Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Intraclass Difference in Pneumonia Risk with Fluticasone and Budesonide in COPD: A Systematic Review of Evidence from Direct-Comparison Studies
Authors Lodise TP, Li J, Gandhi HN, O’Brien G, Sethi S
Received 27 June 2020
Accepted for publication 23 September 2020
Published 11 November 2020 Volume 2020:15 Pages 2889—2900
DOI https://doi.org/10.2147/COPD.S269637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Thomas P Lodise, 1 Jingyi Li, 2 Hitesh N Gandhi, 3 Gerald O’Brien, 3 Sanjay Sethi 4
1Department of Pharmacy Practice, Albany College Pharmacy and Health Sciences, Albany, NY, USA; 2Global Medical Affairs, AstraZeneca, Gaithersburg, MD, USA; 3US Respiratory Medical, AstraZeneca, Wilmington, DE, USA; 4Department of Medicine, University of Buffalo Jacobs School of Medicine and Biomedical Sciences, Buffalo, NY, USA
Correspondence: Sanjay Sethi
Clinical and Translational Research Center, 875 Ellicott St., Room 6045A, Buffalo, NY 14215, USA
Email [email protected]
Background: Inhaled corticosteroids (ICS) are widely used and recommended to treat chronic obstructive pulmonary disease (COPD). While generally considered safe, several studies demonstrated an increased risk of pneumonia with the use of ICS in COPD patients. Although all ICS indicated for COPD carry the class labeling warning of increased pneumonia risk, evidence suggests an intraclass difference in the risk of pneumonia between inhaled budesonide and fluticasone. To date, systematic reviews of direct-comparison studies have not been performed to assess if an intraclass difference exists.
Research Question: This review investigated whether there is an intraclass difference in risk of pneumonia between inhaled fluticasone and budesonide, the 2 most commonly used ICS in COPD.
Study Design and Methods: A search of the medical literature was conducted in PubMed and Embase for the time period of 01/01/69– 05/31/19. The search strategy combined terms that defined the patient/disease type, exposures, outcome, and the study/publication type. Descriptive and comparative statistics reported for fluticasone- and budesonide-containing products in each study, including data for pneumonia event subgroups, were extracted and reported by dose, seriousness, or practice setting. Controlled clinical trials and observational studies meeting the inclusion criteria were assessed for methodologic quality by using the appropriate tool from the list of study quality assessment tools developed by the National Institutes of Health.
Results: The summary relative risk (RR) ratio across 5 included studies (57,199 patients) was 1.13 (95% CI: 1.09– 1.19), representing a 13.5% increased risk of pneumonia among fluticasone users compared to budesonide users. Similarly, summary RR ratio for serious pneumonia implied a 14.4% increased risk of serious pneumonia among fluticasone users compared to budesonide users (pooled RR: 1.14; 95% CI: 1.09– 1.20).
Interpretation: There is likely a clinically important intraclass difference in the risk of pneumonia between fluticasone- and budesonide-containing inhaled medications in COPD.
Keywords: inhaled corticosteroids, pneumonia, COPD
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
The 2 most commonly used inhaled corticosteroids (ICS) in chronic obstructive pulmonary disease (COPD) are fluticasone and budesonide,1 and it is recommended to use them in combination with a long-acting β2-agonist ± a long-acting antimuscarinic antagonist.2 Although ICS can reduce exacerbations in appropriately selected patients with COPD, several studies have demonstrated an increase in the risk of pneumonia with the use of ICS in these patients.3–8 This ICS-induced increase in pneumonia risk is superimposed on the 16-fold increase in pneumonia risk that has been associated with COPD itself in the first year following diagnosis.9 Compared with patients without COPD, outcomes of pneumonia in patients with comorbid COPD include greater severity of pneumonia, more frequent hospitalizations, and increased mortality.10
Although all ICS inhalers indicated for COPD carry the warning of increased risk of pneumonia, evidence suggests that there may be an intraclass difference in the risk of pneumonia between inhaled budesonide and fluticasone.11–15 If such a difference exists, it has significant implications for clinical practice, both at the initial decision to prescribe an ICS and when pneumonia occurs in a patient with COPD who is taking an ICS. A recent randomized controlled trial (RCT) and several well-constructed observational cohort studies provide a direct comparison of pneumonia incidence between budesonide and fluticasone.14,16–20 By conducting a systematic review and analysis of these studies, our objective is to determine the totality of the evidence for differences in drug-specific risk for pneumonia. We excluded the indirect comparison of pneumonia rates in clinical trials and observational studies in which only 1 of these drugs was included because of potential confounding by differences in study populations among these studies.
