21 and from many years of prescription experience.16 The safety profile of intramuscular LA depot formulations has been reported previously.21,25
Discussion
Testosterone is known to promote the growth of prostate cancer cells and ADT is the standard treatment for males with advanced prostate cancer.7,27 Treatment with GnRH analogs results in reduction of serum testosterone levels to those observed after orchiectomy.27 Androgen deprivation can result in decreased mortality and can increase survival rates in males with advanced prostate cancer.7 In the current analysis, the use of the 4- and 6-month intramuscular microsphere depot LA formulations for ADT in patients with prostate cancer produced testosterone levels ≤20 ng/dL in the majority of patients under normal treatment conditions. Across the two studies, 89.4% of patients at 24 weeks on treatment achieved serum testosterone at or below the 20 ng/dL threshold. In the 6-month formulation study, 94.1% of patients were suppressed at week 48.
The standard castrate level has been defined for many years as <50 ng/dL; however, even lower testosterone castrate levels may be beneficial in reducing further the risk of mortality in prostate cancer patients on ADT.5,9,11–13 In a retrospective, hypothesis-generating analysis of 73 males with nonmetastatic prostate cancer on ADT, Morote et al12 demonstrated that survival free of castration-resistant cancer progression was greater in patients who did not have breakthrough testosterone levels >32 ng/dL (1.1 nmol/L). Mean survival-free of androgen independent progression was 137 months for those who had no breakthrough levels >32 ng/dL versus 88 months for those with breakthrough levels >32 ng/dL (P<0.03).
A retrospective analysis by Perachino et al13 of 129 metastatic prostate cancer patients who were treated with goserelin (another GnRH agonist) every 12 weeks suggested that there is a direct correlation between the risk of death and the levels of testosterone in males on ADT. They concluded that the higher the testosterone levels (range 17.5–50.5 ng/dL [0.6–1.8 nmol/L]) in these patients after 6 months of treatment, the greater the risk of death (hazard ratio 1.33, P<0.05). Bertaglia et al9 studied 153 patients receiving ADT and demonstrated that serum testosterone levels <30 ng/dL (1 nmol/L) were associated with a significantly lower risk of death (hazard ratio 0.45, P=0.034). In a secondary analysis of the Canadian PR-7 trial of intermittent versus continuous ADT, Klotz et al11 found that males undergoing continuous ADT whose first year nadir testosterone levels were consistently >0.7 nmol/L (>20 ng/dL) had significantly higher risks of dying from the disease compared with those whose nadir values levels were ≤20 ng/dL. For 0.7–1.7 nmol/L (20–50 ng/dL), the hazard ratio for death was 2.08 versus those whose levels were ≤0.7 nmol/L (≤20 ng/dL), and for those with >1.7 nmol/L (>50 ng/dL), the hazard ratio was 2.93, indicating higher mortality risk for this cohort.
Notwithstanding the aforementioned small trials, robust data clearly establishing the clinical value of achieving and maintaining a serum testosterone level of <20 ng/dL are lacking. The Bethesda Consensus Group, however, stated in 2011 that “a 20 ng/dL threshold for serum testosterone after ADT in patients with advanced prostate cancer was recommended.”28 At least one major prostate cancer clinical guideline (European Association of Urology guidelines on prostate cancer) has also recommended that the target testosterone threshold be lowered to <20 ng/dL (reduced from the previous standard of <50 ng/dL).5 Other guidelines, including the National Comprehensive Cancer Network3 and the American Society of Clinical Oncology,29 are inconsistent regarding the testosterone suppression goal. Additional confirmation regarding the clinical relevance of this lower testosterone threshold is warranted.
Conclusion
In this retrospective pooled analysis of two Phase III trials, intramuscular depot LA achieved and maintained mean serum testosterone levels at the ≤20 ng/dL castrate level in the vast majority (79%–94%) of patients through 48 weeks.
Acknowledgments
AbbVie sponsored this study and participated in the collection, analysis, and interpretation of the data, and in the writing, reviewing, and approval of the final paper. Medical writing and editorial support were provided by Robin Smith, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, NY, USA. Funding for this support was provided by AbbVie.
Disclosure
A Spitz and M Gittleman have been investigators on past AbbVie-sponsored LHRA clinical trials. L Karsh has been an investigator for AbbVie, and is currently an investigator for Astellas, Bayer, Dendreon, Ferring, FKD, Genome Dx, Genomic Health, Heat Biologics, Janssen, Lilly, MdX, Medivation, Pfizer, Spectrum, Takeda, and Tokai; a consultant for AbbVie, Astellas, Bayer, Dendreon, Janssen, Medivation, Spectrum, and Tolmar; and a speaker for Astellas, Bayer, Dendreon, Janssen, Medivation, and Spectrum. S Dragnic, D Gruca, AM Soliman, A Lele, and M Norton are employees of AbbVie and may hold AbbVie stock and/or options. The authors report no other conflicts of interest.
