Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Intestinal Parasitosis and its Association with CD4+ T Cell Count and Viral Load among People Living with HIV in Parasite Endemic Settings of Northwest Ethiopia
Authors Dereb E, Negash M , Teklu T , Damtie D , Abere A , Kebede F, Ewnetu Y, Kasa E
Received 13 August 2021
Accepted for publication 12 November 2021
Published 15 December 2021 Volume 2021:13 Pages 1055—1065
DOI https://doi.org/10.2147/HIV.S328269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Eseye Dereb,1 Markos Negash,2 Takele Teklu,2,3 Debasu Damtie,2,4 Aberham Abere,5 Firehiwot Kebede,1 Yalemwork Ewnetu,1 Eyuel Kasa1
1University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia; 2Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Medical Laboratory Sciences, College of Health Sciences and Medicine, Wolaita Sodo University, Wolaita, Ethiopia; 4The Ohio State University Global One Health LLC, Eastern Africa Regional Office, Addis Ababa, Ethiopia; 5Department of Medical Parasitology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Takele Teklu Tel +251 911-806643
Fax +251 46-5514417
Email [email protected]
Purpose: To study intestinal parasitosis and its association with viral load and CD4+ T cell count in HIV-infected individuals at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia.
Methods: A cross-sectional study was conducted from March to June 2019. Three hundred and sixteen study participants were selected using systematic random sampling technique. Sociodemographic and clinical data were collected using structured questionnaire. Stool samples were collected and examined using direct saline, formol ether concentration technique and modified acid fast staining. CD4+ T cell counts and viral load were determined by fluorescence-activated cell sorting (BD FACS) and COBAS Ampliprep/COBAS TaqMan HI2CAP assay, respectively. Data were entered into Epi Data 3.1 and transferred to SPSS version 20 software for analysis. Bivariable and multivariable analyses were performed using a binary logistic regression model. P values of less than 0.05 were considered statistically significant.
Results: The overall prevalence of intestinal parasitosis was 24.7% (78/316). The most commonly detected parasite was Cryptosporidium species with 5.4% (17/316), followed by Ascaris lumbricoides with 5.1% (16/316). There was a significant association with low CD4+ T cell count (AOR: 3.207; 95% CI: 1.237, 8.317), high viral load (AOR: 2.933; 95% CI: 1.326, 6.489), individuals aged 31– 40 years (AOR: 0.305; 95% CI: 0.124, 0.751) and individuals aged 41– 50 years (AOR: 0.261; 95% CI: 0.101, 0.671).
Conclusion: In this study, prevalence of intestinal parasitic infections was high and was associated with low CD4+ T cell count and high viral load. Therefore, screening of HIV patients, especially those with low CD4+ T-cell count and high viral load, particularly for opportunistic intestinal parasitic infections would be of utmost importance in the efforts to prevent and control opportunistic infections in HIV patients.
Keywords: intestinal parasitosis, HIV, CD4+, viral load, Northwest Ethiopia
Introduction
Intestinal parasitosis (IPs) is a global problem occurring in varying degrees of prevalence in different parts of the world. The problem is disproportionally high in developing countries where there is low socio-economic status, and poor hygienic and sanitation practices.1 From the estimated one-third global population infected by intestinal parasitic infections; the majority live in tropical and sub-tropical parts of the world.2,3 These infections are endemic in many African countries including Ethiopia.4 There seems to be a geographically overlapping high distribution of IPs and Human Immunodeficiency Virus (HIV) infection in sub-Saharan Africa where 71% of people living with HIV (PLWH) live and 65% of new infections and 75% of deaths occurred in 2017.5 This geographic overlap may favor the high IPs with HIV co-infection in the region as reported by several studies.