Methods
Literature Search
A search of the medical literature was conducted in PubMed and Embase for the time period of 01/01/69–05/31/19. The search strategy combined terms that defined the patient/disease type, exposures, outcome, and the study/publication type described in Supplement 1. Studies with fewer than 15 subjects in any treatment arm, crossover studies, case reports or case series, and articles not written in English were excluded. Unpublished non–peer-reviewed materials, such as posters, media presentations, or abstracts from conference proceedings, were screened for any relevant data and were only included if there was a subsequent peer-reviewed publication.
Study Selection
Table 1 summarizes the inclusion and exclusion criteria for the selection of studies included in this review. Observational studies and clinical trials considered for review were required to include patients with COPD who received either fluticasone or budesonide for inhalation. Acceptable methods of identification of COPD in observational studies included medical record documentation or medical coding for diagnosis upon admission, discharge, or billing. For our purposes, any generally accepted guideline definition of COPD (ie, American Thoracic Society-European Respiratory Society [ATS/ERS 2004], Global Initiative for Chronic Obstructive Lung Disease [GOLD] 2018 report, or equivalent) was accepted among the published clinical trials. All levels of COPD severity were included. Included studies must have reported pneumonia outcomes. Studies that did not separately report outcomes data for budesonide and fluticasone were excluded. De-duplicated abstracts were screened to identify publications with the critical components of a COPD population, budesonide and fluticasone treatment, and pneumonia as a safety outcome in clinical trials or as the primary or secondary outcome in observational studies.
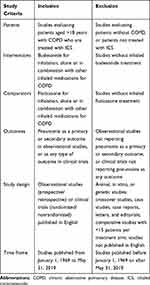 |
Table 1 Inclusion/Exclusion Criteria for Study Selection |
Data Extraction
Extracted information was collected into a data form in Supplement 2. These data included author, publication year, study design, location and database name, inclusion and exclusion criteria, definition of COPD used, type and dose of budesonide and fluticasone included, and measures of outcomes (eg, pneumonia rates and ratios).
Quality Assessment
Controlled clinical trials and observational studies that met the inclusion criteria were assessed for methodologic quality using the appropriate tool from the list of study quality assessment tools developed by the National Institutes of Health.21 Studies determined to be of low quality were excluded from the analysis according to protocol (Supplement 3).
Outcomes
The outcome of pneumonia was defined by the investigators of each study by a clinical diagnosis (chest x-ray, bronchoscopy, blood/sputum culture and specimen), physician diagnosis documented in a medical record, or by medical coding, which typically used International Classification of Diseases codes. Pneumonia severity was defined by the study authors. If severity was not noted within the publication, we applied our own criteria for severity that included hospitalization, a visit to an emergency department, mechanical ventilation, or if it resulted in death.
Statistical Analysis
All descriptive and comparative statistics reported for fluticasone- and budesonide-containing products in each study, including data for pneumonia event subgroups, were extracted and reported by dose, seriousness, or practice setting. These data included events; event proportions or rates; and statistical comparisons reported as ratios (relative risk [RR], hazard ratios [HRs], or odds ratios [ORs]) as reported by the clinical trial, cohort, or case-control studies. If adjusted risk or ORs were not reported, then crude ratios were calculated if sufficient data were reported. The number needed to harm (NNH) was calculated in clinical trials from the difference in reported pneumonia proportions. In cohort studies that reported time-varying event rates per person-year of exposure, the NNH was estimated using the method described by Suissa (2013),22 which converted recurring, event-based number needed to treat calculations with unequal follow-up time to a patient-based number needed to treat.
A summary RR ratio was calculated for the prospective studies comparing the pneumonia risk with fluticasone to the risk with budesonide. Results from the intent-to-treat cohort of RCTs were pooled with results from the observational cohort studies to form a summary RR ratio for pneumonia events between fluticasone and budesonide. Pooled-effect estimates were obtained by a fixed-effects model (Mantel-Haenszel) and a random-effects model (DerSimonian and Laird) using the Metafor package (R version 3.6.3). A random-effects model was to be used if differences in the underlying study populations, methods, and treatment regimens were considered heterogeneous (eTables 1 and 2). A second summary RR ratio was calculated that was limited to outcomes of serious pneumonia defined by events that resulted in hospitalization, emergency department visits, or death (eTables 3 and 4).
