Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Interstitial Granulomatous Drug Reaction Due to Chemotherapy
Received 6 September 2023
Accepted for publication 28 November 2023
Published 20 December 2023 Volume 2023:16 Pages 3625—3628
DOI https://doi.org/10.2147/CCID.S439009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Qiuli Zhang, Jianmin Chang
Department of Dermatology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
Correspondence: Jianmin Chang, Department of Dermatology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, No. 1 DaHua Road, Dong Dan, Beijing, 100730, People’s Republic of China, Tel +86- 010-85133303, Email [email protected]
Abstract: Interstitial granulomatous drug reaction (IGDR) is a drug-related disease with distinctive clinical and histopathological features uncommon in clinical practice. Chemotherapeutics-related IGDR has rarely been reported. Here, we describe one case of interstitial granulomatous drug reaction due to chemotherapy.
Keywords: interstitial granulomatous drug reaction, chemotherapy
Introduction
Interstitial granulomatous drug reaction (IGDR) is a drug-related disease with distinctive clinical and histopathological features uncommon in clinical practice. Magro et al reported and named IGDR firstly in 1988.The pathogenesis of IGDR remains elusive, but it is considered to be related to the drug hypersensitivity of the body. Currently, multiple drugs that can induce IGDR have been reported, including calcium ion channel (CaV channel) blockers, angiotensin-converting enzyme inhibitors (ACEI), β-blocker, hypolipidemic drugs, antihistamines, antidepressants (imipramine),1 antiepileptic drugs (tropoelastin),2 diuretic drugs (hydrochlorothiazide),3 Chinese herbal preparation,4 and immune checkpoint inhibitor.5 IGDR is an uncommon inflammatory drug reaction to a wide range of drugs. Chemotherapeutics-related IGDR has rarely been reported. Patients with IGDR related to chemotherapeutics may face a challenge whether to discontinue the previous chemotherapies. Here, we describe one case of interstitial granulomatous drug reaction due to chemotherapy.
Case Report
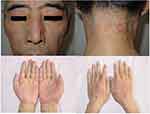
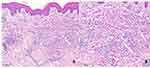
A 58-year-old man visited our hospital for sustaining erythematous papules and plaques in his face, neck, and hands for one week, with mild tenderness to palpation and expanding rashes. Two weeks before the illness onset, the patient was diagnosed with stage IVB classic nodular sclerosis Hodgkin lymphoma, and thus ABVD chemotherapy (adriamycin, bleomycin, vinblastine, and dacarbazine) was administrated. He had no history of other diseases and previous drug allergic reactions. Dermatological examination showed multiple erythematous papules and plaques symmetrically distributed in both upper eyelids, cheeks, dorsal and palms of both hands, and the posterior neck; there were a few white scales on the surface of the lesions (Figure 1). The histopathology of the skin lesions revealed approximately normal epidermis, but diffuse interstitial and perivascular histiocytic infiltrates with scattered neutrophils, nuclear dust, and eosinophils (Figure 2A and B). Complete blood count and urinalysis were normal and he tested negative for ANA, ENA, ANCAs. IGDR was diagnosed based on clinical history and clinical, pathological features. After 3 weeks of treatment with topical 0.1% mometasone furoate once daily, the rashes gradually improved without further exacerbation.
Discussion
IGDR generally spans a long time from drug intake to IGDR onset (occurrence of skin lesion), which was reported to be between 4 weeks and 25 years (mean: 5 years). In the patient reported here, rashes occurred 2 weeks after receiving chemotherapeutics. The latency period was shorter than that reported in the literature, which might be attributable to the patient’s specific condition, the drugs administrated, and environmental factors.
As IGDR is rare in clinical practice, no investigations on the high-risk population have been performed. Patients often present with red to purplish plaques after IGDR onset, common in the popliteal and axillary areas, and can also occur in the proximal extremities, palms, and soles. Other manifestations include scaly erythematous plaques of psoriasis,3 infiltrated erythematous papules/plaques,6 erythrodermic lesions, erythema nodosum-like lesions, generalized erythematous papules/patches, and painful palmoplantar papules/plaques.1 Its various clinical manifestations determine the need to differentiate it from other diseases with similar manifestations such as psoriasis and autoimmune diseases. The histopathology and hematologic examination can help differentiate between these conditions.