Parasitic infections could disturb the balance of anti-HIV immune responses and contribute to HIV replication,6,7 which in turn could accelerate the disease progress to AIDS.8,9 The immune suppression caused by an HIV infection predisposes to various microbial and parasitic infection including certain opportunistic parasitic infections.10–12 The CD4+ T cell count has been used as an important predictor of the level of immunosuppression and for decision of antiretroviral therapy (ART) initiation.13 In 2015, the World Health Organization (WHO) recommended a test and treat strategy in which ART is initiated for all HIV positive patients regardless of their CD4+ T cell count status and clinical conditions.14 The CD4+ T cell count supported with viral load is an important determinant of immune status and treatment outcome in HIV-infected individuals. Several studies have shown that a low CD4+ T cell count of 200 cells/µ1 and a high viral load are associated with infection with opportunistic intestinal parasites, leading to rapid disease progression to ADIS.15–17
Several studies in Ethiopia have reported variable level of IPs/HIV co-infection. A study conducted in Hawassa city, Ethiopia revealed an overall prevalence of 35.8% intestinal parasitic infection with a 25.5% and 10.3% rate of single and multiple infection.18 A very recent study conducted at Debre Tabor General Hospital in Ethiopia reported a 25.3% prevalence of IPs among HIV infected patients.19 From opportunistic parasitic infections Cryptosporidium parvum, Isospora belli, and Microsporidium were commonly identified parasites while Ascaris lumbricoides, Trichuris trichiura, Entamoeba histolytica/dispar, and Giardia Lamblia were the most identified pathogenic parasites in HIV patients.18–20
Study reports revealed strong association of CD4+ T cell count with intestinal parasitic infections, especially opportunistic intestinal parasitic infection in HIV positive patients.21,22 Moreover, several studies reported higher prevalence of IPs among pre-ART HIV positive patients compared to on ART patients as the highly active anti-retroviral therapy (HART) partially restores the immune function of the patients.15,23–25 Studies in Ethiopia so far have reported the prevalence and associations of intestinal parasitosis with ART status and to some-extent with CD4+ T cell counts while the association of IPs with the viral load in HIV patients remains limited particularly in our study area. Hence, this study was intended to assess the association of IPs with viral load and CD4+ T cell count in HIV positive patients as these two markers are important predictors of disease progression and treatment response.
Materials and Methods
Study Setting
An institutional-based cross-sectional study was conducted from March to May 2019 at the University of Gondar Comprehensive Specialized Hospital ART clinic and VCT center, which is in Gondar town, Amhara National Regional State, Ethiopia. The total population of Gondar is estimated to be 360,600.26 The hospital provides health, research, and community services for more than 5 million residents in the town and inhabitants in the neighboring zones. Presently, the University of Gondar Comprehensive Specialized Hospital provides a service for 5584 ART patients at the ART clinic. In the hospital, all confirmed HIV positives are eligible for ART regardless of their CD4+ count, viral load and clinical conditions.14 At the baseline, patients shall be thoroughly evaluated and, for the rest of their lives, periodically monitored for toxicity, intolerance, response or failure to treatment will be monitored. Starting from the first follow-up clinical assessment will be continued and every six months HIV viral load (at 6 months and 12 months after initiating ART and every 12 months thereafter) and CD4+ cell count if conducted.
Sample Size Determination and Sampling Techniques
The sample size for the study was estimated by taking the prevalence of 29.1% from the previous study on parasite HIV co-infection16 with a confidence interval of 95% and a margin of error of 5% using a single population proportion formula. The minimum sample size required for the study was 316. The approximate number of participants who were expected to attend the ART service during the three months of the study period was determined by reviewing patient flows for the previous four months. The number of patients was proportionally allocated to each day according to the total number of clients to be served during the study period. Study participants were selected by systematic random sampling at every k intervals from the daily follow-up list of HIV patients coming to ART clinic per day. The k value was calculated as k = N/n, 1396/316 = 4. Study participants were selected every four intervals until the sample size was saturated.
Operational Definitions
Pre-ART: Newly HIV positive individuals who are screened on VCT center and ready to start ART.