Statement of IRB Approval
IRB approval was not necessary for this study because it was a systematic review.
Results
Of the 519 articles identified from the initial database search, 445 were excluded. Of these excluded articles, 92 abstracts were reconfirmed by the reviewer to lack pneumonia data. Of the remaining 74 studies retrieved, 1 RCT14 and 5 observational cohort studies16–20 met the eligibility criteria and were included in the systematic review (Figure 1). Characteristics of the included studies are reported in Table 2, and all included studies were considered to be of fair to good quality. The FULFIL trial14 was the only RCT that directly compared fluticasone and budesonide and reported pneumonia outcomes.
 |
Table 2 Characteristics of Head-to-Head Studies of Fluticasone and Budesonide in COPD |
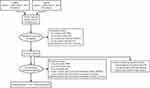 |
Figure 1 Study selection diagram. |
RCT and Observational Cohort Studies
In the FULFIL study, Lipson et al compared the efficacy and safety of fluticasone furoate 100 μg, umeclidinium 62.5 μg, and vilanterol 25 μg administered once-daily in a fixed dose inhaler (n=911) against a combination inhaler of budesonide 400 μg and formoterol 12 μg twice daily (n=899) for 24 weeks. In the intent-to-treat population through Week 24, the overall incidence of pneumonia was 2.2% for fluticasone users versus 0.8% for budesonide users (crude ratio 2.75). The NNH for the absolute difference in pneumonia events was 71 patients treated for 24 weeks with the fluticasone/umeclidinium/vilanterol combination compared with the budesonide/formoterol combination (95% confidence interval [CI], 40–328 patients). The incidence of on-treatment serious pneumonia in the intent-to-treat population at 24 weeks was 1.0% in fluticasone combination users and 0.3% in budesonide combination users (crude ratio 3.33). The calculated NNH for serious pneumonia, based on this study result, is 143 patients (95% CI, 60–1417 patients).
Using linked primary care record data in Sweden from the period of 1999–2009, the retrospective cohort study by Janson et al matched fluticasone/salmeterol users to budesonide/formoterol users (n=2734 each) by multiple criteria, including pneumonia and COPD exacerbation history.17 In this analysis, a higher pneumonia risk was reported with fluticasone combination use compared with budesonide combination therapies.17 The reported adjusted RR for fluticasone users compared with budesonide users was 1.73 (95% CI, 1.57–1.90) for a pneumonia diagnosis, 1.74 (95% CI, 1.56–1.94) for a pneumonia-related hospital admission, 1.56 (95% CI, 1.39–1.75) for a diagnosis of pneumonia in primary care, and 1.75 (95% CI, 1.53–2.00) for a diagnosis of pneumonia in hospital outpatient care. The calculated NNH for a pneumonia diagnosis was estimated to be 22 patients treated with fluticasone combination to yield 1 additional patient with pneumonia compared with budesonide.
Yang et al (2017)16 used the Taiwan National Health Insurance Research Database to compare the incidence of pneumonia in matched populations of 7295 patients per cohort who were users of a fixed 2-drug combination of fluticasone or of budesonide for the study period of 1997–2010. The adjusted risk of serious pneumonia was higher in patients using fluticasone combination inhalers compared with those using budesonide (adjusted HR, 1.13; 95% CI, 1.08–1.20). The calculated NNH for a pneumonia diagnosis was estimated to be 69 patients treated with fluticasone combination compared with budesonide. Likewise, the risk of pneumonia requiring mechanical ventilation was higher for fluticasone versus budesonide (adjusted HR, 1.14; 95% CI, 1.05–1.24).
Kern et al (2015)19 analyzed pneumonia as a secondary outcome using the HealthCore Integrated Research Environment (HIRE) managed care database. Matched COPD patients were followed for 12 months. Unlike the other observational cohort studies, the authors used logistic regression models to calculate adjusted ORs rather than RRs. The proportion of patients diagnosed with pneumonia during the 12 months following the initiation of therapy was not statistically different between the 2 treatment groups (budesonide, 17.3%; fluticasone, 19.0%; adjusted OR, 0.92; 95% CI, 0.81–1.04; p=0.19). Pneumonia-related hospitalizations occurred in 8.9% of budesonide patients versus 10.3% of the fluticasone group (adjusted OR, 0.87; 95% CI, 0.75–1.02; p=0.09), respective proportions with pneumonia-related emergency department visits were 1.0% versus 1.3% (adjusted OR, 0.80; 95% CI, 0.51–1.23; p=0.31), and pneumonia-related outpatient/office visits were 12.0% versus 12.6% (adjusted OR, 0.97; 95% CI, 0.84–1.12; p=0.64).