References
Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V; Panel Members; European Society for Medical Oncology. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 2013;24(5):1141–1162. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
NCCN guidelines version 2.2016 prostate cancer. 2016. Fort Washington, PA 19034, USA: National Comprehensive Cancer Network®. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 15, 2016. | ||
Horwich A, Parker C, de Reijke T, Kataja V; ESMO Guidelines Working Group. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi106–vi114. | ||
Mottet N, Bellmunt J, Briers E, et al. EAU Guidelines on prostate cancer. 2015. European Association of Urology. Available from: http://uroweb.org/wp-content/uploads/EAU-Guidelines-Prostate-Cancer-2015-v2.pdf. Accessed February 18, 2016. | ||
Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. | ||
Labrie F, Belanger A, Luu-The V, et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev. 2005;26(3):361–379. | ||
Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59(4):572–583. | ||
Bertaglia V, Tucci M, Fiori C, et al. Effects of serum testosterone levels after 6 months of androgen deprivation therapy on the outcome of patients with prostate cancer. Clin Genitourin Cancer. 2013;11(3):325–330.e321. | ||
Crawford ED, Moul JW, Sartor O, Shore ND. Extended release, 6-month formulations of leuprolide acetate for the treatment of advanced prostate cancer: achieving testosterone levels below 20 ng/dl. Expert Opin Drug Metab Toxicol. 2015;11(9):1465–1474. | ||
Klotz L, O’Callaghan C, Ding K, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015;33(10):1151–1156. | ||
Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178(4 Pt 1):1290–1295. | ||
Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2010;105(5):648–651. | ||
Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56(6):1021–1024. | ||
Persad R. Leuprorelin acetate in prostate cancer: a European update. Int J Clin Pract. 2002;56(5):389–396. | ||
AbbVie Inc. Lupron-Depot® (leuprolide acetate for depot suspension) [prescribing information]. North Chicago, IL: AbbVie, Inc; 2016. Available from: http://www.rxabbvie.com/pdf/lupronuro_pi.pdf. Accessed July 8, 2016. | ||
Tolmar Pharmaceuticals Inc. Eligard-leuprolide acetate [prescribing information]. Fort Collins, CO: Tolmar Pharmaceuticals, Inc; 2014. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b78d1919-9dee-44fa-90f9-e0a26d32481d. Accessed April 14, 2016. | ||
Sartor O, Dineen MK, Perez-Marreno R, Chu FM, Carron GJ, Tyler RC. An eight-month clinical study of LA-2575 30.0 mg: a new 4-month, subcutaneous delivery system for leuprolide acetate in the treatment of prostate cancer. Urology. 2003;62(2):319–323. | ||
Chu FM, Jayson M, Dineen MK, Perez R, Harkaway R, Tyler RC. A clinical study of 22.5 mg. La-2550: A new subcutaneous depot delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2002;168(3):1199–1203. | ||
Sharifi R, Soloway M. Clinical study of leuprolide depot formulation in the treatment of advanced prostate cancer. The Leuprolide Study Group. J Urol. 1990;143(1):68–71. | ||
Spitz A, Young JM, Larsen L, Mattia-Goldberg C, Donnelly J, Chwalisz K. Efficacy and safety of leuprolide acetate 6-month depot for suppression of testosterone in patients with prostate cancer. Prostate Cancer Prostatic Dis. 2012;15(1):93–99. | ||
Braeckman J, Michielsen D. Efficacy and tolerability of 1- and 3-month leuprorelin acetate depot formulations (Eligard®/Depo-Eligard®) for advanced prostate cancer in daily practice: a Belgian prospective non-interventional study. Arch Med Sci. 2014;10(3):477–483. | ||
Crawford ED, Sartor O, Chu F, Perez R, Karlin G, Garrett JS. A 12-month clinical study of LA-2585 (45.0 mg): a new 6-month subcutaneous delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol. 2006;175(2):533–536. | ||
Tunn UW. A 6-month depot formulation of leuprolide acetate is safe and effective in daily clinical practice: a non-interventional prospective study in 1273 patients. BMC Urol. 2011;11:15. | ||
Sharifi R, Knoll LD, Smith J, Kramolowsky E. Leuprolide acetate (30-mg depot every four months) in the treatment of advanced prostate cancer. Urology. 1998;51(2):271–276. | ||
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. | ||
Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355(9214):1491–1498. | ||
Djavan B, Eastham J, Gomella L, et al. Testosterone in prostate cancer: the Bethesda consensus. BJU Int. 2012;110(3):344–352. | ||
Resnick MJ, Lacchetti C, Penson DF. Prostate cancer survivorship care guidelines: American Society of Clinical Oncology practice guideline endorsement. J Oncol Pract. 2015;11(3):e445–e449. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.