ART patients: The person who was found to be HIV positive and started ART.27
Viral load: The number of copies of HIV in one milliliter of blood (copies/mL) (high viral load: greater than 1000 copies/mL).28
ART Adherence: The extent to which the client’s/patient’s behavior is consistent with the prescribed health program as agreed upon through a shared decision-making process between the client/patient and the health-care provider (good adherence: >95%, moderate adherence: 85–94% and poor adherence: <85%).29
Data Collection Procedure
Informed consent was obtained from all participants above 18 years of age. Data were collected by an experienced nurse working in the ART clinic with a structured questionnaire to collect sociodemographic, clinical, environmental, and personality variables. Information on ART adherence and duration on ART was obtained by reviewing their medical records. Trained medical laboratory personnel performed CD4+ T-cell counts using fluorescence-activated cell sorting (BD FACS) according to the manufacturer’s instructions. In brief, blood specimens were collected aseptically by venipuncture into evacuated tubes containing ethylenediaminetetraacetic acid (EDTA) anticoagulant, completely expanding the vacuum in the tubes. The blood specimens were mixed well to prevent clotting and were labeled. The samples were then placed on a gentle blood rocker to ensure that the samples are uniformly distributed while awaiting the analysis. After quality control had been performed and passed CD4+ T cell count analysis was performed on blood samples using a FACS count–automated machine (Becton Dickinson, USA). Once the reagent pair of tubes had been labeled and mixed using vortex with the pair upright and upside down for about 5 seconds, the tubes were then mixed with the patient’s blood sample by inverting the tube five times after being opened using a coring station. About 50 μL blood was pipetted into each tube, and the tubes were vortexed in the upright position for 5 seconds. Then the tubes were incubated at room temperature in the dark for 60–120 minutes, 50 μL of fixative solution added to each, and mixed upright using the vortex for 5 seconds. After 30 minutes of incubation, the FACS count was run and results were printed out. Viral load was determined using the COBAS Ampliprep/COBAS TaqMan HI2CAP assay, a nucleic acid amplification assay for quantification of HIV-1 RNA in plasma. The assay uses the COBAS Ampliprep instrument for automated sample processing and the COBASTaqMan analyzer for automated amplification and detection. CD4+ T cell categorization and viral load definition were made based on the WHO criteria.
An adequate stool sample (40 g of formed stool and 10 mL of diarrheal stool) was collected from each participant using carefully labeled, dry, leak-proof, and grease-free transparent stool caps and examined macroscopically for adult intestinal parasites, consistency, and other physical abnormalities. A direct saline and iodine wet mount of each sample was used to detect intestinal parasites microscopically. The wet mounts were examined under a light microscope at ×10 and ×40 magnifications for trophozoites and adult worms. Formol-ether concentration method was performed on a portion of each stool sample according to standard procedures. The smear was prepared from the sediment and observed under a light microscope with a magnification of 10× and 40× magnifications for ova, cyst, and larval stage of intestinal parasites. Modified Ziehl-Neelsen staining was performed on a small portion of the concentrated stool sample for detection of oocytes of opportunistic intestinal parasites according to standard operating procedures. A thin smear was prepared directly from the sediment of the concentrated stool and observed under a light microscope with a magnification of 100× for oocytes.
Quality Control
All laboratory materials and reagents have been checked for the expiry date and stored appropriately. Laboratory analyses were carried out using standard operating procedures. Specimens were also checked for labeling identification numbers and procedures of collection. Intestinal coccidian parasite identifications were checked using the colored atlas. A blood specimen was collected by an appropriate tube and transported to the testing laboratory according to standardized procedures. All assays were performed according to the manufacturer’s instructions and strictly followed procedure and steps based on standard operating procedures to perform each test. Running controls (positive and negative) were performed during each batch of tests. To prevent infection during sample collection and analysis, appropriate personal protective equipment was used. Adequate stool specimen was collected using carefully labeled, dry, leak-proof, and grease-free transparent stool caps. The specimen was kept free from water, soil, and urine contamination. Specimens contaminated with water, urine, and soil were rejected and the study participants were requested to bring another. Positive and negative controls were used to check the quality of the microscope and the staining solutions. A direct stool examination was performed within 30 min to avoid delay. All microscopic findings and questionnaire-based information were encoded and reported appropriately. CD4+ T cell categorization and viral load definition were made based on the WHO criteria.
Data Analysis
Using Epi Data 3.1, the data was double-entered and cleaned before being transferred to SPSS version 20 for analysis. The overall sociodemographic, clinical characteristics and specific prevalence of intestinal parasite infectionswere calculated using descriptive statistics. The association between the independent and dependent variables was measured and tested using OR and 95% CI. The relative contribution of each selected variable to the outcome of interest was assessed using logistic regression, and p-values <0.05 were considered statistically significant.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki. Ethical clearance was obtained from the School of Biomedical and Laboratory Science, College of Medicine and Health Sciences, University of Gondar. Permission to conduct the study was also obtained from University of Gondar Comprehensive Specialized Hospital. Written informed consent was obtained from study participants. During the study period, the patients’ results were sent to their follow-up center at ART clinic and parasite-infected subjects were linked to ART clinic by communicating with the team leader of ART clinic for further management of parasite infection.