Roberts et al (2011)18 used the PharMetrics Integrated Database of medical claims to examine pneumonia-related hospital, emergency department, and office visit utilization in COPD patients who initiated fluticasone and budesonide combination products. No statistical difference was reported in pneumonia-related hospitalizations in budesonide versus fluticasone users (1.8% vs 1.9%, respectively; p=0.652). The difference in the number of office visits for pneumonia trended toward significance, with higher proportions among fluticasone than budesonide users (3.6% versus 2.7%, respectively; p=0.052). Emergency department utilization was equivalent (0.2% each; p=1.000).
Hirano et al (2018)20 compared the incidence of pneumonia among patients using fluticasone, budesonide, and “Other” ICS treatments alone, and in combination with a long-acting β2-agonist. Neither fluticasone nor budesonide treatment were risk factors for pneumonia in this study, either alone or in combination. However, this study was much smaller than the studies discussed above. The 3-year incidence of pneumonia among the 193 fluticasone/salmeterol users was 5.7% compared to 0.0% among 30 budesonide/formoterol users (p=0.1961).
Pooled Summary of Pneumonia Risk
A summary and plot of the comparisons between fluticasone and budesonide for the clinical trial and observational cohort studies are presented in Figure 2. Roberts et al (2011)18 was excluded from the “Any Pneumonia” outcome in Figure 2 because the data for all pneumonias combined were not reported and could not be derived, but data were included for “Serious Pneumonia,” as defined by pneumonia-related hospitalization.
 |
Figure 2 Risk of pneumonia associated with fluticasone and budesonide in head-to-head studies. |
There is low non-significant heterogeneity (I2=36.05%; Q statistic=6.25, p=0.18) among 1 RCT14 and 4 observational cohort studies16,17,19,20 included for “any pneumonia.” The summary RR across these studies using a fixed-effects model was 1.13 (95% CI, 1.09–1.19), representing a 13.5% increased risk of pneumonia among fluticasone users compared to budesonide users (Table 3). Pooled analysis of only serious pneumonia events, reported in 5 studies,14,16,17 showed low non-significant heterogeneity (I2=0.00%; Q statistic=3.903, p=0.419) and yielded an increased risk with fluticasone of 14.4% with a fixed-effects model (RR, 1.14; 95% CI, 1.09–1.20; Table 4).
 |
Table 3 Any Pneumonia – Fixed-Effects Model |
 |
Table 4 Serious Pneumonia – Fixed-Effects Model |
Discussion
This systematic review based on direct-comparison studies suggests an intraclass difference between budesonide and fluticasone for pneumonia risk in patients with COPD. Overall, there was an estimated 13.5% higher risk of any pneumonia among patients with COPD treated with fluticasone compared to budesonide. Although differences in follow-up period across studies prevented estimation of an overall NNH, the NNH across the studies ranged from 22–71 patients treated with fluticasone, yielding 1 additional patient with pneumonia compared with budesonide. The risk of serious pneumonia events was 14.4% higher among patients with COPD treated with fluticasone compared to budesonide.23–26 Collectively, these findings have important clinical implications as annual incidence of pneumonia is 10 times greater in COPD patients than in the general population,27 and this risk is further doubled in COPD patients taking an ICS.28 An estimated 250 million patients worldwide have COPD and 40%–50% of these patients are taking ICS; our findings therefore suggest that the use of fluticasone over budesonide could yield at least 3.5 million additional pneumonia cases. ICS choice on initiation of therapy and substitution of one ICS for another could be important strategies to mitigate the risk of pneumonia and associated complications in COPD.23–26