Histopathologically, IGDR leads to diffuse infiltrations of interstitial histiocytes and lymphocytes in the dermis, sometimes with granulomas and multinucleated giant cells accompanying denatured collagens and elastin fibers. Additionally, there is a 50% chance of eosinophil infiltration and lymphocyte atypia (epidermotropic, interstitial, or in the dermal-epidermal junction), as well as interface dermatitis changes and necrotic keratinocytes. No vasculitis or mucin deposition is reported generally.
The diagnosis of IGDR is mainly based on the clinical presentations, histopathological findings, and medication history of patients. In histopathology, IGDR should be distinguished from interstitial granulomatous dermatitis (IGD) and interstitial granuloma annulare. Some scholars have believed that IGDR is a disease independent of drug-induced IGD. IGD is usually accompanied by arthritis or joint pain with concomitant system diseases, such as rheumatoid arthritis and systemic lupus erythematosus. Although histopathologically, eosinophil infiltration and lymphocyte atypia are not common. Interstitial granuloma annulare is generally present with skin-colored or red papules that can form annular or non-annular plaques or sometimes patches. Pathologically, the lesions are focal with shallow invasion, and no vacuolar degeneration of basal cells, lymphocyte atypia, or epidermotropic phenomenon is presented. Histological features that favor IGDR include vacuolar interface dermatitis with diffuse interstitial infiltrate of histiocytes and lymphocytes, prominently eosinophils, with or without lymphoid atypia.
After the withdrawal of implicated drugs, skin lesions can subside gradually within 1–40 weeks (mean: 8 weeks),3 and the period is substantially longer than the conventional drug reaction time. Thus, prompt identification and withdrawal of related drugs is conducive to the remission and resolution of skin lesions. If the causative drug is necessary or cannot be withdrawn because of various causes, or there is no alternative, topical glucocorticoids, or oral glucocorticoids, dapsone, and Alitretinoin may have some role in relieving IGDR.3,6 For our patient, he could not stop chemotherapy to control his primary disease and topical corticosteroid was effective.
Conclusion
Chemotherapeutics can result in interstitial granulomatous drug reaction. We give a case report help clinicians to better diagnose this entity. A careful history and histological findings are crucial for diagnosis. The most important treatment for IGDR is withdrawal of offending medication. But treatment with topical corticosteroids may be effective for patients who cannot discontinue the chemotherapeutics to treat primary malignant diseases.
Consent Statements
Written informed consent was provided by the patient to have the case details and accompanying images published. Institutional approval was not required to publish the case details.
Funding
There is no funding to report.
Disclosure
No potential conflict of interest was reported by the author(s).
References
1. Pascucci A. Granulomatous drug eruption associated with imipramine. JAAD Case Rep. 2018;4(2):152–154. doi:10.1016/j.jdcr.2017.11.004
2. Brown-Joel ZO, Stone MS. Reactive granulomatous dermatitis in association with topiramate ingestion. Clin Exp Dermatol. 2019;44(7):833–834. doi:10.1111/ced.13923
3. Grose E, Ramien M. Interstitial granulomatous drug reaction related to hydrochlorothiazide. Dermatol Online J. 2019;25(7):
4. Lee HW, Yun WJ, Lee MW, et al. Interstitial granulomatous drug reaction caused by Chinese herbal medication. J Am Acad Dermatol. 2005;52(4):712–713. doi:10.1016/j.jaad.2004.11.028
5. Trinidad C, Nelson KC, Glitza Oliva IC, et al. Dermatologic toxicity from immune checkpoint blockade therapy with an interstitial granulomatous pattern. J Cutan Pathol. 2018;45(7):504–507. doi:10.1111/cup.13150
6. Aria AB, Chen L, Huen AO. A case report of bosutinib-induced interstitial granulomatous drug reaction in a patient with chronic myelogenous leukemia: a case report. SAGE Open Med Case Rep. 2018;6:2050313X18795075. doi:10.1177/2050313X18795075
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.