Result
Socio-Demographic Characteristics of the Study Participants
Three hundred sixteen (316) study participants living with HIV/AIDS were enrolled in this study. Among them, 59.8% (189/316) were females. Most of the participants, 35.8% (113/316), were within the age group of 31–40 years with a mean age of 38.32±10.07. Among the study participants, 86.1% (272/316) had been on ART; 89.6% (283/316) are living in urban areas; 38.3% (121/316) were married, and 21.5% (68/316) were daily laborers (Table 1).
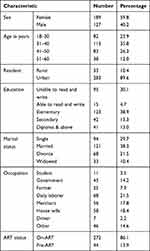 |
Table 1 Sociodemographic Characteristics of the Study Participants at University of Gondar Specialized Hospital Northwest Ethiopia, 2019 |
Prevalence of Intestinal Parasites
The overall prevalence of intestinal parasitosis was 24.7% (78/316). Eleven different intestinal parasite species were identified. The most predominant parasite detected is Cryptosporidium species, which accounts for 5.4% (17/316) followed by Ascaris lumbricoides (A. lumbricoides) 5.1% (16/316). In contrast, the least prevalent parasites were Schistosoma mansoni (S. mansoni) and Microsporidia, which comprise 0.3% (1/316) of each (Figure 1). Mixed infection was identified in 2.2% (7/316), of which A. lumbricoides and S. mansoni detected in one, E. histoletica/dispar and hookworm detected in one, G. Lamblia and E. histolytica/dispar detected in one, A. lumbricoides and T. Trichiura detected in two, S. stercoralis and E. histolytica/dispar detected in two study subjects.
 |
Figure 1 Species of intestinal parasite detected among HIV/AIDS patients at University of Gondar specialized hospital ART clinic, northwest Ethiopia, 2019. |
Intestinal Parasites Infections in Relation to CD4+ T Cell Count, Viral Load, and ART Status
The relationship between CD4+ count and viral load with intestinal parasites infection is shown in Table 2. Twenty four point four percent (19/78) co-infected patients were immunocompetent (CD4+ >500 cell/µL), 44.9% (35/78) presented mild to advanced immunosuppression (CD4+ 201–499 cell/µL), and 30.8% (24/78) presented severe immunosuppression (CD4+ <200 cell/µL). Cryptosporidium species was a leading cause of intestinal parasitosis among severe and mild to advanced immunocompromised HIV patients followed by S. stercoralis. However, A. lumbricoides was a leading cause of intestinal parasitosis among immunocompetent (CD4+ >500 cell/µL) HIV patients followed by Hookworm 15% (3/19).
Viral load <1000 copies per mL are considered with a low probability to develop AIDS and death, in this case, 34.6% (27/78) had their viral load >1000 copies/mL, of which 25.9% (7/27) was infected with Cryptosporidium species followed by S. stercoralis 22.2% (6/27). Of 51 intestinal parasites infected HIV positive individuals who had viral load <1000 copies per mL, the dominantly detected parasite was A. lumbricoides 27.5% (14/51). The majority (75%) of parasites were detected in stage I. In stage III and stage IV the only parasite detected was S. stercoralis alone or co-infected with other parasites (Table 3). The prevalence was found to be 22.1% (60/272) on ART patients and 40.9% (18/44) in Pre-ART patients (Table 2).
Intestinal Parasitosis and Its Association with CD4+ T Cell Count, Viral Load, and Other Factors on HIV Positive Study Participants
In bivariate logistic regression clinical stages, age, ART status, ART adherence, viral load, and CD4 count had shown a significant association with an intestinal parasite. Age, CD4+ T cell count, and viral load remained significantly associated with the intestinal parasite in the multivariable logistic regression analysis. Consequently, the odds of intestinal parasite were 3 times higher in study participants with CD4+ T cell count <200 cells/μL as compared to those who have CD4+ T cell count >500 cells/μL (AOR = 3.207; 95% CI = 1.237, 8.317; P < 0.000). As well, study participants with a viral load >1000 copies/mL were 3 times more likely infected with intestinal parasite than having viral load <1000 copies/mL (AOR = 2.933; 95% CI = 1.326, 6.489; P = 0.008). The odds of the age range of 31–40 years was 70% less likely to be infected with intestinal parasite as compared to those who have the age range of 51–60 years (AOR = 0.305; 95% CI = 0.124, 0.751; P = 0.01). Likewise, the age range of 41–50 years was 74% less likely infected with intestinal parasite as compared to those who have the age range of 51–60 years (AOR = 0.261; 95% CI = 0.101, 0.671; P = 0.005) (Table 4).