The findings from this study are consistent with the larger body of literature. The meta-analysis by Yang (2019) is one of the most comprehensive analyses on the topic of pneumonia associated with ICS. It included 25 RCTs comprising approximately 5000 patients using fluticasone furoate or fluticasone propionate (FP) from 1999 to 2019.15 They found that in 12 studies with fluticasone, the risk of pneumonia was 84% greater than with placebo (RR, 1.84; 95% CI, 1.47–2.30). However, in 11 budesonide studies, the risk differential was not significant (RR, 1.23; 95% CI, 0.95–1.54). The authors also examined the risk by dose and found an increased risk at all levels for fluticasone, but none at low and medium doses of budesonide treatment (there were no data for high-dose budesonide). However, that analysis did not include data from the FULFIL or UPLIFT trials. In a retrospective analysis of the UPLIFT study (4-year, double-blind, parallel-group study in COPD patients with moderate-to-severe airflow limitation who were randomized to either placebo or tiotropium), patients were divided into 3 groups based on their medications at entry: no ICS (n=2292), FP (n=1981), and other ICS (n=1719). The risk of pneumonia was 38% higher in fluticasone users than in the no-ICS group (RR, 1.38; 95% CI, 1.20–1.58; p<0.001). The pneumonia event rate was also higher in the fluticasone group versus the other ICS group (0.077 vs 0.058, respectively; p<0.001). In addition to the clinical literature, large population-based nested case-control studies conducted in Canada1 and Taiwan29 reported that fluticasone is associated with significantly higher risk of pneumonia, whereas the risk with budesonide is comparatively much lower among patients with COPD. Notably, Suissa et al (2013) established a dose-response effect that the risk of serious pneumonia increases with larger daily dose of inhaled fluticasone, but not for budesonide, based on data from the Régie de l’assurance maladie du Québec database that includes ≥160,000 patients with COPD followed for up to 18 years.30 More interestingly, using the same database, Suissa reported a significant reduction in pneumonia risk among patients where fluticasone was discontinued (ie, a positive “de-challenge”; RR, 0.58; 95% CI, 0.54–0.61) but less so with budesonide (RR, 0.87; 95% CI, 0.78–0.97).30 The dose-response effect and potential positive de-challenge between fluticasone and the higher risk of pneumonia, along with consistent findings across observational cohort and nested case-control studies, suggest the associations observed in these studies might be causal, confirming the evidence from clinical studies and the FULFIL trial.
One potential explanation for the observed difference in the pneumonia risk between fluticasone and budesonide is the more potent and sustained immunosuppressive effect of fluticasone versus budesonide in airway tissue, likely due to differences in the availability of the drugs to that tissue.31 FP molecules persist in the airway lining fluid and are slowly absorbed into airway tissue, whereas budesonide is quickly absorbed, thus reducing the duration of the local immunosuppressive effect.32 Fluticasone also has a fluorine moiety in its molecular structure that differentiates it from budesonide and makes it chemically more lipid soluble.33 The relatively higher lipophilicity and slower dissolution rate allow fluticasone to persist in local lung tissue longer than budesonide.32 Another potential mechanism is the differential effects of these ICS on macrophage receptor expression. Provost (2019)34 reported that budesonide prevented reduction in bacterial recognition receptor expression by both Nontypeable Haemophilus influenzae and Streptococcus pneumoniae, whereas fluticasone was only able to prevent these reductions with the pneumococcus. The greater pharmacologic potency of fluticasone may also play a role in pneumonia risk. Janson et al (2017)35 noted that FP might be 10–100 times more potent than budesonide in its anti-inflammatory and immunosuppressive activity when assessed in vitro. This preclinical evidence is corroborated by observations of a greater association between non-tuberculous mycobacterial pulmonary disease and fluticasone than budesonide from 2 population-based case-control studies conducted in Denmark and Canada.36,37
Several things should be noted when interpreting the findings from this analysis. The summary pooled risk estimate is subject to limitations that require careful consideration. These data were pooled from 1 RCT and 5 observational cohort studies that used real-world data. Because they are not RCTs, the observational studies are inherently limited by the available data that could result in biases due to unmeasured confounders and misclassification of exposures and outcomes. However, such biases should equally affect fluticasone and budesonide unless practitioners are aware of an intraclass difference over time.