Discussion
In this study, the prevalence of intestinal parasitosis and its association with CD4+ count, viral load, and WHO clinical stages of the disease among people living with HIV/ADIS were analyzed. The overall prevalence of intestinal parasites among people living with HIV/AIDS was 24.7%. The high prevalence of parasitic infection among HIV patients might be associated with the HIV/AIDS infection as the spectrum of immunodeficiency progresses, HIV-infected individuals become susceptible to a variety of opportunistic infections hence previously considered nonpathogenic or with transient pathogenic potential in immune-competent individuals, are opportunistically becoming aggressive and causing debilitating illnesses in HIV/AIDS patients.21,22 In another way, even though they are not measured in our study as indicated from previous studies in Gondar,30 the absence of toilet in HIV-positive/AIDS individuals homes, high contaminated water, bad management of waste, and bad drainage system might be contributed to this high prevalence. Our finding is in line with the studies conducted in Tigray Ethiopia (26.4%),31 South Africa (24.7%),32 and Ghana (25.2%).33 However, the result was higher than the reports in Nigeria (11.4%, 15.4%)23,34 and Kombolcha (13.9%).35 In another way, the prevalence was lower than the studies conducted in Burkina Faso (73.3%),36 Nigeria (60.8%),17 Hawassa (47.8%),20 Gondar (29.1%),16 Butajira Ethiopia (35.9%),15 India (32.5%)37 and Jimma (37%).38
Opportunistic parasitic infection is found to be high among those with low CD4+ count and high viral load. Patients with CD4+ T lymphocyte counts below 200 cells/l were shown to have a higher risk of intestinal parasitosis in the current investigation. The leading causes of intestinal parasitosis among HIV patients with CD4+ counts less than 200 cells/μL and 201–499 cells/μL were found to be Cryptosporidium species and S. stercoralis. Nevertheless, A. lumbricoides was the leading cause of intestinal parasitosis in HIV patients with CD4+ count >500 cells/μL. The ultimate goal of initiating ART in HIV patients is to restore CD4+ cell count and protective immunological responses against a wide range of pathogens, resulting in a reduction in the incidence of opportunistic infection and an improvement in the survivability of the patients. However, in some patients with the dysregulated immune response after initiation of ART may result in immune reconstitution inflammatory syndrome; a paradoxical worsening of an existing infection or disease progress or appearance of a new infection/disease process soon after initiation of therapy.39 In line with the studies conducted in Felege Hiwot Referral Hospital in Bahir Dar,40 Ghana,33 and Brazil22 the present study shows that the incidence of intestinal parasites was much higher in people with CD4+ cell counts below 200 cells/µL, demonstrating the importance of CD4+ T lymphocytes in fighting HIV and slowing the course of AIDS, which explains why patients with low CD4+ T cell counts are more likely to be infected by intestinal Coccidia, and why patients with CD4+ T cell counts lower than 500 cells/µL have a higher parasite prevalence. This could be further explained by the fact that the immune system is weakened when CD4+ T cell numbers are low, and as a result, the host cannot eradicate microorganisms that thrive and cause disease.
HIV RNA (viral load) and CD4+ T cell count are the two surrogate markers of the person’s immune status, ART efficiency, and prognosis of AIDS infection development. The intended use of ART is to improve the patient’s immune system, down-regulation of viral replications, and slow the progression of the disease, thus decreasing the incidence of opportunistic infections including intestinal parasitosis. In the present study, patients on ART with viral load >1000 cp/mL showed almost a threefold increased risk of developing intestinal parasitosis compared to those with viral load <1000 cp/mL (AOR 2.933; 95% CI 1.326, 6.489). This finding differs from the result of the study conducted in Mexico41 which showed no significant association between intestinal parasitosis and viral load. The difference might be emanated from either of the three points; 1) the Mexico study used Zinc Sulphate floatation technique for concentration while this study used formol-ether concentration technique or 2) The Mexico study used 30,000 cp/mL of viral load as cut-off value, while this study used 1000 cp/mL or 3) Sample size difference as Mexico study used 109 HIV patient while this study used 316 HIV patients as study participants. Regarding the cut-off value and sample size, it is recommendable to use the WHO standard cut-off value, ie, 1000 cp/mL for viral load29 with recommended concentration techniques on a large sample size.
In this study, the prevalence of intestinal parasitosis was significantly high in Pre-ART as compared to on ART patients. Our finding supports the scientific notion that states increasing the immune status of HIV-infected patients with ART may help to reduce the acquisition of parasite infection.13 Similarly, the prevalence of intestinal parasites was reported to be higher among patients who did not receive ART treatment.34 Our data are also in agreement with the previous results from Gondar and Gojjam.16,24
WHO staging is the strongest predictor of disease progression, survival, and risk of death; however, in this study, WHO staging had failed to associate with intestinal parasitosis significantly in both bivariable and multivariable logistic regression models. This might be because of good adherence to ART and had better awareness about HIV/AIDS and opportunistic infection given by health-care providers when they come to their follow-up at the outpatient department.
Conclusion
The prevalence of intestinal parasites, particularly opportunistic intestinal parasites, was found to be high among HIV/AIDS patients in the current study. Low CD4+ T cell count and high viral load were found to be significantly associated with the risk of developing opportunistic intestinal parasitosis. Compared to HIV patients on ART, Pre-ART patients with a low CD4+ T cell count and a high viral load level had a higher prevalence of intestinal parasitosis. As a result, screening HIV patients for opportunistic intestinal parasitosis using stool concentration techniques would be critical in the effort to prevent and control opportunistic infections among HIV patients, particularly among those with low CD4+ T cell count and high viral load levels.
Acknowledgments
The authors would like to thank University of Gondar for the financial support and the study participants. We would also like to thank University of Gondar Comprehensive Specialized Hospital ART clinic and VCT center staff for their support and good will during data collection.
Disclosure
All authors declared no conflicts of interest for this work.
References
1. Adamu H, Endeshaw T, Teka T, Kifle A, Petros B. The prevalence of intestinal parasites in paediatric diarrhoeal and non-diarrhoeal patients in Addis Ababa hospitals, with special emphasis on opportunistic parasitic infections and with insight into the demographic and socio-economic factors. Ethiopian J Health Develop. 2006;20(1):39–46. doi:10.4314/ejhd.v20i1.10010
2. Chan MS. The global burden of intestinal nematode infections fifty years on. Parasitol Today. 1997;13(11):438–443. doi:10.1016/S0169-4758(97)01144-7
3. Tefera T, Mebrie G. Prevalence and predictors of intestinal parasites among food handlers in Yebu town, southwest Ethiopia. PLoS One. 2014;9(10):e110621. doi:10.1371/journal.pone.0110621
4. Yeshitila YG, Zewde H, Mekene T, Manilal A, Lakew S, Teshome A. Prevalence and associated risk factors of intestinal parasites among schoolchildren from two primary schools in Rama Town, Northern Ethiopia. Can J Infect Dis Med Microbiol. 2020;2020:25.
5. Dwyer-Lindgren L, Cork MA, Sligar A, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189–193. doi:10.1038/s41586-019-1200-9
6. Gopinath R, Ostrowski M, Justement SJ, Fauci AS, Nutman TB. Filarial infections increase susceptibility to human immunodeficiency virus infection in peripheral blood mononuclear cells in vitro. J Infect Dis. 2000;182(6):1804–1808. doi:10.1086/317623
7. Harms G, Feldmeier H. HIV infection and tropical parasitic diseases deleterious interactions in both directions? Trop Med Int Health. 2002;7(6):479–488. doi:10.1046/j.1365-3156.2002.00893.x
8. Karanja DM, Colley DG, Nahlen BL, Ouma JH, Secor WE. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am J Trop Med Hyg. 1997;56(5):515–521. doi:10.4269/ajtmh.1997.56.515
9. Tawill SA, Gallin M, Entmann KD, Kipp W, Bamuhiiga J, Büttner DW. Impaired antibody responses and loss of reactivity to Onchocerca volvulus antigens by HIV-seropositive onchocerciasis patients. Trans R Soc Trop Med Hyg. 1996;90(1):85–89. doi:10.1016/S0035-9203(96)90488-5
10. Mayer KH, Karp CL, Auwaerter PG, Mayer KH. Coinfection with HIV and tropical infectious diseases. II. Helminthic, fungal, bacterial, and viral pathogens. Clin Infect Dis. 2007;45(9):1214–1220. doi:10.1086/522180
11. Nielsen NO, Simonsen PE, Dalgaard P, et al. Effect of diethylcarbamazine on HIV load, CD4%, and CD4/CD8 ratio in HIV-infected adult Tanzanians with or without lymphatic filariasis: randomized double-blind and placebo-controlled cross-over trial. Am J Trop Med Hyg. 2007;77(3):507–513. doi:10.4269/ajtmh.2007.77.507
12. Nkenfou CN, Nana CT, Payne VK. Intestinal parasitic infections in HIV infected and non-infected patients in a low HIV prevalence region, West-Cameroon. PLoS One. 2013;8:e57914. doi:10.1371/journal.pone.0057914
13. World Health Organization. [2019-02-20]. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach - 2010 revision; 2010. Available from: https://www.who.int/hiv/pub/arv/adult2010/en/webcite.