In addition, the studies by Kern et al (2015)19 and Hirano et al (2018)20 reported pneumonia as patient proportions, whereas Janson et al (2013)38 and Yang et al (2017)16 reported pneumonia as events per person-time. A patient with pneumonia can only be counted once, but in the latter 2 studies, multiple pneumonia events were counted if they recurred in the same patient. Unit transformations were not made in these calculations because data were inadequately reported in the publications. Janson also normalized rates to events per person-year without reporting the total number of events and patient-years for the fluticasone and budesonide cohorts. Yang reported total events and total person-years and thus carried the greatest weight when entered into the model. Unlike RCTs, observational studies with aggregated real-world data are subject to wide variances in individual patient exposure to an ICS. Thus, 100 patient-years of exposure is not equivalent to 100 patients treated for 1 year in an RCT. Each of these variations can serve to distort the standard error, variance, and weight calculations that form the fixed/random-effects model and pooled rate estimate. Therefore, the summary risk estimate should only be used to inform the overall risk of pneumonia at a population level.
Conclusions
The evidence from this systematic review of direct comparisons of fluticasone and budesonide, in addition to evidence from pre-clinical, clinical, and observational literature that we have discussed, provides a totality of evidence that the risk of pneumonia in COPD patients is higher with fluticasone than budesonide. In addition, in several of the analyses, the risk of pneumonia associated with budesonide was seen numerically, but was not statistically different from placebo. Differences in the pharmacologic, pharmacodynamic, and pharmacokinetic profiles of fluticasone and budesonide support the biological plausibility of the differences in the risk of pneumonia between these drugs. The differential in the risk of pneumonia for these 2 treatments has been consistently reported across different study designs and definitions of pneumonia. The lower apparent likelihood of developing pneumonia with budesonide should play a role in our decision-making regarding the choice of ICS-containing therapies in COPD.
Abbreviations
aOR, adjusted odds ratio; aRR, adjusted risk ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; d/c, discontinued use; EMR, electronic medical record; FEV1, forced expiratory volume in 1 second; FL, fluticasone; FP, fluticasone propionate; HIR, HealthCore Integrated Research Environment; HR, hazard ratio; ICD, International Classification of Diseases; ICS, inhaled corticosteroids; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting antimuscarinic antagonist; LTRA, leukotriene receptor antagonist; OR, odds ratio; RCT, randomized controlled trial; RR, relative risk; SABA, short-acting β2-agonist; SD, standard deviation.
Acknowledgments
Editorial support was provided by Shane Walton, PhD, of MedErgy (Yardley, PA), which was in accordance with Good Publication Practice (GPP3) and funded by AstraZeneca (Wilmington, DE).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by AstraZeneca (Wilmington, DE, USA). AstraZeneca was involved in the study design, collection, analysis, and interpretation of data and the development and review of the manuscript. The decision to submit the manuscript for publication was made by the authors.
Disclosure
Jingyi Li and Hitesh Gandhi are employees of AstraZeneca. Gerald O’Brien was an employee of AstraZeneca at the time of this study; he is now affiliated with Liquidia Technologies, Morrisville, NC. Thomas P Lodise is a consultant for AstraZeneca. Dr. Sethi has received research funding (to Jacobs School of Medicine, University at Buffalo, State University of New York) from Cipla, Sanofi, and GlaxoSmithKline; has been a consultant and/or participated in advisory boards for AstraZeneca, Boehringer Ingelheim, Circassia, Gilead, GlaxoSmithKline, Merck, Nabriva, Novavax, Paratek, Sunovion, and Theravance; has been a consultant for Aradigm and Pulmonx; and has been a speaker for AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. He has received royalties from UpToDate and Taylor and Francis. The authors report no other conflicts of interest in this work.
References
1. Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029–1036. doi:10.1136/thoraxjnl-2012-202872
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report). 2020.
3. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
4. Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106(2):257–268. doi:10.1016/j.rmed.2011.07.020
5. Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176(2):162–166. doi:10.1164/rccm.200611-1630OC
6. Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647. doi:10.1183/09031936.00193908
7. Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(2):144–149. doi:10.1164/rccm.200602-244OC
8. Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6(5):320–329. doi:10.1080/15412550903140881
9. Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. doi:10.1378/chest.128.4.2099
10. Restrepo MI, Sibila O, Anzueto A. Pneumonia in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul). 2018;81(3):187–197. doi:10.4046/trd.2018.0030
11. Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi:10.1164/rccm.200707-973OC
12. Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–565. doi:10.2165/00003495-200969050-00004
13. Tashkin DP, Rennard SI, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs. 2008;68(14):1975–2000. doi:10.2165/00003495-200868140-00004
14. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
15. Yang M, Du Y, Chen H, Jiang D, Xu Z. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019;77:105950. doi:10.1016/j.intimp.2019.105950
16. Yang HH, Lai CC, Wang YH, et al. Severe exacerbation and pneumonia in COPD patients treated with fixed combinations of inhaled corticosteroid and long-acting beta2 agonist. Int J Chron Obstruct Pulmon Dis. 2017;12:2477–2485. doi:10.2147/COPD.S139035
17. Janson C, Larsson K, Lisspers KH, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ. 2013;346:f3306. doi:10.1136/bmj.f3306
18. Roberts M, Mapel D, Petersen H, Blanchette C, Ramachandran S. Comparative effectiveness of budesonide/formoterol and fluticasone/salmeterol for COPD management. J Med Econ. 2011;14(6):769–776. doi:10.3111/13696998.2011.622817
19. Kern DM, Davis J, Williams SA, et al. Comparative effectiveness of budesonide/formoterol combination and fluticasone/salmeterol combination among chronic obstructive pulmonary disease patients new to controller treatment: a US administrative claims database study. Respir Res. 2015;16:52. doi:10.1186/s12931-015-0210-x
20. Hirano R, Fujita M, Matsumoto T, On R, Watanabe K. Inhaled corticosteroids might not increase the risk of pneumonia in patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis. 2018;13:3503–3509. doi:10.2147/COPD.S180349
21. APhA. Focus on COPD: Pharmacists Helping Patients Improve Outcomes and Reduce Readmissions. 2017. Available from: https://www.pharmacist.com/focus-copd. Accessed October 7, 2020.
22. Suissa S. Number needed to treat in COPD: exacerbations versus pneumonias. Thorax. 2013;68(6):540–543. doi:10.1136/thoraxjnl-2012-202709
23. World Health Organization. Burden of COPD. Available from: https://www.who.int/respiratory/copd/burden/en/. Accessed April 15, 2019.
24. World Health Organization. Chronic obstructive pulmonary disease (COPD). 2017. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
25. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340(25):1941–1947. doi:10.1056/NEJM199906243402502
26. Van Andel AE, Reisner C, Menjoge SS, Witek TJ. Analysis of inhaled corticosteroid and oral theophylline use among patients with stable COPD from 1987 to 1995. Chest. 1999;115(3):703–707. doi:10.1378/chest.115.3.703
27. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806–1812. doi:10.1093/cid/cix647
28. Festic E, Scanlon PD. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am J Respir Crit Care Med. 2015;191(2):141–148. doi:10.1164/rccm.201409-1654PP
29. Wang CY, Lai CC, Yang WC, et al. The association between inhaled corticosteroid and pneumonia in COPD patients: the improvement of patients’ life quality with COPD in Taiwan (IMPACT) study. Int J Chron Obstruct Pulmon Dis. 2016;11:2775–2783.
30. Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177–1183. doi:10.1378/chest.15-0627
31. Maassen van den Brink KI, Boorsma M, Staal-van den Brekel AJ, Edsbäcker S, Wouters EF, Thorsson L. Evidence of the in vivo esterification of budesonide in human airways. Br J Clin Pharmacol. 2008;66(1):27–35. doi:10.1111/j.1365-2125.2008.03164.x
32. Dalby C, Polanowski T, Larsson T, Borgstrom L, Edsbacker S, Harrison TW. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10:104. doi:10.1186/1465-9921-10-104
33. Tashkin DP, Strange C. Inhaled corticosteroids for chronic obstructive pulmonary disease: what is their role in therapy? Int J Chron Obstruct Pulmon Dis. 2018;13:2587–2601. doi:10.2147/COPD.S172240
34. Provost KA, Smith M, Miller-Larsson A, Gudleski GD, Sethi S. Bacterial regulation of macrophage bacterial recognition receptors in COPD are differentially modified by budesonide and fluticasone propionate. PLoS One. 2019;14(1):e0207675. doi:10.1371/journal.pone.0207675
35. Janson C, Stratelis G, Miller-Larsson A, Harrison TW, Larsson K. Scientific rationale for the possible inhaled corticosteroid intraclass difference in the risk of pneumonia in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3055–3064. doi:10.2147/COPD.S143656
36. Andrejak C, Nielsen R, Thomsen VO, Duhaut P, Sorensen HT, Thomsen RW. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–262. doi:10.1136/thoraxjnl-2012-201772
37. Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J. 2017;50(3):1700037. doi:10.1183/13993003.00037-2017
38. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584–594. doi:10.1111/joim.12067
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