14. World Health Organization. [2019-02-20]. Guideline on when to start antiretroviral therapy on pre-exposure prophylaxis for HIV; 2015. Available from: https://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/webcite.
15. Gedle D, Kumera G, Eshete T, Ketema K, Adugna H, Feyera F. Intestinal parasitic infections and its association with undernutrition and CD4 T cell levels among HIV/AIDS patients on HAART in Butajira, Ethiopia. J Health Popul Nutr. 2017;36(1):1–10. doi:10.1186/s41043-017-0092-2
16. Eshetu T, Sibhatu G, Megiso M, et al. Intestinal parasitosis and their associated factors among people living with HIV at University of Gondar Hospital, Northwest-Ethiopia. Ethiop J Health Sci. 2017;27(4):411–420. doi:10.4314/ejhs.v27i4.12
17. Joseph A, Ano-Edward G. Correlation of viral load to intestinal parasitosis in HIV seropositive patients attending UITH, Ilorin. Ann Trop Pathol. 2016;7(1):49.
18. Shimelis T, Tassachew Y, Lambiyo T. Cryptosporidium and other intestinal parasitic infections among HIV patients in southern Ethiopia: significance of improved HIV-related care. Parasit Vectors. 2016;9(1):1–7. doi:10.1186/s13071-016-1554-x
19. Alemayehu E, Gedefie A, Adamu A, et al. Intestinal parasitic infections among HIV-infected patients on antiretroviral therapy attending debretabor general hospital, Northern Ethiopia: a cross-sectional study. HIV/AIDS (Auckland, NZ). 2020;12:647.
20. Alemu G, Alelign D, Abossie A. Prevalence of opportunistic intestinal parasites and associated factors among HIV patients while receiving ART at Arba Minch Hospital in southern Ethiopia: a cross-sectional study. Ethiop J Health Sci. 2018;28(2):147–156. doi:10.4314/ejhs.v28i2.6
21. Bissong M, Nguemain N, Ng’awono T, Kamga F. Burden of intestinal parasites amongst HIV/AIDS patients attending Bamenda regional Hospital in Cameroon. Afr J Clin Exp Microbiol. 2015;16(3):97–103. doi:10.4314/ajcem.v16i3.3
22. Barcelos NB, Silva LD, Dias RF, Menezes HR, Rodrigues RM. Opportunistic and non-opportunistic intestinal parasites in HIV/AIDS patients in relation to their clinical and epidemiological status in a specialized medical service in Goiás, Brazil. Revista Do Instituto de Medicina Tropical de São Paulo. 2018;8:60.
23. Jegede EF, Oyeyi ET, Bichi AH, Mbah HA, Torpey K. Prevalence of intestinal parasites among HIV/AIDS patients attending Infectious Disease Hospital Kano, Nigeria. Pan Afr Med J. 2014;17. doi:10.11604/pamj.2014.17.295.3707
24. Tadesse A, Worku A, Girma A. Prevalence of intestinal parasites and associated factors among adult pre-ART and ART patients in Goncha Siso Enesie Woreda, East Gojjam, Northwest Ethiopia, 2014. J AIDS Clin Res. 2017;8:10.
25. Missaye A, Dagnew M, Alemu A, Alemu A. Prevalence of intestinal parasites and associated risk factors among HIV/AIDS patients with pre-ART and on-ART attending dessie hospital ART clinic, Northeast Ethiopia. AIDS Res Ther. 2013;10(1):7. doi:10.1186/1742-6405-10-7
26. EthioVisit. Ethiopia Administrative Regions, Cities and Population. Available from: http://www.ethiovisit.com/ethiopia/ethiopia-regions-and-cities.html.
27. Stafford KA, Nganga LW, Tulli T, Foreit KGF. Factors associated with outcomes of Pre-ART HIV care. J Int Assoc Prov AIDS Care (JIAPAC). 2018;17:2325958218759602. doi:10.1177/2325958218759602
28. Health fmo. National Guidelines for Comprehensive HIV Prevention catF; 2017.
29. Achappa B, Madi D, Bhaskaran U, Ramapuram JT, Rao S, Mahalingam S. Adherence to antiretroviral therapy among people living with HIV. N Am J Med Sci. 2013;5(3):220. doi:10.4103/1947-2714.109196
30. Yallew WW, Terefe MW, Herchline TE, et al. Assessment of water, sanitation, and hygiene practice and associated factors among people living with HIV/AIDS home based care services in Gondar city, Ethiopia. BMC Public Health. 2012;12:1057. doi:10.1186/1471-2458-12-1057
31. Gebrewahid T, Gebrekirstos G, Teweldemedhin M, Gebreyesus H, Awala A, Tadla K. Intestinal parasitosis in relation to CD4 count and anemia among ART initiated patients in St. Mary Aksum general hospital, Tigray, Ethiopia. BMC Infect Dis. 2019;19(1):350. doi:10.1186/s12879-019-3989-0
32. Adeleke OA, Yogeswaran P, Wright G. Intestinal helminth infections amongst HIV-infected adults in Mthatha General Hospital, South Africa. Afr J Prim Health Care Fam Med. 2015;7(1):1–7. doi:10.4102/phcfm.v7i1.910
33. Tay SC, Aryee ENO, Badu K. Intestinal Parasitemia and HIV/AIDS Co-Infections at Varying CD4+ T-Cell Levels. HIV/AIDS Res Treat Open J. 2017;4(1):40–48. doi:10.17140/HARTOJ-4-126.
34. Olopade BO, Idowu CO. Intestinal parasites among HIV-infected patients at obafemi awolowo university teaching hospitals complex, Ile-Ife. Ann Trop Pathol. 2017;8(1):34. doi:10.4103/atp.atp_19_17
35. Gebretsadik D, Haileslasie H, Feleke DG. Intestinal parasitosis among HIV/AIDS patients who are on anti-retroviral therapy in Kombolcha, North Central, Ethiopia: a cross-sectional study. BMC Res Notes. 2018;11(1):613. doi:10.1186/s13104-018-3726-6
36. Zida A, Yacouba A, Sawadogo M, Diallo I, Sangare I. Opportunistic and other intestinal parasites infections among HIV-positive patients in the era of combination antiretroviral therapy and preventive treatment in Ouagadougou, Burkina Faso. J HIV Clin Sci Res. 2017;4(2):008–0014.
37. Khalil S, Mirdha BR, Sinha S, et al. Intestinal parasitosis in relation to anti-retroviral therapy, CD4+ T-cell count and diarrhea in HIV patients. Korean J Parasitol. 2015;53(6):705. doi:10.3347/kjp.2015.53.6.705
38. Tadesse G, Zeynudin A, Mekonnen Z, Taha M, Adamu H, Kebede A. Intestinal parasitosis among HIV sero positive in Jimma, Ethiopia. J Trop Dis. 2013;01(04). doi:10.4172/2329-891X.1000122
39. DeSimone JA, Pomerantz RJ, Babinchak TJ; IRIR Definition. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med. 2000;133:447. doi:10.7326/0003-4819-133-6-200009190-00013
40. Kiros H, Nibret E, Munshea A, Kerisew B, Adal M. Prevalence of intestinal protozoan infections among individuals living with HIV/AIDS at Felegehiwot Referral Hospital, Bahir Dar, Ethiopia. Int J Infect Dis. 2015;35:80–86. doi:10.1016/j.ijid.2015.04.012
41. Quihui-Cota L, Valencia ME, Crompton DW, et al. Prevalence and intensity of intestinal parasitic infections in relation to nutritional status in Mexican schoolchildren. Trans R Soc Trop Med Hyg. 2004;98(11):653–659. doi:10.1016/j.trstmh.2003.12.017 PMID: 15363645.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



